Research Article - Biomedical Research (2017) Volume 28, Issue 20
Protective effects of sildenafil and resveratrol on ovarian ischemiareperfusion injury in rats
Nazile Erturk1, Hulya Elbe2, Mehmet Erman Erdemli3, Serdar Aktas4, Gurkan Yigitturk5, Zeynep Aksungur3, Alev Suzen6, Feral Ozturk2
1Department of Paediatric Surgery, Faculty of Medicine, Mugla Sitki Kocman University, Turkey
2Department of Histology and Embryology, Faculty of Medicine, Mugla Sitki Kocman University, Turkey
3Department of Medical Biochemistry, Faculty of Medicine, Omer Halisdemir University, Turkey
4Department of Pharmacology and Toxicology, Faculty of Veterinary Medicine, Adnan Menderes University, Turkey
5Department of Histology and Embryology, Faculty of Medicine, EGE University, Turkey
6Department of Paediatric Surgery, Mugla Sitki Kocman University Education and Research Hospital, Turkey
- *Corresponding Author:
- Nazile Erturk
Asst. Prof., Faculty of Medicine
Department of Pediatric Surgery
Mugla Sitki Kocman University, Turkey
Accepted date: December 11, 2017
Abstract
Aim: This study investigated the protective effects of sildenafil (SIL) and resveratrol (RSV) on ovarian ischemia-reperfusion (I/R) injury.
Materials and Methods: Forty-Eight Wistar albino rats were divided equally into groups: control (sham), ischemia (2 h ischemia), I/R (2 h ischemia+2 h reperfusion), I/R+RSV (10 mg/kg intraperitoneally), I/R+SIL (1.4 mg/kg intraperitoneally), and I/R+RSV+SIL. RSV and SIL were administered 30 min before the end of the ischemia period, and reperfusion was carried out for 2 h. The ovaries were then removed and evaluated histopathologically for mean histopathological damage score (MHDS) and anti-inducible nitric oxide (NO) synthase (iNOS) antibody. Malondialdehyde (MDA) level, glutathione (GSH) activity, total antioxidant status (TAS), total oxidant status (TOS), and NO level were measured.
Results: Histopathological findings such as enema, inflammation, haemorrhage, and congestion were detected in the ischemia and I/R groups. Compared with controls, these groups had significantly higher MHDSs. Compared with the ischemia and I/R groups, the treatment groups had significantly decreased MHDSs. iNOS immunostaining was most evident in the ischemia and I/R groups, and TOS and MDA and NO levels were significantly increased, whereas GSH activity and TAS were significantly decreased compared with those in controls. TOS and MDA and NO levels were significantly decreased, and GSH activity and TAS were significantly increased in the treatment groups.
Conclusion: RSV and SIL decreased histopathological and biochemical damage and exerted protective effects on I/R-induced ovarian damage. SIL and RSV act simultaneously to reduce tissue injury.
Keywords
Ovarian torsion, Ischemia-reperfusion, Sildenafil, Resveratrol, NO
Introduction
Ovarian torsion (OT) occurs when ovaries twist twice around vascular structures. Without timely diagnosis, OT disrupts ovarian blood flow and eventually causes tissue necrosis [1]. Hence, early diagnosis and treatment are vital for preserving ovarian viability. OT affects women in all age groups, with 15% of cases occurring in children [2]. OT has an incidence of nearly 4.9/100,000 among females aged 1 to 20 years [3].
Even when tissues become cyanotic during OT, torsioned ovaries should be detorsioned and tissue perfusion reestablished to avert future infertility [4]. The aim of treatment is not only to provide tissues with adequate blood flow but also to restore tissue perfusion [4]. Tissue injury occurring during reperfusion after ovarian torsion–detorsion is called ischemiareperfusion (I/R) injury [5], and it is defined as the microvascular and parenchymal cellular dysfunction of ischemic organs after ischemia [4,6-8]. I/R injury is mediated by reactive oxygen species (ROS) that form as a result of lipid peroxidation [8,9] and release inflammatory substances. Eliminating the effects of ROS may prevent or minimize I/R injury [5]. Various therapeutic agents have been used to reduce tissue injury after ovarian I/R injury [4,5,7,10,11] and ovarian function returns to normal after detorsioning in a majority of OT cases (~95%) [12]. However, because the clinical signs of OT are nonspecific, detorsion surgery is often delayed and ischemia progresses. Developing novel pharmacological agents to prevent I/R injury in delayed cases of OT is of great clinical importance.
Sildenafil (SIL) is a specific and selective inhibitor of phosphodiesterase type V, an enzyme that regulates cyclic guanosine monophosphate (cGMP) catabolism in the vascular bed. SIL increases cellular cGMP levels, thereby augmenting the effects of nitric oxide (NO). Increased cGMP levels mediate the relaxation of smooth muscle cells and increase blood flow to tissues [13].
