Research Article - Biology & Medicine Case Reports (2017) Volume 1, Issue 1
Oral serum bovine immunoglobulin improves IBS-D symptoms analyzed from patient medical charts.
- *Corresponding Author:
- Burnett BP
Redhill Biopharma, Inc. 8045 Arco Corporate Drive
Ste. 120 Raleigh, NC 27617, USA
Tel: 954-299-1105
E-mail: bburnett@redhillus.com
Accepted date: August 11, 2017
Citation: Good L, Shaw A, Wei D, et al. Oral serum bovine immunoglobulin improves IBS-D symptoms analyzed from patient medical charts. Biol Med Case Rep. 2017;1(1):16-23.
DOI: 10.35841/biology-medicine.1.1.16-23
Visit for more related articles at Biology & Medicine Case ReportsAbstract
Purpose: To examine clinical practice effectiveness of a medical food containing serum-derived bovine immunoglobulin (SBI) for the management of symptoms in irritable bowel syndrome with diarrhea (IBS-D) patients.
Methods: From 165 IBS-D patient medical charts [mean age=59.6 years (range: 19-98), female (n=109), Caucasian (n=144)], recorded daily stools/consistency, abdominal pain, and patient/ physician reported quality of life (QoL) scores were retrospectively collected on those prescribed SBI for a minimum of 8 weeks. A generalized estimating equations model was used to analyze and compare changes for scores of symptoms and QoL.
Results: Where data existed in charts, 46% of patients on SBI had a combined mean score reduction in daily stools, improvement in stool consistency (Bristol Stool Scale from >5 to <5) and decrease in abdominal pain (?30%). Significant improvements (p<0.001) in individual symptom and QoL scores with a high response were also reported for daily stools (76%), consistency (78%), abdominal pain (69%), as well as patient (79%) and physician QoL (73%). Eleven patients experienced 13 adverse events (AEs) while receiving SBI with nausea (n=6) most prevalent. No serious AEs were reported, but 4 patients discontinued SBI.
Conclusion: In this retrospective chart analysis of IBS-D patients who took a medical food containing SBI for 8 weeks, there were statistical improvements in daily stool number, stool consistency, abdominal pain and QoL scores supporting its use IBS-D.
Keywords
Irritable bowel syndrome, Diarrhea, Abdominal pain, Treatment response, Functional bowel, Serum-derived immunoglobulin.
Introduction
Irritable bowel syndrome (IBS) is a chronic gastrointestinal (GI) disorder affecting 10-25% of the general population [1,2]. Patients with IBS can alternate over time between constipation (IBS-C) or diarrhea (IBS-D) predominant, a so-called IBS mixed (IBS-M) phenotype [3]. The exact cause of IBS is not known; however, diverse etiologies such as alterations in intestinal motility, hypersensitivity, brain-gut interactions, various food sensitivities, malabsorption, intestinal inflammation, altered gut barrier function, and dysbiosis have all been implicated [2]. While prescription and over-the-counter standard-of-care (SOC) agents are available to treat this condition, many IBS patients are refractory to these therapies adversely affecting their overall quality of life (QoL) [4]. IBS-D is defined by Rome criteria as 25% of bowel movements with Bristol stool form types 6 or 7 (loose or watery) and less than 25% of bowel movements with Bristol stool form types 1 or 2 (hard stools) accompanied by a history of abdominal pain or worsening abdominal pain even with defecation [5]. Bloating, flatulence, urgency, fecal incontinence, excessive straining during defecation, incomplete evacuation, and mucus are also common in IBS-D patients.
Serum-derived bovine immunoglobulin/protein isolate (SBI) is the key ingredient in a medical food (EnteraGam®) intended for the management of chronic diarrhea and loose stools in conditions such as IBS-D [6]. Generally Recognized as Safe (GRAS) status is required for all foods including medical foods consumed in the United States. The FDA has reviewed the SBI protein formulation as part of a petition for GRAS and issued a letter of no challenge to the safety findings for use in food [7]. The postulated mechanism of action of SBI begins with immunoglobulin (Ig)-G (IgG) binding and neutralizing microbial antigens associated with intestinal dysbiosis [8-10], something no other food-derived protein source can do. In a co-culture model, SBI sterically hindered translocation of an antigen through a damaged epithelial layer to prevent immune induction [11]. In colitis animal models, SBI consumption led to decreased proinflammatory cytokine and chemokine production, increased anti-inflammatory proteins, and attenuated tissue damage [12-14]. SBI has also been tested in a randomized controlled trial, an open label study and several case series in IBS-D patients [15-21].
The purpose of this retrospective chart analysis was to evaluate, in a private practice setting, the impact of the medical food nutritional intervention containing SBI on patients diagnosed with IBS-D for its effect on symptoms as well as improvement in QoL for those who consumed the product for a minimum of 8 weeks.
