Research Article - Research and Reports in Gynecology and Obstetrics (2017) Research and Reports in Gynecology and Obstetrics (Special Issue 2-2017)
Naked eye visual inspection with acetic acid versus cervical smear as a screening test for cervical intraepithelial neoplasia.
Ali Nakash1,2*, Ali Falih Al-Assadi2, Zina Abdul Hussein AL-Safi2, Jasim M AL-Diab21Department of Obstetrics and Gynecology, Kingston Hospital, UK
2Department of Obstetrics and Gynecology, Basrah University, Iraq
- *Corresponding Author:
- Ali Nakash
Department of Obstetrics and Gynecology
North Middlesex Hospital
London
United Kingdom
Tel: 0208 3044811; 07853946928
E-mail: anakashuk@yahoo.co.uk
Accepted Date: April 26, 2017
Citation: Nakash A, Al-Assadi AF, AL-Safi ZAH, et al. Naked eye visual inspection with acetic acid versus cervical smear as a screening test for cervical intraepithelial neoplasia. Res Rep Gynaecol Obstet. 2017;1(2):1-8
Abstract
Background: Worldwide, cervical cancer is the second commonest cancer among women. Cervical cancer generally develops slowly over a period of 10-15 years. It is preceded by detectable and treatable precursor conditions. The presence of effective screening programs makes the cervical cancer a preventable condition. Because of the lack of trained cytopathologists and cytology labs, an alternative method of screening is needed for countries with very limited resources and infrastructure. However, simple visual inspection of the cervix after application of acetic acid has been effectively used in resource-poor settings for cervical cancer screening.
Aim of the study: To evaluate the use of naked eye visual inspection with acetic acid (VIA) as an alternative to cervical cytology as a screening test for diagnosis of cervical intraepithelial neoplasia. This study is a prospective comparative study carried out in Basra maternity and children hospital included 156 women between the ages of (25-65) years old, who were attending the outpatient department for deferent gynaecological problems. Both screening tests Pap smear sampling and VIA was performed, and compared the results to histopathology as a diagnostic test and results were analysed statistically. The study included 156 women, Pap smear and VIA was done for all patients respectively and the results were 31 women with positive pap smear and 85 women with positive VIA. All the positive acetowhite (85) and 20 negative acetowhite randomly selected and underwent cervical biopsy and histopathology. The total number included in biopsy sampling was 105 patients.
Results: The results were positive for CIN and cancer in 52 cases. After comparing the results of Pap smear and histopathology the true positive Pap smear results were 24 cases out of 52 and the true positive VIA was 44 cases from 52. The sensitivity of VIA was higher than Pap smear. After dividing the result of VIA to low threshold and high threshold the specificity will increase without loss of sensitivity, and this increment was statistically significant.
Keywords
Cervical intraepithelial neoplasia, Pap smear, Visual inspection with acetic acid, Cervical screening, Cervical cancer, Acetowhite
Introduction
Cervical cancer
It is the second commonest cancer among women, worldwide, with only breast cancer occurring more commonly [1], while cervical intra epithelial neoplasia (CIN) is a pre-malignant condition of the cervix. It is usually asymptomatic and detected by routine cytological screening [2]. The concept of CIN was introduced first in 1968, when Richart indicated that all dysplasia have the potential for progression. The term CIN is equivalent to the term dysplasia [3], which means disordered growth and development of the epithelial lining of the cervix [2]. Consequently squamous metaplasia should not be diagnosed as dysplasia or CIN because it does not progress to invasive cancer [4]. Approximately 10% of women with CIN have concomitant pre invasive neoplasia of the vulva, vagina or anus. Conversely 40-60% of patient with vulval intraepithelial neoplasia (VIN) or vaginal intraepithelial neoplasia (VAIN) have synchronous CIN [3]. World-wide, cervical cancer comprises 12% of all cancers in women [5]. Cervical cancer accounts for about 500,000 new cases diagnosed annually. Of the 288,000 death due to cervical cancer each year, more than 80% occur in developing world where the least resources exist for management, and this proportion is expected to increase to 90% by 2020 [6]. Cervical carcinoma remains a significant cause of mortality [7].