NO is a highly reactive molecule with a range of physiological functions. This messenger plays a key role in the modulation of vascular tone, neurotransmission, and the immune system [14]. In mammals, NO is synthesized from L-arginine by a family of enzymes referred to as the NO synthases [14,15]. In addition to the constitutive endothelial and neuronal forms, an inducible NO synthase (iNOS) also exists [14]. iNOS activity has been demonstrated in a wide array of cells and tissues, including macrophages, chondrocytes, Kupffer cells, hepatocytes, neutrophils, pulmonary epithelium, colonic epithelium, vasculature, and various neoplastic tissues [15].
Resveratrol (3,5,4)-trans-trihydroxystilbene (RSV) is a natural phytoalexin found in grapes, peanuts, mulberries, and red wine. In addition to its anti-inflammatory effects, it has anticancer and antioxidant properties [16]. Although its exact mechanism of action is unknown, it is thought to act by inhibiting ribonucleotide reductase and DNA polymerase through antioxidant activity, as well as via the transcription of cyclooxygenase 2 [17]. Several recent studies have indicated that RSV has a protective effect against renal, ovarian, and testicular I/R injury [16-18].
Although studies examining the effects of SIL and RSV on I/R-induced ovarian injury have been conducted, no study has yet investigated their combined effects. Moreover, we found no studies of the effects of SIL and RSV on NO. The aim of the present study was to use histological and biochemical methods to investigate the individual and combined effects of SIL and RSV on ovarian I/R injury.
Nowadays maternal age for pregnancy is older than previous [19]. Because of that ovarian reserve is more important. Interventions to protect each follicle are valuable. The aim of present study protocol was based on the prevention ovary in cases ovarian torsion.
Materials and Methods
Animals
After the approval of the study protocol by the Adnan Menderes University Animal Studies Local Ethics Committee (13.01.2016/010), 48 Wistar albino rats were obtained from the Experimental Animal Laboratory (Ankara, Turkey). The rats were 70 days old on average and weighed 200-250 g. The animals were maintained at the appropriate temperature and humidity with a 12 h dark-light cycle and ad libitum access to food and water for 7 days before the experiment. Then, the rats were randomly divided into 6 groups of 8 rats each: (1) control (sham), (2) torsion (ischemia-I), (3) torsion–detorsion (I/R), (4) torsion–detorsion+SIL (I/R+SIL), (5) torsion–detorsion+RSV (I/R+RSV), and (6) torsion–detorsion+SIL+RSV (I/R+SIL +RSV).
The rats were fasted for 6 h before the surgical procedure. After being weighed, the rats were anesthetized with intramuscular ketamine hydrochloride (50 mg/kg; Ketalar; Eczacibasi, Istanbul, Turkey) and xylazine hydrochloride (10 mg/kg; Rompun; Bayer Turk Ilac Ltd., Istanbul, Turkey).
Surgical and pharmacological procedures
After appropriate preoperative sterilization, the abdominal cavity was entered through a subumbilical median incision (2.5 cm). In the control group, both ovaries were observed, and the abdomen was closed using 3/0 silk suture (sham operation). After 2 h, relaparotomy was carried out and both ovaries were surgically removed. Bilateral ovarian I/R was achieved with the slip-knot technique, as described by Coskun et al. [20], using 3/0 silk suture: “to achieve complete vascular occlusion, two adjacent sutures were passed underneath the ovary; one loop occluding the blood flow between the uterine horn and ovary, and the other loop occluding the blood flow between the ovary and omentum. The arm of the suture was passed under the other arm to form a loop-like bowknot”.
In the ischemia group, both ovaries were surgically removed during relaparotomy at the end of a 2 h ischemia period. In the I/R groups, both ovaries were subjected to ischemia for 2 h and reperfusion for 2 h. Then, both ovarian tissues were surgically removed. Thirty minutes before the end of the ischemia period, SIL (1.4 mg/kg; Sigma Chemicals, USA) was administered orally to the animals in group 4, RSV (10 mg/kg; Sigma Chemicals) in group 5, and SIL (1.4 mg/kg) +RSV (10 mg/kg) in group 6. The dosing and administration procedures followed previously described protocols [4,10]. Both ovaries were removed at the end of the I/R period.
The rats were killed at the completion of all surgical procedures. The excised ovarian tissue was liberated from other soft tissues, after which the right ovary was preserved at -80 °C in a deep freezer for biochemical analyses and the left was placed in a 10% formaldehyde solution for histological examinations.
Histological evaluation
The samples selected for histological examination were placed in buffered 10% formalin solution and embedded in paraffin. The paraffin blocks were cut at 5 μm, mounted on slides, and stained with haematoxylin-eosin (H–E) for evaluation of general ovarian structure, including severity of ovarian damage such as oedema, inflammation, haemorrhage, and congestion. Mean histopathological damage score (MHDS) was calculated. For this analysis, each slide for each criteria was semiquantitatively graded as follows: absent (0), mild (1), moderate (2), and severe (3). The maximum score was 12. All sections were examined using a Nikon Eclipse 80i light microscope and Nikon Image Analysis system.