Methods
Patient population
This retrospective institutional review board (IRB)-reviewed chart analysis was designed to collect symptom data from medical charts of patients who had been prescribed commercial SBI therapy (EnteraGam®) for management of IBS-D (ClinicalTrials.gov Identifier: NCT02661425). Data collected from patient medical charts was restricted to patients who were at least 18 years of age at the initiation of SOC or SBI therapy and had been previously diagnosed with IBS-D using Rome III criteria. Any newly diagnosed patients with IBS-D who took SBI for a minimum of 8 weeks were included in the analysis. In addition, patients who failed on SOC and then immediately took SBI for a minimum of 8 weeks were also included. The most prevalent medications used as SOC prior to immediate administration of SBI in decreasing order of prevalence were protein pump inhibitors, antidiarrheal agents (atropine/ diphenyloxalate, loperamide), antibiotics (i.e., rifaximin), probiotics, antispasmodics, and cholestyramine. Only patients who gave their informed consent for use of their recorded medical chart data were included in the analysis. Patients were excluded from the evaluation if they had not taken SBI daily as prescribed for a minimum of 8 weeks per protocol or had not consented to the use of their recorded chart data. Patients were also excluded from analysis if they started a different SOC therapy for IBS-D while taking SBI.
Experimental dietary product
Serum-derived bovine immunoglobulin/protein isolate (SBI) is the principle ingredient in EnteraGam® [6], the medical food that was administered to patients who were analyzed in this retrospective chart analysis. Patients did not modify their diet in any other way. Each commercial EnteraGam® packet (10 g net weight) contains a light-colored powder consisting of 5 g of SBI derived from United States Department of Agriculture-approved edible plasma, 5 g dextrose (also known as glucose), as well as trace amounts of sunflower lecithin, a healthy fat used in the spray drying process. SBI is composed of >90% protein which consists primarily of polyclonal immunoglobulins (e.g. >50% IgG) along with other proteins and peptides that are similar to those commonly consumed by humans in beef products.
Analysis design
This retrospective medical chart analysis was performed under an IRB-reviewed (Sterling IRB, Atlanta, GA) protocol and designed to gather symptom data from existing medical charts for all IBS-D patients who were administered SBI for at least 8 weeks. No IRB approval of the protocol was necessary since this was a retrospective analysis of medical chart data under 45 Code of Federal Regulations (CFR) 46.101(b) and no human experimentation was performed. The IRB did review the protocol as appropriate under this regulation. The analysis of patient medical chart data did comply with the Health Insurance Portability and Accountability Act of 1996 Privacy Rule (46 CFR Part 160; Part 164 (subparts a,e)) under United States FDA regulations.
A data collection tool (DCT) was used to accumulate medical chart data (see supplementary material). The DCT collected information on the following if present in the medical charts: demographics (age, sex, race) and anthropomorphic information (height, weight); symptom data [number of daily stools, stool consistency (Bristol Stool Scale: 1-separate hard lumps, like nuts; 2-lumpy, sausage like; 3-sausage with cracks on the surface; 4-smooth, soft sausage snake-like; 5-soft blobs; 6-mushy; 7-watery, no solid pieces), abdominal pain (11 point scale: 0=no pain and 10=worst pain possible)], urgency (episodes per day), fecal incontinence (episodes per day), flatulence (episodes per day), bloating (0= none; 1= mild; 2= moderate; 3= severe), fatigue (0= none; 1= mild; 2= moderate; 3= severe)]; and patient-reported scores and physician-assessed impressions of QoL (1= poor; 2=fair; 3= good; 4= excellent). The DCT also captured information from patient medical charts on comorbidities, medications including those for GI conditions, and adverse events (AEs). Medical chart data was recorded from medical charts using the DCT by the physician, physician assistant or nurse practitioner with the aid of a medical science liaison (MSL) at all times assuring private patient information. The supervising physician then examined the DCT and signed off to attest to the accuracy of the captured medical chart information. Double data entry was then performed by two MSLs and verified by a third person before being sent to the statistician.
Outcome measures
The primary outcome was defined by a combined reduction in the number of daily stools, an improvement stool consistency by Bristol Scale Score from >5 to <5, and a 30% or greater decrease in abdominal pain after a minimum of 8 weeks of SBI administration. Patients were required to have data in their charts regarding the characteristic symptoms for IBS-D, number of daily stools, stool consistency and abdominal pain, to assess their combined response to SBI for the primary outcome measure. Secondary outcome measures (i.e., number of daily stools, stool consistency, abdominal pain intensity, urgency, fecal incontinence, flatulence, bloating, fatigue, and patient-reported, as well as physician-assessed QoL scores) were required to have a one-point improvement in individual symptom or the QoL scale scores.
Safety
Safety data (i.e., therapy-emergent AEs and serious adverse events, SAEs) using the DCT from medical charts was collected on SBI if patients took at least one dose. Any new side effect information recorded during SBI administration and not already associated with an existing comorbidity was considered an AE.
Statistical analysis
A generalized estimating equations (GEE) model was used to analyze changes in mean symptom and QoL scores. The GEE model is an extended form of generalized linear model and can account for the unknown correlation among patients. In the GEE model, the following patient characteristics were controlled for: age distributions (age 18-44, 45-54, 55-64, 65- 74, and >=75 years), gender (female vs. male), and race (white vs. non-white). Additionally, the GEE model adjusted the cluster effect from the individual clinical practice sites where outcomes were correlated for patients who were treated at the same site to minimize site bias. Subgroup analyses were performed to demonstrate the robustness of the effect among patient cohorts with more restrictive criteria applied for the combined primary outcome measure and secondary individual symptom scores. Comparisons of significance prior to and after a minimum of 8 weeks of SBI administration was also performed using a t-test.