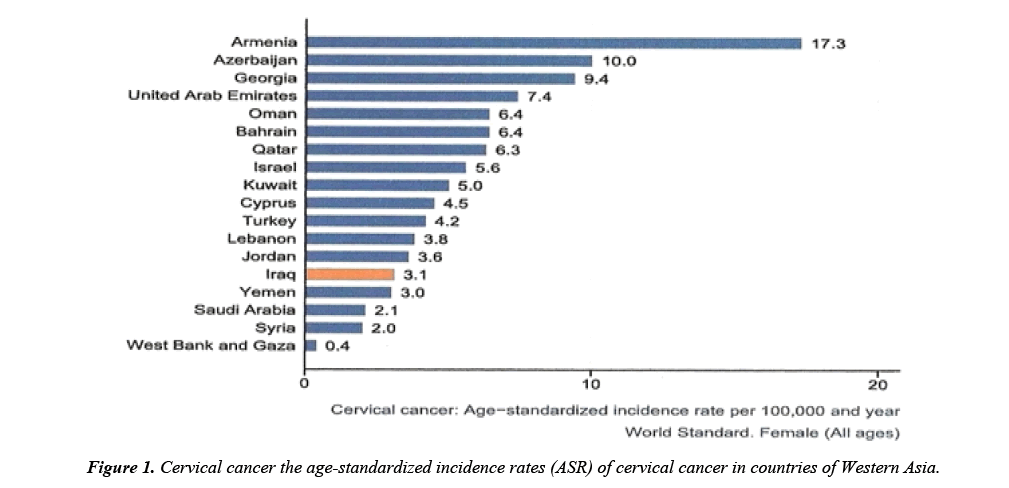
The situation is compounded by the fact that in under developed countries 75% present in an advanced stage, which is the contrary of what happen in the developed countries where 75% present in early stage thanks to screening programmes available for cervical cancer. The highest incidence is observed in Latin America, the Caribbean, sub-Saharan Africa, and Southern and Southeast Asia. According to WHO/Information centre on HPV and Cervical Cancer the age-standardized incidence rates (ASR) of cervical cancer in countries of Western Asia as shown in Figure 1 [8].
The prevalence of CIN varies from as low as 1.05% in family planning or general gynaecology clinics to as high as 13.7% in sexually transmitted disease clinics [2].
Cervical cancer generally develops slowly over a period of 10- 15 years [9]. It is preceded by detectable and treatable precursor conditions in which certain cells in the cervix develop abnormal characteristics but not yet cancerous [10].
The diagnosis of CIN is usually made in women in their 20s. Carcinoma institute is diagnosed in women 25 to 35 years of age and invasive cancer usually after the age of 40. The median age of diagnosis of cervical cancer is 52 and the average is 45 [11].
The presence of effective screening programs and the administration of prophylactic vaccine against HPV make the cervical cancer a preventable condition [12].
Understanding the natural history of various degrees of CIN is central to the appropriate clinical management of patients. Approximate rates of spontaneous regression, persistence and progression of CIN as in Table 1 [2]. During embryogenesis upward migration of stratified squamous epithelium from the urogenital sinus and vaginal plate is thought to replace mullerian epithelium. Squamous columnar junction (SCJ) rarely remains restricted to the external os. Instead, it is a dynamic point that changes in response to puberty, pregnancy, menopause and hormonal stimulation. In neonates SCJ is located on exocervix. The adolescent cervix is believed to be more susceptible to carcinogenic stimuli because of the active process of squamous metaplasia, which occur within the transformation zone during periods of endocrine change, but under the influence of HPV, cellular alterations occur that result in a typical transformation zone. The anterior lip of the cervix is twice as likely to develop CIN as the posterior lip and CIN rarely originates in the lateral angles. Once CIN occurs it can progress horizontally to involve the entire transformation zone but it usually does not replace the original squamous epithelium. The only way to determine where the original SCJ was located is to look for nabothian cysts or cervical cleft openings, which indicate the presence of columnar epithelium [3].