Immunohistochemical (IHC) evaluation
For IHC analysis, sections were mounted on polylysine-coated slides. After rehydration, the samples were transferred to citrate buffer (pH 7.6) and heated in a microwave oven for 20 min. After cooling for 20 min at room temperature, the sections were washed with phosphate buffered saline (PBS), maintained in 0.3% H2O2 for 7 min, and washed again with PBS. The sections were incubated with an anti-rabbit iNOS antibody (ab15323, 1:100; Abcam) for 60 min. They were then rinsed in PBS and incubated with biotinylated goat antipolyvalent for 10 min and streptavidin peroxidase for 10 min at room temperature.
Staining was completed with chromogen+substrate for 15 min and the slides were counterstained with Mayer’s haematoxylin for 1 min, rinsed in tap water, and dehydrated. The antibody was used according to the manufacturer’s instructions. Cytoplasmic staining for iNOS in ovarian cells was identified as a brown colour. The relative intensity of iNOS immunostaining within cells was scored as follows: absent (0), slight (1), moderate (2), and severe (3). All sections were examined with the Nikon Eclipse 80i light microscope and Nikon Image Analysis system.
Biochemical analysis
Malondialdehyde (MDA) levels and glutathione (GSH) activity: The remaining samples were homogenized in ice-cold 0.1 M Tris HCl buffer (pH 7.5) containing protease inhibitor and phenylmethylsulfonyl fluoride (1 mM) with a homogenizer (IKA Ultra Turrax T 25 basic) at 16,000 rpm for 2 min at 4°C. The homogenates were used to measure the MDA level and the GSH activity. MDA, referred to as a thiobarbituric acid reactive substance, was measured with thiobarbituric acid at 535 and 520 nm in a spectrophotometer, as previously described [21]. The results were reported as nanomoles per gram of wet tissue. Reduced GSH assay GSH concentrations in the homogenates were measured according to the spectrophotometric method of Ellman [22]. Results were reported as nanomoles per gram of wet tissue.
Total oxidant status (TOS) and total antioxidant status (TAS): TOS was measured with enzyme-linked immunosorbent assay set to 25°C according to the manufacturer’s instructions, with a mix of 500 mL reagent 1 (measuring buffer) and 75 mL. The absorbance was measured at 530 nm. Then, 25 mL of reagent 2 (pro-chromogen solution) was added. After 10 min of incubation, TOS was determined by measuring the absorbance at 530 nm [23].
TAS measurement also followed the kit procedure, in which the enzyme-linked immunosorbent assay was set at 25°C, 500 μL of reagent 1 (measurement buffer) and 30 μL of serum were mixed, and the absorbance was measured at 660 nm. Then, 75 μL of reagent 2 (coloured 2,2-azinobis [3- ethylbenzothiazoline-6-sulfonic acid] diammonium salt solution) was added to the mixture. After 10 min of incubation, TAS was determined by measuring the absorbance at 660 nm (24). The results were reported in micromoles per liter.
NO levels: NO is a labile compound that is converted rapidly to the stable end-products nitrate (NO3) and nitrite (NO2). Because total nitrite was considered an index of endogenous NO production, NO levels were measured as total nitrite (25). The results were reported in nanomoles per gram of wet tissue.
Statistical Analysis
Statistical analysis was carried out using SPSS for Windows version 14.0 (SPSS Inc., Chicago, IL, USA). All data are expressed as arithmetic means ± standard error of the mean. Normality for continuous variables in groups was determined with the Shapiro–Wilk test. The variables did not show normal distribution (p<0.05). The Kruskal–Wallis and Mann–Whitney U tests were used to compare variables among the studied groups. A p value of <0.05 was considered statistically significant.
Results
Histopathological results
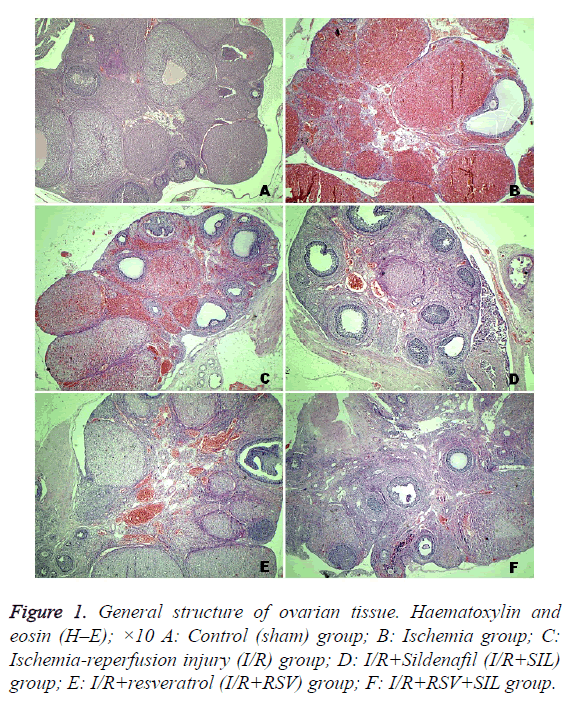
Tissue from the control group had a normal histological appearance. The follicles and corpus luteum were healthy (Figure 1A). On the contrary, tissue from the ischemic ovaries was bluish-black. In the ischemia and I/R group ovaries, severe histopathological findings were detected, including interstitial oedema, inflammatory cell infiltration, marked haemorrhage, and congested blood vessels (Figures 1B and 1C). Compared with the MHDS of 0.12 ± 0.12 in the control group, those in the ischemia group (8.87 ± 0.22) and I/R group (8.25 ± 0.36) were significantly different (p<0.05). The ovarian tissues in the I/R+SIL, I/R+RSV, and I/R+SIL+RSV groups showed minimal histopathological change (Figures 1D-1F) and had significantly decreased MHDSs (5.62 ± 0.18, 5.25 ± 0.16, and 4.87 ± 0.29, respectively) compared with those in the damage groups (p<0.05). The MHDS data are shown in Table 1.