Results
In this retrospective medical chart analysis, data were collected from medical charts on 165 patients identified as diagnosed with IBS-D who were given a nutritional intervention in the form of a protein-containing medical food, SBI. These patient medical charts analyzed were from 11 different gastroenterology practices distributed across 8 states (1-Connecticut, 3-Florida, 1-Georgia, 1-Missouri, 1-Tennessee, 1-New York, 1-Texas, 1-Virginia) in the U.S. The mean age of the population was 59.6 years (range: 19-98) and patients were primarily female (n=109) and Caucasian (n=144; Hispanic=9; Black=4; Asian=3; Unknown=5). The most prevalent GI comorbidities in this cohort of patients in decreasing order of prevalence were gastroesophageal reflux disease, hemorrhoids, and esophagitis. The most prevalent non-GI comorbidities were anxiety and depression. No patient was considered malnourished or protein deficient based on current blood work in medical charts. Twentysix patients did not comply with healthcare provider instructions to take SBI daily for a minimum of 8 weeks and were excluded from analysis. Forty patients who were prescribed other IBS-D medications at the same visit as SBI were excluded from the analysis as the healthcare provider chose to administer multiple agents in combination. Four patients discontinued SBI due to an AE (see below) and were excluded from analysis. Of 95 remaining patients, the number of patients reporting symptom scores characteristic of IBS-D (daily stool number, stool consistency and abdominal pain), other symptoms associated with the condition (urgency, fecal incontinence, flatulence, bloating, fatigue), and QoL assessments was not consistent from chart to chart accounting for differences in the data collection numbers shown in Table 1. Therefore, the number of charts reporting symptoms to evaluate combined scores for daily stool number, stool consistency and abdominal pain (n=44) is less than those collected on these individual symptoms, 67, 76, 61, respectively (Table 1).
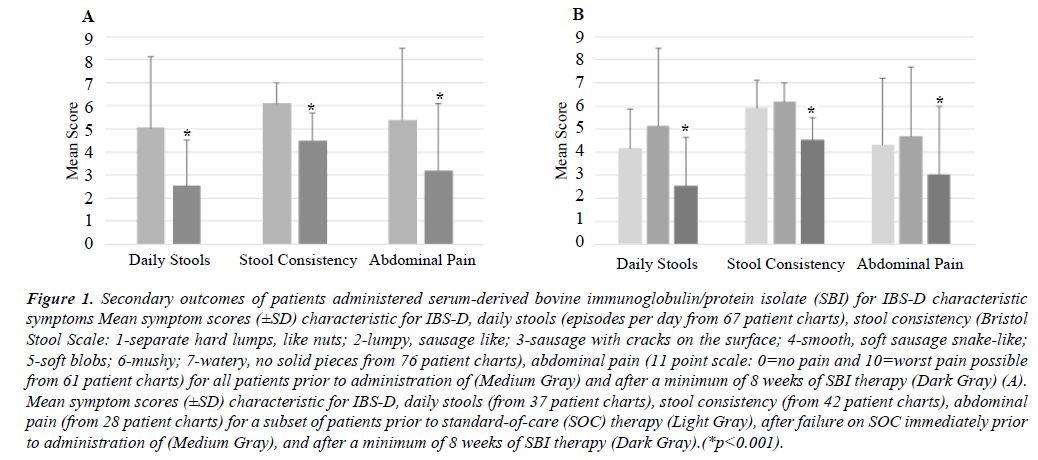
There was no effect found regarding age, gender, or geographic location. After a minimum of 8 weeks of SBI administration, a significant response (p<0.001) was found for improvement in the combined primary outcome of reducing daily stools, improving Bristol Scale Scores from >5 to <5, and a ≥30% decrease in abdominal pain was found in 46% of patients (20 of 44) where symptom medical chart data was available. When response rates for secondary outcomes of individual IBS-D characteristic symptoms for patients who took SBI a minimum of 8 weeks were analyzed, there was also a high and significant response. Seventy-six percent (51of 67 patients) showed a reduction in the number of daily stools declining from 5.11 ± 3.4 to 2.54 ± 2.1 (mean change= -2.37; p<0.001). There was a 78% (59 of 76 patients) response for improvement in stool consistency using Bristol Scale scoring from 6.2 ± 0.8 to 4.5 ± 1.0 (mean change= -1.63, p<0.001). For abdominal pain, 69% (42 of 61 patients) had decreased pain scores from 4.7 ± 3.0 to 3.0 ± 3.0 (mean change= -1.66, p<0.001) using an 11-point scale (Figure 1A).
Figure 1: Secondary outcomes of patients administered serum-derived bovine immunoglobulin/protein isolate (SBI) for IBS-D characteristic symptoms Mean symptom scores (±SD) characteristic for IBS-D, daily stools (episodes per day from 67 patient charts), stool consistency (Bristol Stool Scale: 1-separate hard lumps, like nuts; 2-lumpy, sausage like; 3-sausage with cracks on the surface; 4-smooth, soft sausage snake-like; 5-soft blobs; 6-mushy; 7-watery, no solid pieces from 76 patient charts), abdominal pain (11 point scale: 0=no pain and 10=worst pain possible from 61 patient charts) for all patients prior to administration of (Medium Gray) and after a minimum of 8 weeks of SBI therapy (Dark Gray) (A). Mean symptom scores (±SD) characteristic for IBS-D, daily stools (from 37 patient charts), stool consistency (from 42 patient charts), abdominal pain (from 28 patient charts) for a subset of patients prior to standard-of-care (SOC) therapy (Light Gray), after failure on SOC immediately prior to administration of (Medium Gray), and after a minimum of 8 weeks of SBI therapy (Dark Gray).(*p<0.001).