Table 1. Approximate rates of spontaneous regression, persistence and progression of CIN.
| CIN1 | CIN2 | CIN3 | |
|---|---|---|---|
| Regression to normal | 60% | 40% | 30% |
| Persistence | 30% | 35% | 48% |
| Progression to CIN3 | 10% | 20% | - |
| Progression to cancer | <1% | 5% | 22% |
At present there are two cervical cytology techniques: Firstly, the Conventional Pap Smear. This cytology method requires special care to avoid air drying of the cells, a leading cause of poor slide quality. The false negative errors may occur in sampling, preparation and interpretation. Sampling errors may occur because the lesion is too small to exfoliate or the device used did not pick up the cells and transfer to glass slide. Preparation errors due to poor fixation on the glass slide leading to air drying. The slide may be also obscured by thick vaginal discharge, blood or mucus, thick slide lead to poor fixation as the fixative failed to penetrate the cell sample [13].
The second most recent technique is the Liquid based cytology. The collected cell sample is rinsed in a vial contain a liquid preservative, the sample then processed under the control of the cytology laboratory to provide a thin layer of cervical cells without debris on a glass slide. Thin layer cytology have proven to be more sensitive than conventional glass slide pap smears because the cells doesn’t clump on top of each other in the liquid based medium and there is less debris on the resulting slide. More intra epithelial lesions are identified [10].
Computer assisted diagnosis has come up as new idea based on the believe that optical scanning by computer could be used for Papanicolaou smear interpretation, but differences in staining and the overlap of cells has made practical application very difficult. However, in the past several years, two different computer-based systems for cervical cytology use have been approved by the Food and Drug Administration. The widespread use of liquid-based cervical cytology specimens has greatly enhanced the applicability of these optical image recognition systems. Although the initial cost of these computer-based systems is large, considerable savings should be realized by the around-the-clock work and a reduced need for cytotechnologists, who could concentrate on the diagnostic evaluation of high-risk slides identified by the computer on primary screening. More clinical experience with these techniques is needed, but early results are promising [14].
Aim of Study
The aim of this study is to evaluate the use of naked eye visual inspection by acetic acid as an alternative to cervical cytology as a single screening test for diagnosis of cervical intraepithelial neoplasia.
Materials and Methods
A prospective comparative study was carried out in Basra maternity and children hospital, during the period from the 1st of March 2009 to the 1st of November 2010. The study included (156) women ages 25-65 years, who were attending the outpatient department for deferent gynaecological problems, about 32(20.5%) of women with history of menorrhagia, 28(17.9%) of women were complaining of intermenstrual bleeding (IMB), 25(16%) were complaining of postcoital bleeding (PCB), 12(7.6%) were complaining of postmenapausal (PMB), 11(7%) with chronic pelvic pain, and 48(30.7%) were complaining of vaginal discharge.
Exclusion criteria for the study
Virgin, pregnant woman, previous history of cervical cancer or CIN, prior total hysterectomy, women with severe cervicitis until they had completed treatment, moderate to severe vaginal bleeding.
The patients included in the study were given information about the study, agreed to participate. A reproductive and health history was taken including menstrual history, vaginal bleeding pattern (IMB, menorrhagia, or PCB), age of patient, current pregnancy status or contraceptive method, parity, level of education of woman and her husband with occupation of both, history of smoking and family history of cervical cancer. The procedure and the reason for it should be carefully explained to the woman beforehand. This starts off with assembling equipment, vaginal speculum (Cusco's speculum), sterile rubber gloves, adequate light source about 100 watt (halogen or flash light), cotton swabs, labeled slides, Ayres spatula, Cuplan’s jar contain 95% ethyl alcohol, freshly prepared 5% acetic acid solution (5 ml of glacial acetic acid with 95 ml of distilled water). The woman is asked to lie in a modified lithotomy position onto the examination table after she has emptied her bladder.