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |
|---|---|---|---|---|---|---|
| MHDSs | 0.12 ± 0.12 | 8.87 ± 0.22a | 8.25 ± 0.36a | 5.62 ± 0.18a,b,d | 5.25 ± 0.16a,c | 4.87 ± 0.29a,e |
Table 1: The mean histopathological damage score (MHDS) of all groups.
IHC findings
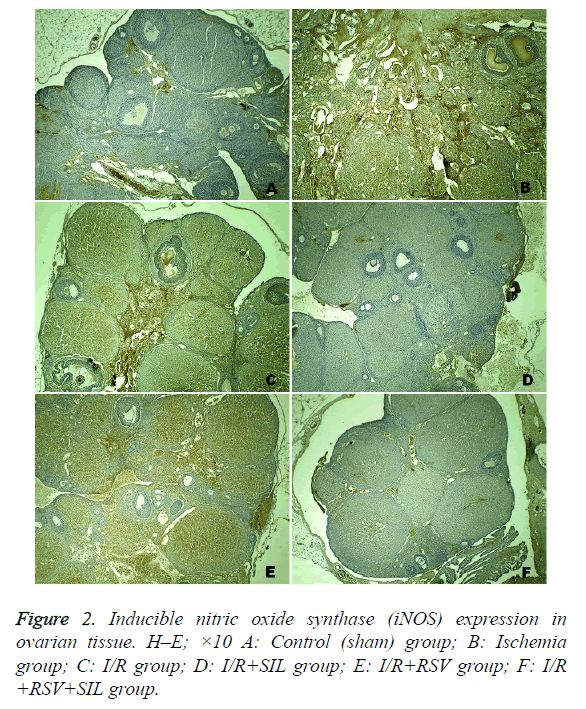
Positive iNOS immunostaining was weak in the ovary sections of the control group and was most evident in the ischemia and I/R groups. Comparatively, in the same areas in the I/R+SIL, I/R+RSV, and I/R+SIL+RSV groups’ tissues, iNOS immunostaining intensity was decreased (Figure 2). Although iNOS expression was significantly reduced in the I/R+RSV group compared with that in the ischemia and I/R groups, it was also significantly different from the control and I/R groups administered SIL (groups 4 and 6). Comparison among the treatment groups showed that iNOS expression in the SILadministered groups (groups 4 and 6) was significantly lower than that in the RSV-administered group (group 5; p=0.005 and p=0.008, respectively). The iNOS immunostaining scores are shown in Table 2.
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |
|---|---|---|---|---|---|---|
| Score | 0.62 ± 0.18 | 2.37 ± 0.18a | 2.12 ± 0.22b | 0.62 ± 0.18c,d | 1.62 ± 0.18e,f | 0.87 ± 0.12g,h,j |
Table 2: The iNOS immunostaining scores of all groups.