Interestingly, when individual symptoms characteristic of IBS-D were analyzed for patients who had scores in the medical charts for SOC therapies immediately prior to SBI administration, there was no improvement. The number of daily stools increased from 4.2 ± 1.7 to 5.1 ± 3.4 (mean change= +0.9, 37 patients), stool consistency worsened from 5.9 ± 1.2 to 6.2 ± 0.8 (mean change= +0.3, 42 patients), and abdominal pain increased slightly from 4.3 ± 2.9 to 4.7 ± 3.0 (mean change= +0.4, 28 patients) in this subset of patients (Figure 1B). The time of each of the SOC administration varied from minimum of 2 weeks (i.e., rifaximin) to >3 months (i.e., antidiarrheal agents). Similar improvements of individual characteristic IBS-D individual symptom scores, however, were observed among patients in this SOC subset of patients after administration of SBI for a minimum of 8 weeks (Figure 1B). The number of daily stools declined from 5.05 ± 3.1 to 2.52 ± 2.0 (mean change= -2.53, p<0.001, 37 patients), stool consistency improved from 6.1 ± 0.9 to 4.5 ± 1.2 (mean change= -1.61, 42 patients, p<0.001), and abdominal pain decreased from 5.4 ± 3.1 to 3.2 ± 2.9 (mean change= -2.23, 28 patients, p<0.001).
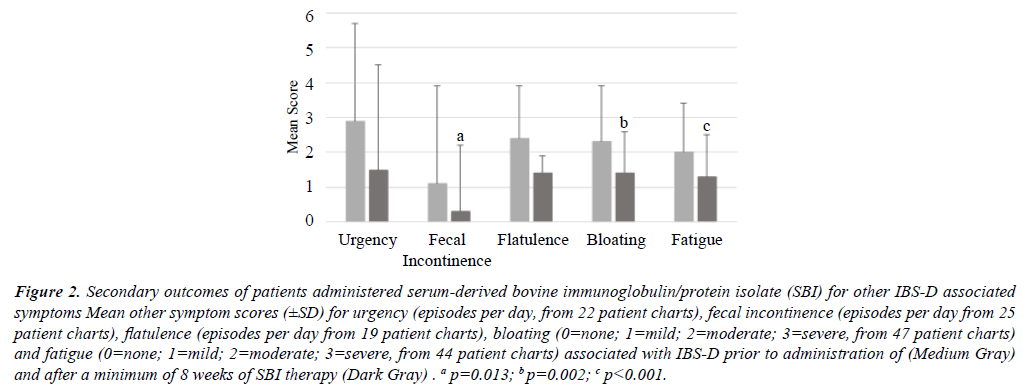
When response rates for secondary outcomes of other individual symptoms collected from medical charts (Table 1) typically found in IBS-D patients (urgency, fecal incontinence, flatulence, bloating and fatigue) were analyzed for those who consumed SBI a minimum of 8 weeks, response rates were generally above 50%. Sixty-four percent (14 of 22 patients) showed a reduction in episodes per day of urgency from 2.9 ± 2.8 to 1.5 ± 3.0 (mean change= -1.36). There was 36% (9 of 25 patients) who fewer episodes of fecal incontinence per day decreasing from 1.1 ± 1.5 to 0.3 ± 0.5 (mean change= -0.80, p=0.13) and 42% (8 of 19 patients) had fewer events of flatulence per day going from 2.4 ± 2.8 to 1.4 ± 1.9 (mean change= -0.95). For bloating, 53% (25 of 47 patients) had improved scores from 2.3 ± 1.6 to 1.4 ± 1.2 (mean change= -0.89, p=0.002) and 55% (24 of 44 patients) had less fatigue down from a score of 2.0 ± 1.4 to 1.3 ± 1.2 (mean change= -0.77, p<0.001) (Figure 2). In the case where there were less than 25 patients with other symptom scores associated with IBS-D in their charts, accurate statistical analysis was not possible. While no improvement in mean scores for these other symptoms associated with IBS-D were observed in the subset of patients after first taking SOC therapies, the same pattern of improvement in symptoms was observed after SBI intake for a minimum of 8 weeks (data not shown).