Biochemical findings
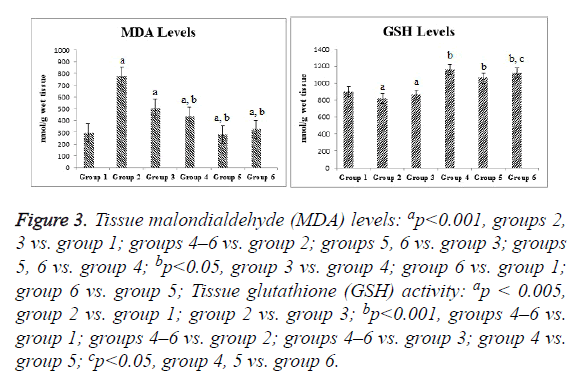
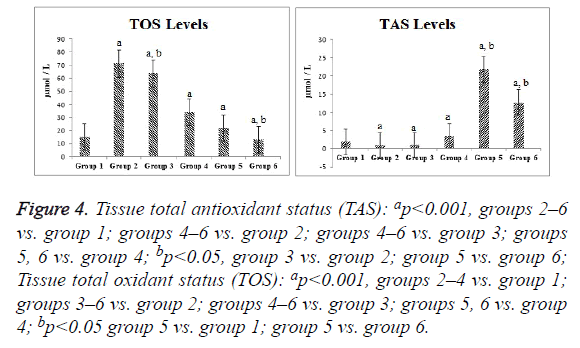
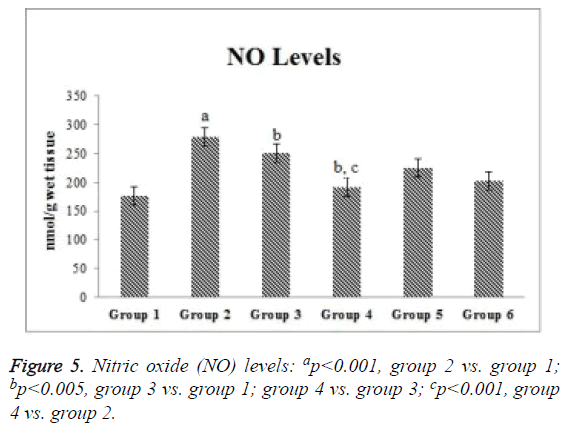
Compared with the control group, the ischemia and I/R groups showed significantly increased TPS and MDA and NO levels and significantly decreased GSH activity and TAS (p<0.05). Compared with the ischemia and I/R groups, the treatment groups showed significantly decreased TOS and MDA and NO levels (p<0.05), and significantly increased TAS and GSH activity (p<0.05). A significant difference was found among the treatment groups with regard to all biochemical parameters (p<0.05). The mean tissue biochemical values of all of the groups are shown in Figures 3-5.
Figure 3: Tissue malondialdehyde (MDA) levels: ap<0.001, groups 2, 3 vs. group 1; groups 4–6 vs. group 2; groups 5, 6 vs. group 3; groups 5, 6 vs. group 4; bp<0.05, group 3 vs. group 4; group 6 vs. group 1; group 6 vs. group 5; Tissue glutathione (GSH) activity: ap < 0.005, group 2 vs. group 1; group 2 vs. group 3; bp<0.001, groups 4–6 vs. group 1; groups 4–6 vs. group 2; groups 4–6 vs. group 3; group 4 vs. group 5; cp<0.05, group 4, 5 vs. group 6.
Figure 4: Tissue total antioxidant status (TAS): ap<0.001, groups 2–6 vs. group 1; groups 4–6 vs. group 2; groups 4–6 vs. group 3; groups 5, 6 vs. group 4; bp<0.05, group 3 vs. group 2; group 5 vs. group 6; Tissue total oxidant status (TOS): ap<0.001, groups 2–4 vs. group 1; groups 3–6 vs. group 2; groups 4–6 vs. group 3; groups 5, 6 vs. group 4; bp<0.05 group 5 vs. group 1; group 5 vs. group 6.
Discussion
OT is the fifth most common cause of gynaecological emergencies and its diagnosis is often delayed owing to nonspecific symptoms [5]. When OT is not diagnosed in a timely manner, it can impair ovarian blood flow and cause tissue necrosis [1]. OT is not considered an isolated condition because unilateral torsion and oophorectomy also affect contralateral ovulation [26]. Early diagnosis and treatment are vital for preserving ovarian viability [2]. In a series of 14 patients with OT, Celik et al. [27] demonstrated that, despite their necrotic appearance, the ovaries of 13 patients returned to normal function after detorsion.
In women of reproductive age, detorsioning and providing conservative treatment is of utmost importance for preserving fertility after OT. The level of tissue injury depends on the duration and severity of OT. Coskun et al. [20] reported that critical ischemia occurs 2 h after ovarian I/R injury induced by the slip-knot technique. They first detected haemorrhage, the early sign of necrosis, 2 h after ischemia and 1 h after reperfusion, although Uguralp et al. [17], who used a microvascular clamp model, reported that ischemia developed after 4 h of ischemia and 1 h of reperfusion. Celik et al. [4], who used the slip-knot technique, observed advanced haemorrhage after 3 h of ischemia and 1 h of reperfusion.
Coskun et al. [20] reported that, owing to the fragile nature of the ovarian vascular structures in rats, the microvascular clamp technique was ineffective for creating vascular occlusion, and they demonstrated the superior effectiveness of the slip-knot technique for creating I/R injury. This technique is advantageous because it achieves complete vascular occlusion. Furthermore, the knot can be unfastened for reperfusion without severing vascular structures. We therefore used the slip-knot technique in our study.
ROS have been implicated in the pathophysiology of ovarian I/R injury. Normal cell functions are maintained with lipid and protein structures in the cell membrane. Lipid peroxidation is a free radical process that leads to the degradation of polyunsaturated fatty acids in cell membranes. If the peroxidation of unsaturated fatty acids occurs with ROS, particularly hydroxyl radicals and singlet oxygen, the membrane is destroyed [16]. Several studies investigated tissue injury resulting from ovarian I/R and reported various histopathological findings, including interstitial oedema, vascular congestion, haemorrhage, inflammatory cell infiltration, follicular cellular degeneration, and follicular loss [4,5,7,10,11]. We also detected oedema, marked inflammatory cell infiltration, severe haemorrhage, and congested blood vessels in the ovarian I/R group in the present study.