Figure 2: Secondary outcomes of patients administered serum-derived bovine immunoglobulin/protein isolate (SBI) for other IBS-D associated symptoms Mean other symptom scores (±SD) for urgency (episodes per day, from 22 patient charts), fecal incontinence (episodes per day from 25 patient charts), flatulence (episodes per day from 19 patient charts), bloating (0=none; 1=mild; 2=moderate; 3=severe, from 47 patient charts) and fatigue (0=none; 1=mild; 2=moderate; 3=severe, from 44 patient charts) associated with IBS-D prior to administration of (Medium Gray) and after a minimum of 8 weeks of SBI therapy (Dark Gray) . a p=0.013; b p=0.002; c p<0.001.
| Symptom or QoL | Patients (n) with Scores Recorded in Medical Chart |
|---|---|
| Daily Stool Number | 67 |
| Stool Consistency | 76 |
| Abdominal Pain | 61 |
| Urgency | 22 |
| Fecal Incontinence | 25 |
| Flatulence | 19 |
| Bloating | 47 |
| Fatigue | 44 |
| Patient-Reported QoL | 53 |
| Physician-Assessed QoL | 52 |
| Daily Stool Number, Stool Consistency and Abdominal Pain | 44 |
QoL=Quality of Life; SBI=Serum-Derived Bovine Immunoglobulin/Protein Isolate
Table 1. The number of patient charts with reported symptom and quality of life scores who consumed SBI for a minimum of 8 weeks.
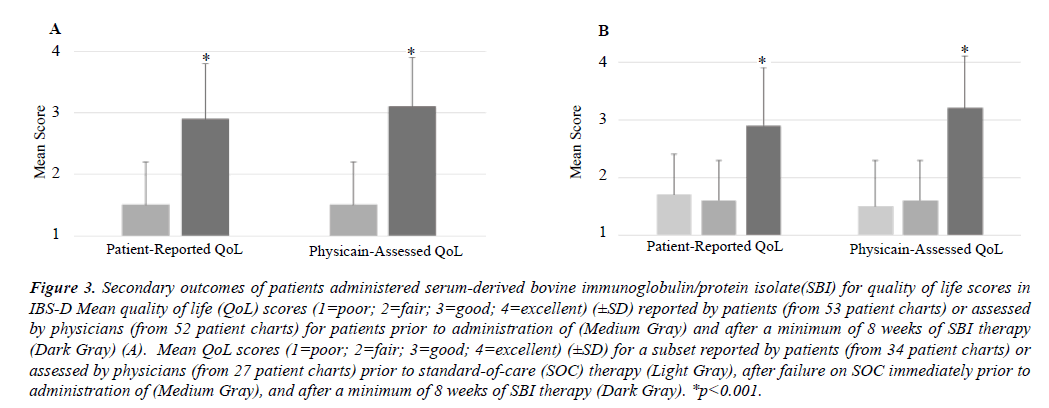
When QoL scores were analyzed from medical charts using a scale from 1-4 (poor, fair, good, excellent) for those who consumed SBI for a minimum of 8 weeks, 79% (42 of 53 patients) of patients reported an improvement from 1.5 ± 0.7 to 2.9 ± 0.9 (mean change= +1.42, p<0.001), while physicians assessed that 73% (38 of 52 patients) of patients had improvement from 1.5 ± 0.7 to 3.1 ± 0.8 (mean change= +1.6, p<0.001) (Figure 3A). For patients who had QoL scores in the medical charts for SOC therapies prior to immediate SBI administration, there was no improvement in patient-reported (1.7 ± 0.7 vs. 1.6 ± 0.7, mean change= -0.1, 34 patients) or physician-assessed (1.5 ± 0.8 vs. 1.6 ± 0.7, mean change= +0.1, 27 patients) scores on SOC. A similar pattern of improvement, however, was found after a minimum of 8 weeks SBI consumption. There was a 79% (27 of 34 patients) response for improvement in QoL reported patient scores with an increase from 1.6 ± 0.7 to 2.9 ± 1.0 (mean change= +1.3, p<0.001) and a 78% (21 of 27 patients) response as judged by physicians with scores improving from 1.6 ± 0.7 to 3.2 ± 0.9 (mean change= +1.6, p<0.001) (Figure 3B).
Figure 3: Secondary outcomes of patients administered serum-derived bovine immunoglobulin/protein isolate(SBI) for quality of life scores in IBS-D Mean quality of life (QoL) scores (1=poor; 2=fair; 3=good; 4=excellent) (±SD) reported by patients (from 53 patient charts) or assessed by physicians (from 52 patient charts) for patients prior to administration of (Medium Gray) and after a minimum of 8 weeks of SBI therapy (Dark Gray) (A). Mean QoL scores (1=poor; 2=fair; 3=good; 4=excellent) (±SD) for a subset reported by patients (from 34 patient charts) or assessed by physicians (from 27 patient charts) prior to standard-of-care (SOC) therapy (Light Gray), after failure on SOC immediately prior to administration of (Medium Gray), and after a minimum of 8 weeks of SBI therapy (Dark Gray). *p<0.001.
Two patients out of 165 total were purported to have consumed at least one dose of SBI, but did not return for a follow up visit and were not included in the AE analysis. There were 11 patients who had a combined total of 13 AEs collected from medical charts out of 163 patients (8%) who received at least one dose of SBI (Table 2). Out of the total 13 AEs recorded, 1 patient had nausea and increased abdominal pain while another had nausea and a headache. The predominant AE observed in patients after consuming SBI was nausea (n=6; 3.7%). Four out of 163 patients discontinued SBI (2.5%) therapy prior to 8 weeks due to an AE. There were no serious AEs (SAEs) noted in the medical charts. There were no significant mean changes in weight while on SBI.
| Adverse Event | SBI (N =163), n (%) | Discontinued, n (%) |
|---|---|---|
| Nausea | 6 (3.7) | 3 (1.8) |
| Increased Abdominal Pain | 2 (1.2) | -- |
| Constipation | 1 (0.6) | -- |
| Cramping | 1 (0.6) | -- |
| Headache | 1 (0.6) | -- |
| Frequent Urination | 1 (0.6) | -- |
| Metallic Taste | 1 (0.6) | 1 (0.6) |
| Total | 13 (8.0) | 4 (2.5) |
SBI=Serum-Derived Bovine Immunoglobulin/Protein Isolate
Table 2. Adverse events in medical charts recorded for patients who consumed at least one dose of SBI.