SIL is a vasoactive medication used widely for the treatment of erectile dysfunction. Because it relaxes blood vessels and regulates blood flow, it has also recently been used for hypoxia-induced disorders such as pulmonary hypertension, chronic heart failure, and indomethacin-induced gastropathy [10]. A number of reports have also suggested that SIL may have anti-inflammatory properties via inhibition of ROS and inflammatory cytokine production [16]. The protective effect of SIL against I/R injury has been shown experimentally in testis, liver, lung, brain, and kidney tissues [28-33]. On the contrary, a limited number of studies of ovarian injury reported that SIL reduces tissue injury after I/R [4,10]. In an ovarian I/R study by Incebiyik et al. [10], the highest injury score was observed in the torsion group, whereas a significant reduction in injury score occurred in the groups administered SIL. The authors detected no significant differences between the SIL doses of 0.7 mg/kg and 1.4 mg/kg, which reported that there is no a dose-dependent effect Celik et al. [4] used SIL at a dose of 1.4 mg/kg and observed statistically significant differences in histopathological findings; they observed no infiltration after SIL treatment, a result similar to that in the control group. We also found the highest injury score in the ischemia group (group 2). SIL treatment at a dose of 1.4 mg/kg significantly lowered the tissue injury score.
Experimental I/R studies have indicated that antioxidants have a protective and therapeutic role. RSV is a compound found naturally in grapes that has anti-inflammatory, anticarcinogenic, and antioxidant properties [16]. The free radical scavenging activity of RSV inhibits the peroxidation of membrane lipids via nitrite and superoxide anion production at high doses [16-18]. Some studies have reported protective effects of RSV against renal, testicular, and ovarian I/R injury [20-22]. In a study of OT, Hascalik et al. [16] found that RSV treatment significantly lowered rates of acute polymorphonuclear leukocyte infiltration, vascular dilatation, and oedema, although complete reversal of the effects of OT did not occur. Our results also showed a significant reduction in the rate of histopathological findings in the RSVadministered ovarian I/R group.
On the contrary, compared with SIL administration, the use of RSV was more effective in reducing injury severity. Although several studies examined the effects of both SIL and RSV on ovarian I/R injury, our study is the first to use both agents simultaneously, and we found the lowest histopathological injury score in the I/R+SIL+RSV group. We suggest that SIL and RSV act simultaneously to reduce tissue injury.
MDA is the final product of lipid peroxidation. Free oxygen radicals that are produced in excess for various reasons during OT more frequently affect membrane lipids containing unsaturated fatty acids than other biomolecules. Interaction with membrane lipids leads to a rise in membrane permeability and severe cell damage [34]. We demonstrated that MDS levels were increased by ischemia and reduced by RSV and SIL. The I/R+SIL+RSV group showed no significant difference compared with the other treatment groups, which indicates that these agents did not have a synergistic action. The elevated tissue MDA concentration in the ischemia and I/R groups shows that oxidative stress developed. Many researchers also reported that ovarian MDA levels are increased in I/R injury [4,16,34,35]. Celik et al. [4] reported that MDA levels in I/R injury were reduced by SIL administration.
Our study also showed that GSH activity is reduced by ischemia and increased by RSV and SIL administration. However, RSV and SIL did not show a synergistic action. Yapca et al. [34], Hascalik et al. [16], and Nayki et al. [35] reported decreased levels of GSH activity in ovaries with I/R injury. Hascalik et al. [16] also reported that GSH activity is increased by RSV administration. Celik et al. [4] reported that various antioxidant parameters (superoxide dismutase, glutathione peroxidase) are increased by SIL administration.
We demonstrated a similarity between TAS and GSH activity in ovarian tissue, and found that RSV and SIL increased the antioxidant capacity. TOS showed parallelism with MDA levels and the oxidant levels were increased in the I/R groups, although mainly the ischemia group. A comparison of TAS values showed that RSV and SIL lacked a synergistic action, whereas a synergistic action occurred in terms of TOS. Incebiyik et al. [10], Sak et al. [11], and Eser et al. [5] reported that ischemia increased TOS and antioxidants increased TAS. Incebiyik et al. [10] demonstrated no significant difference in TAS induced by various SIL doses.
In our study, ovarian tissue that was weakly stained by the iNOS antibody in the control group was intensely stained in the ischemia and I/R groups. iNOS staining intensity was reduced in the treatment groups. We found that iNOS expression was reduced in the SIL-administered groups. On the contrary, RSV administration reduced iNOS expression, but it remained significantly higher than that in the control group. Compared with the control tissue, that in the group treated with both agents had similar iNOS expression.
SIL induces iNOS expression [36]. However, the effect of SIL on iNOS level is controversial. Our comprehensive literature search yielded no previous study investigating the effects of SIL on NO in I/R injury. Studies of renal I/R reported that pharmacological blockage of iNOS lessens tissue injury [37,38]. Abdel-Latif et al. [39] reported IHC results showing that iNOS expression was decreased by SIL in cyclosporineinduced renal injury in rats. On the contrary, Choi et al. [40] reported IHC results showing that iNOS expression was increased by SIL in renal I/R injury in rats. Kucuk et al. [41] reported that SIL reduced renal iNOS levels in I/R injury. In the present study, NO levels were increased in the ischemia and I/R groups. On the contrary, a significant reduction was measured in the I/R+SIL group. These findings were consistent with IHC findings.