Discussion
SBI has been tested as a nutritional intervention compared to a soy protein isolate in one double-blind, placebo-controlled trial [15]. At 10 g SBI per day, there was a significant reduction the number of days per week patients experienced IBS-D characteristic symptoms (loose stools, abdominal pain) as well as other symptoms typically associated with IBS-D (flatulence, bloating, urgency), whereas a soy protein placebo had no significant effect after 6 weeks on any symptoms. There were no SAEs reported, though 3 subjects (2 SBI, 1 placebo) withdrew due to nausea. Valentin et al. [16] found in an open-label study that patients taking SBI 5 g twice daily that there were statistical improvements in stools per day, ease of passage, and evacuation during an 8-week study. There was also a numerical reduction of worst pain severity the last 2 weeks of the study, but it did not reach statistical significance (P=0.078). There were no SAEs and 20 therapy-emergent AEs which included cramping (2); gas (2); headache (2); nausea (2); and one patient for each with abdominal or back pain, acid reflux, bloating, cold sore, feeling sick, leaking, metallic taste in mouth, sinus infection, sores on tongue, and stomach flu. One patient withdrew due to metallic taste, mouth sore and nausea. In recent retrospective reports of SBI use collected from medical charts, there was approximately a 70% overall response rate to therapy in drug-refractory IBS-D patients [17-20]. For example, Hilal et al. [17] and Good et al. [18] reported patients with IBS-D, IBS-M, and IBS with bloating had about 75% overall management of their condition. Similarly, in patients who were refractory to traditional pharmaceutical therapies including rifaximin diagnosed with small intestinal bacteria overgrowth (SIBO) comorbid with IBS-D or IBS-M, there was 75% response rate within 4 weeks to 5 g SBI twice daily [19]. Responses in the analysis were similar whether the patients were lactulose breath test positive and were diagnosed with SIBO or negative and diagnosed with IBS-D or IBS-M. In another population of drug refractory IBS-D patients who were administered SBI 5 g per day for 16 weeks, 82% of patients reported a moderate to complete response to therapy, with the majority of condition management occurring in the first 4-6 weeks [20]. Finally, two case reports of drug-refractory, post-C. difficile infectious IBS-D patients demonstrated that their chronic diarrhea was completely managed after SBI administration over 2-4 weeks [21].
While the exact cause of IBS-D is unknown, dietary triggers [22], accelerated intestinal transit [23], visceral hypersensitivity [24,25], and alterations to the gut microbiome [26] have all been implicated in the pathophysiology of IBS-D. In addition, some studies have shown that there is a subset of IBS-D patients where inflammation is present compared to healthy subjects [27,28]. Because of this multifactorial etiology, many clinical approaches are used to reduce abdominal pain and diarrhea in IBS-D patients associated with different aspects of the pathophysiology. These include bulking agents like fiber (i.e., psyllium, pectin), antidiarrheal agents (i.e. atropine/diphenyloxalate, loperamide, eluxadoline), dietary modification (i.e., FODMAP), serotonergic agents (alosetron), antidepressants (i.e., tricyclic antidepressants, selective serotonin reuptake inhibitors), analgesics (peppermint oil), and non-systemic antibiotics (rifaximin) [29-31].
The FDA recently issued guidance for the clinical evaluation of drugs to treat IBS [32]. In this guidance, there were two recommended components for endpoints in IBS-D: (1) At least a 30% reduction compared with baseline in weekly average worst abdominal pain intensity using an 11-point (i.e., 0 to 10) numeric rating scale; and (2) a 50% reduction in the number of days per week with at least one stool with a consistency rating of Type 6 or 7 by Bristol Stool Scale compared with baseline. An early clinical study of alosetron prior to this guidance utilized an eight-point global improvement scale that asked subjects how their symptoms after treatment compared to the previous 3 months, from substantially worse to substantially improved, found between 43-50% response rates depending on dose compared to 31% in the placebo group (all p<0.02) [33]. The TARGET 1 and 2 studies of rifaximin used an endpoint for adequate relief of global IBS symptoms for at least 2 of 4 weeks following treatment and found 41% response rate compared to 32% in the placebo group (all p<0.05) [34]. In TARGET 3, an open-label phase of the rifaximin study evaluated its readministration and relapse as well as a response rate using a composite endpoint where there was a 30% or greater reduction in abdominal pain and at least a 50% improvement in the number of days per week in which the Bristol Stool scale was type 6 or 7 (loose or watery stools) [35]. TARGET 3 found a 42% response rate to rifaximin using the composite endpoint. Trials of eluxadoline found, using a composite response of a 30% or greater reduction in abdominal pain and improvement in stool consistency using Bristol Stool scale criteria as above on the same day for at least 50% of the days, response rates to this agent were between 24% to 32% depending on dose and time on therapy (12 vs. 26 weeks) compared to placebo responses of 16% to 20% (all p<0.05) [36]. A recent FDA warning regarding eluxadoline has been issued by the FDA for 120 reports of serious cases of pancreatitis or death, all of which occurred in clinical practices after approval of this agent in May of 2015 [37].