Conclusion
Nowadays maternal age for pregnancy is older than previous. Because of that ovarian reserve is more important. Interventions to protect each follicle are valuable. The results of this study suggest that the administration of SIL and RSV before reperfusion may have protective effects against ovarian I/R injury in OT, as indicated by increases in TOS and MDA and NO levels, and reduced GSH activity and TAS. These agents also reduced histopathological ovarian injury of I/R origin. SIL and RSV may be appropriate treatment agents for oxidative stress induced by ovarian I/R injury, including adnexal torsion. Our results suggest that SIL and RSV act synergistically to reduce tissue injury. In conclusion, the results of this study show that both SIL and RSV effectively protected rat ovaries against reperfusion-induced injury via their potent antioxidant properties.
References
- Spinelli C, Buti I, Pucci V, Liserre J, Alberti E, Nencini L, Alessandra M, Lo Piccolo R, Messineo A. Adnexal torsion in children and adolescents: new trends to conservative surgical approach-our experience and review of literature. Gynecol Endocrinol 2013; 29: 54-58.
- Gocmen A, Karaca M, Sari A. Conservative laparoscopic approach to adnexal torsion. Arch Gynecol Obstet 2008; 277: 535-538.
- Guthrie BD, Adler MD, Powell EC. Incidence and trends of pediatric ovarian torsion hospitalizations in the United States, 2000-2006. Pediatrics. 2010; 125: 532-538.
- Celik M, Aksoy AN, Aksoy H, Aksoy Y, Halici Z. Sildenafil reduces ischemia-reperfusion injury in rat ovary: biochemical and histopathological evaluation. Gynecol Obstet Invest 2014; 78: 162-167.
- Eser A, Hizli D, Haltas H, Namuslu M, Kosus A, Kosus N, Kafali H. Effects of curcumin on ovarian ischemia-reperfusion injury in a rat model. Biomed Rep 2015; 3: 807-813
- Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol 2000; 190: 255-266.
- Sagsoz N, Kisa U, Apan A. Ischaemia-reperfusion injury of rat ovary and the effects of vitamin C, mannitol and verapamil. Hum Reprod 2002; 17: 2972-2976.
- Ozkisacik S, Yazici M, Gursoy H, Culhaci N. Does gradual detorsion protect the ovary against ischemia-reperfusion injury in rats? Pediatr Surg Int 2014; 30: 437-440.
- Perrelli MG, Pagliaro P, Penna C. Ischemia/reperfusion injury and cardioprotective mechanisms: Role of mitochondria and reactive oxygen species. World J Cardiol 2011; 3: 186-200.
- Incebiyik A, Seker A, Camuzcuoglu H, Kocaslan S, Camuzcuoglu A, Hilali NG, Vural M, Taskin A, Aksoy N. Does sildenafil have protective effects against ovarian ischemia-reperfusion injury in rats? Arch Gynecol Obstet 2015; 291: 1283-1288.
- Sak ME, Soydinc HE, Sak S, Evsen MS, Alabalik U, Akdemir F, Gul T. The protective effect of curcumin on ischemia-reperfusion injury in rat ovary. Int J Surg 2013; 11: 967-970.
- Oelsner G, Shashar D. Adnexal torsion. Clin Obstet Gynecol 2006; 49: 459-463
- Corbin JD, Francis SH. Pharmacology of phosphodiesterase-5 inhibitors. Int J Clin Pract 2002; 56: 453-459.
- Raposo C, Nunes AK, Luna RL, Araújo SM, da Cruz-Höfling MA, Peixoto CA. Sildenafil (Viagra) protective effects on neuroinflammation: the role of iNOS/NO system in an inflammatory demyelination model. Mediators Inflamm 2013; 2013: 321460.
- Lechner M, Lirk P, Rieder J. Inducible nitric oxide synthase (iNOS) in tumor biology: the two sides of the same coin. Semin Cancer Biol 2005; 15: 277-289.
- Hascalik S, Celik O, Turkoz Y, Hascalik M, Cigremis Y, Mizrak B, Yologlu S. Resveratrol, a red wine constituent polyphenol, protects from ischemia-reperfusion damage of the ovaries. Gynecol Obstet Invest 2004; 57: 218-223.
- Uguralp S, Mizrak B, Bay Karabulut A. Resveratrol reduces ischemia reperfusion injury after experimental testicular torsion. Eur J Pediatr Surg 2005; 15: 114-119.
- Kitada M, Koya D. Renal protective effects of resveratrol. Oxid Med Cell Longev 2013; 2013: 568093.
- Martin JA, Hamilton BE, Ventura SJ, Menacker F, Park MM. Births: Final Data for 2000. Natl Vital Stat Rep 2002; 50:1-101.