Though this retrospective medical chart analysis was not a randomized, placebo-controlled study, we attempted to restrict data collection and analysis to similar combined outcome measures as required in IBS clinical studies for daily stool number, stool consistency improving from a Bristol Scale score of >5 to <5 and a 30% improvement in abdominal pain after 8 weeks of SBI administration. The 8-week minimum administration of SBI was chosen based on responses observed in two trials and cases series [15-21]. There was a 46% response rate for all patients who consumed SBI for a minimum of 8 weeks using the combined endpoint. There was also 69% to 78% response in patients for the primary individual symptoms characteristic of IBS-D (daily stool frequency/consistency, abdominal pain) for patients who consumed SBI for a minimum of 8 weeks. Other symptoms associated with IBS-D, such as urgency, fecal incontinence, flatulence, bloating, and fatigue, were more difficult to assess since the number of patients with recorded responses in their medical charts were not enough to reach statistical significance in each case. Enough patients had scores in their medical charts for fecal incontinence, bloating and fatigue, however, that there was an observable statistical improvement among patients with recorded data who were administered SBI for a minimum of 8 weeks. Patient-reported and physician-assessed QoL responses were also statistically significant and similar in all patients administered SBI or in the subset who had SOC failures first immediately prior to SBI administration with between a 73% to 79% response rate.
Advantages
One advantage of this retrospective analysis in terms of safety was that patients were not excluded based on comorbidity or poly-pharmacy. There was also a low rate of recorded AEs in medical charts for patients administered SBI as a nutritional intervention. The safety of SBI and recorded AEs found in this analysis were also similar to that observed in previous clinical trials with IBS-D [15,16] and in retrospective case studies of drug-refractory IBS patients [17-21] with nausea as the predominant AE. Indeed, post-marketing surveillance over a 4-year period has shown that there have been few AEs (<0.2%) and no SAEs associated with over 3 million doses of SBI prescribed [6]. The primary in-market AEs reported was nausea, mild constipation or diarrhea, increased urination, and headache (data on file).
Conclusion and Limitations
The limitations of this type of retrospective medical chart analysis is that it was not a double-blind, placebo-controlled trial, recording of specific symptoms by each healthcare provider at each site was not uniform, and the population was heterogenous in some cases having received multiple, different SOC agents prior to SBI. Even though the unique design of this IRB-reviewed retrospective chart analysis was performed with a specific, strict collection protocol, bias could be inherent in the selection of patients from medical charts. Coupled with previously published positive data of SBI outcomes, however, this new analysis along with the safety profile of the nutritional product suggest that this medical food should be considered as an option for use in IBS-D patients. A large, well-controlled study is needed to substantiate efficacy against placebo [38].
#The SBI Study Group
The following investigators contributed medical chart information to this retrospective analysis: Patricia Burgunder, ARNP; Jeffrey Fenyves, MD; Paul Feuerstadt, MD; Santander Gill, MD; Ernesto Guerra, MD; Raouf Hilal, MD; Michael LeVine, MD; Patricia Mitchell, PA; Nadia Sarran, Clinical Coordinator; Ira Shafran, MD; Jason Roy, ARNP; William Salt, MD; and Leonard Weinstock, MD.
Acknowledgments
The authors wish to acknowledge editorial assistance by Alex Brewer III, PhD, Bryon Petschow, PhD and Christopher Detzel, PhD. This research and preparation of the manuscript was supported by Entera Health, Inc. Financial support in the form of educational grants provided to SBI Study Group investigators to pay for the labor of collection of medical chart data by Entera Health, Inc.
Disclosure of Potential Conflicts of Interest
Bradshaw T, Burnett BP, Jackson BE, Shaw A, Vasquez RE were employees of Entera Health, Inc which manufactures a medical food product which contains serum-derived bovine immunoglobulin/protein isolate when this analysis was performed.
References
- Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71-80.
- Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: A clinical review. JAMA. 2015;313:949-58.
- Engsbro AL, Simren M, Bytzer P. Short-term stability of subtypes in the irritable bowel syndrome: prospective evaluation using the Rome III classification. Aliment Pharmacol Ther. 2012;35:350-359.
- Mönnikes H. Quality of life in patients with irritable bowel syndrome. J Clin Gastroenterol. 2011;45:98-101.
- Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150:1393-1407.
- EnteraGam® Full Product Information; http://enteragam.com/assets/lib/EnteraGam_Product_Information.pdf, serum derived bovine immunoglobulin. 2017.
- Kurlander J
- Petschow BW, Burnett B, Shaw AL, et al. Serum-derived bovine immunoglobulin/protein isolate: Postulated mechanism of action for management of enteropathy. Clin Exp Gastroenterol. 2014;7:181-190.
- Horgan A, Maas KJ, Henderson AL, et al. Serum-derived bovine immunoglobulin/protein isolate binds to pathogen associated molecular patterns. FASEB, San Diego. CA. 2014;26-30.
- Henderson AL, Horgan A, Detzel CJ, et al. Serum-derived bovine immunoglobulin/protein isolate binds and neutralizes Clostridium difficile toxins A and B. Poster session presented at Digestive Disease Week. IL. 2014;3-6.
- Detzel CJ, Horgan A, Henderson AL, et al. Bovine immunoglobulin/protein isolate binds pro-inflammatory bacterial compounds and prevents immune activation in an intestinal co-culture model. 2015;10(4).
- Henderson AL, Wymore-Brand M, Darling RJ. Attenuation of colitis by serum-derived bovine immunoglobulin/protein isolate in a defined microbiota mouse model. Dig Dis Sci. 2015;3726-5.
- Perez-Bosque A, Miró L, Maijó M, et al. Dietary intervention with serum-derived bovine immunoglobulins protects barrier function in a mouse model of colitis. Am J Physiol Gastrointest Liver Physiol. 2015;G1012-G1018.
- Pérez-Bosque A, Miró L, Maijó M. et al. Oral Serum-derived bovine immunoglobulin/protein isolate has immunodulatory effects on the colon of mice that spontaneously develop colitis. 2016;11(5).
- Wilson D, Evans M, Weaver E, et al. Evaluation of serum-derived bovine immunoglobulin protein isolate in subjects with diarrhea-predominant irritable bowel syndrome. Clini Med Insights. Gastroenterol. 2013;6:49-60.
- Valentin N, Camilleri M, Carlson P, et al. Potential mechanisms of effects of serum-derived bovine immunoglobulin/protein isolate therapy in patients with diarrhea-predominant irritable bowel syndrome. Physiol. 2017;5(5).
- Hilal R, Mitchell P, Guerra E, et al. Case series of 10 drug-refractory IBS patients who respond to oral serum-derived bovine immunoglobulin/protein isolates (SBI). Open J Gastroenterol. 2014;4:321-328.
- Good L, Rosario R, Panas R. New therapeutic option for irritable bowel syndrome: Serum-derived bovine immunoglobulin: A case study. World J Gastroenterol. 2015;21: 3361-3366.
- Weinstock LB, Jasion VS. Serum-derived bovine immunoglobulin/protein isolate therapy for patients with refractory irritable bowel syndrome. Open J Gastroenterol. 2014;4:329-334.
- Shafran I, Burgunder P, Young H. Nutritional management of refractory IBS-D patients by the medical food serum-derived bovine immunoglobulin (SBI) in a 28-patient cohort. The James W. Freston AGA Conference. 2015.
- Crawford C, Panas R. Post-infectious irritable bowel syndrome with functional diarrhea following C. difficile infections: Case studies of responses using serum-derived bovine immunoglobulin. J Gastroenterol Hepatol. 2015;4(4):1577-1581.
- Fritscher-Ravens A, Schuppan D, El-Richmann M, et al. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2014;147:1012-1020.
- Manabe N, Wong BS, Camilleri M, et al. Lower functional gastrointestinal disorders: evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol Motil. 2010;22:293- e82.
- Wilder-Smith CH, Schindler D, Lovblad K, et al. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595-1601.
- Vermeulen W, De Man JG, Pelckmans PA, et al. Neuroanatomy of lower gastrointestinal pain disorders. World J Gastroenterol. 2014;20:1005-1020.
- Collins SM. A role for the gut microbiota in IBS. Nat Rev Gastroenterol Hepatol. 2014;11:497-505.
- Martinez C, Lobo B, Pigrau M, et al. Diarrhoea-predominant irritable bowel syndrome: an organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut. 2012.
- An S, Zong G, Wang Z, et al. Expression of inducible nitric oxide synthase in mast cells contributes to the regulation of inflammatory cytokines in irritable bowel syndrome with diarrhea. Neurogastroenterol Motil 2016.
- Ford AC, Moayyedi P, Lacy BE, et al. Task force on the management of functional bowel disorders. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109:S2-S26.
- Lacy BE. Diagnosis and treatment of diarrhea-predominant irritable bowel syndrome. Intl J Gen Medi. 2016;9:7-17.
- Pimentel M. potential mechanisms of action of rifaximin in the management of irritable bowel syndrome with diarrhoea. Aliment Pharmacol Ther. 2016;43:37-49.
- http://enteragam.com/assets/lib/EnteraGam_Product_Information.pdf
- Krause R, Ameen V, Gordon SH, et al. A randomized, double-blind, placebo-controlled study to assess efficacy and safety of 0.5 mg and 1 mg alosetron in women with severe diarrhea-predominant IBS. Am J Gastroenterol. 2007;102:1709-1719.
- Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22-32.
- Lembo A, Pimentel M, Rao SS. Efficacy and safety of repeat treatment with rifaximin for diarrhea-predominant irritable bowel syndrome: Results of the TARGET-3 study. 2016;151:1113-1121.
- Program and abstracts of the American College of Gastroenterology 2014 Annual Scientific Meeting, Philadelphia.2014;17-22.
- Lembo AJ, Lacy BE, Zuckerman MJ, et al. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med. 2016;374:242-253.
- http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm154991.htm