- Coskun A, Coban YK, Ciralik H. Critical ischemic time for the rat ovary: experimental study evaluating early histopathologic changes. J Obstet Gynaecol Res 2009; 35: 330-334.
- Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 1978; 86: 271-278.
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959; 82: 70-77.
- Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem 2005; 38: 1103-1111.
- Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem 2004; 37: 277-285.
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15 N] nitrate in biological fluids. Anal Biochem 1982; 126: 131-138.
- Lagana AS, Sofo V, Salmeri FM, Palmara VI, Triolo O, Terzić MM, Patrelli TS, Lukanovic A, Bokal EV, Santoro G. Oxidative Stress during Ovarian Torsion in Pediatric and Adolescent Patients: Changing The Perspective of The Disease. Int J Fertil Steril 2016; 9: 416-423.
- Celik A, Ergün O, Aldemir H, Ozcan C, Ozok G, Erdener A, Balýk E. Long-term results of conservative management of adnexal torsion in children. J Pediatr Surg 2005; 40: 704-708.
- Beheshtian A, Salmasi AH, Payabvash S, Kiumehr S, Ghazinezami B, Rahimpour S, Tavangar SM, Dehpour AR. Protective effects of sildenafil administration on testicular torsion/detorsion damage in rats. World J Urol 2008; 26: 197-202.
- Inan M, Uz YH, Kizilay G, Topcu-Tarladacalisir Y, Sapmaz-Metin M, Akpolat M, Aydogdu N. Protective effect of sildenafil on liver injury induced by intestinal ischemia/reperfusion. J Pediatr Surg 2013; 48: 1707-1715.
- Shih PK, Cheng CM, Li HP, Huang MF, Chiu CW, Chen JX, Chen NW, Chou SH. Pretreatment with sildenafil alleviates early lung ischemia-reperfusion injury in a rat model. J Surg Res 2013; 185: e77-83.
- Yildiz H, Durmus AS, Simsek H, Yaman I. Effects of sildenafil citrate on torsion/detorsion-induced changes in red blood cell and plasma lipid peroxidation, antioxidants, and blood hematology of male rats. Eur J Obstet Gynecol Reprod Biol 2011; 159: 359-363.
- Yazdani A, Khoja Z, Johnstone A, Dale L, Rampakakis E, Wintermark P. Sildenafil Improves Brain Injury Recovery following Term Neonatal Hypoxia-Ischemia in Male Rat Pups. Dev Neurosci 2016; 38: 251-263
- Medeiros PJ, Villarim Neto A, Lima FP, Azevedo IM, Leão LR, Medeiros AC. Effect of sildenafil in renal ischemia/reperfusion injury in rats. Acta Cir Bras. 2010; 25: 490-495.
- Yapca OE, Turan MI, Borekci B, Akcay F, Suleyman H. Bilateral ovarian ischemia/reperfusion injury and treatment options in rats with an induced model of diabetes. Iran J Basic Med Sci 2014; 17: 294-302.
- Nayki UA, Nayki C, Cetin N, Cimen FK, Coban A, Mammadov R, Tas IH, Malkoc I. Effect of Kineret(R) on ovarian ischemia reperfusion injury in a rat model. J Obstet Gynaecol Res 2016; 42: 1525-1533.
- Morsy MA, Ibrahim SA, Amin EF, Kamel MY, Rifaai RA, Hassan MK. Sildenafil Ameliorates Gentamicin-Induced Nephrotoxicity in Rats: Role of iNOS and eNOS. J Toxicol 2014; 2014: 489382.
- Chatterjee PK, Patel NS, Kvale EO, Cuzzocrea S, Brown PA, Stewart KN, Mota-Filipe H, Thiemermann C. Inhibition of inducible nitric oxide synthase reduces renal ischemia/reperfusion injury. Kidney Int 2002; 61: 862-871.
- Walker LM, Walker PD, Imam SZ, Ali SF, Mayeux PR. Evidence for peroxynitrite formation in renal ischemia-reperfusion injury: studies with the inducible nitric oxide synthase inhibitor L-N(6)-(1-Iminoethyl)lysine. J Pharmacol Exp Ther 2000; 295: 417-422.
- Abdel-latif RG, Morsy MA, El-Moselhy MA, Khalifa MA. Sildenafil protects against nitric oxide deficiency-related nephrotoxicity in cyclosporine A treated rats. Eur J Pharmacol 2013; 705: 126-134.
- Choi DE1, Jeong JY, Lim BJ, Chung S, Chang YK, Lee SJ, Na KR, Kim SY, Shin YT, Lee KW. Pretreatment of sildenafil attenuates ischemia-reperfusion renal injury in rats. Am J Physiol Renal Physiol. 2009; 297: F362-F370.
- Küçük A, Yucel M, Erkasap N, Tosun M, Koken T, Ozkurt M, Erkasap S. The effects of PDE5 inhibitory drugs on renal ischemia/reperfusion injury in rats. Mol Biol Rep 2012; 39: 9775-9782.