Inspection of external genetilia was done to rule out presence of lesions, warts, papules, ulceration, discharge, redness, swelling and excoriation. Then full pelvic examination was done in the usual way. A sterile Cusco's speculum was carefully inserted in the vagina, and avoids use of antiseptic solution for sterilization of genetilia.
Inspection of the cervix was done for cervicitis, ectropian, nabothian cyst, cervical ulcer or erosion, polyp, outgrowth, and bleeding. The four vaginal fornices then examined to make sure they are free from any growth or abnormal visual finding. The gross appearance of the cervix is classified into: Normal, abnormal, suspicious of Malignancy.
Normal cervix
A normal cervix appears smooth, round, pink, lubricated with clear mucoid secretion and has a central hole (the external os).
Abnormal cervix
This category includes all benign looking lesions, such as: Hypertrophy, redness or congestion, irregular surface, distortion, simple erosions (that do not bleed on touch), cervical polyps (with smooth surface), and abnormal discharge (foul smelling, dirty/greenish, white/cheesy, blood stained).
Suspicious of malignancy
Malignancy should be suspected when there is erosion that bleeds on touch, a growth with an irregular surface.
Then both screening tests Pap smear sampling and VIA was performed. A Pap smear sample was done using a conventional disposable wooden Ayres spatula; scrape the cervix around the entire transformation zone and smearing the cells onto a labelled glass slide. The smear is fixed with 95% ethyl alcohol for 20-30 minutes. The woman should have this test when she is not menstruating; the best time is between day 10 and 20 after the first day of the last menstrual period. Those with vaginal bleeding on the day of the test, they were postponed until the bleeding stops.
For about two days before a Pap test, the patient should avoid douching or using vaginal medicines, spermicidal foams, creams, or gels. The patient should not have sexual intercourse for 1 to 2 days before Pap test, because these may hide abnormal cells and cause unclear results. After completing the Pap smear, VIA was done and it involves gentle application of 5% acetic acid using cotton swab to avoid bleeding. The woman is informed that she might feel a slight stinging sensation.
After 1-2 minutes a naked eye evaluation was performed under 100-watt illumination. The transformation zone is carefully checked for any dense non movable acetowhite areas in the mucosa. If acetowhite areas are identified on the cervix after 1-2 minute, the test is positive. Criteria for categorization VIA test result were show in Table 2 (WHO, 2002).
Table 2. Criteria for categorization VIA test result.
| VIA test outcome | Criteria |
|---|---|
| Negative (-) | No aceto white lesions. Acetowhitening on endocervical polyps, nabothian cysts. |
| Single positive (+) (low threshold) |
Prominent white line like acetowhitening of the squamo columnar junction. |
| Double positive (++) (high threshold) |
Faint, translucent, ill defined, irregular acetowhite lesions on the cervix. Definite, angular, geographic acetowhite lesions far away from the squamo columnar junction. Opaque, dense, dull, definite, well-defined acetowhite lesions touching the squamo columnar Junction or close to the external os. Large, circumificial, well-defined, thick, dense acetowhite lesions. Growth on the cervix turns acetowhite. |
Then a punch biopsy was taken from the cervix of all patients with positive acetowhite areas and 20 patients with negative aceitowhite area as a random sample. These samples were kept in container with 10% formalaldehyde, and sent to the pathologist. Questioner was filled out with clinical finding at a pelvic evaluation and results of VIA. The woman after completion of the test can go back to her normal activities. The pap smears are processed and stained by Iraqi constant and stable Papanicolaou stain in following steps: 1) Ethanol in downward concentration 80%, 70%, and 50% for 10 second in each concentration. 2) Distilled water for 10 second. 3) Haematoxylin for 1 minute. 4) Tap water for 1 minute. 5) Acid alcohol for 5 second. 6) Tap water for 1 minute. 7) Carbonic lithium for 1 minute. 8) Tap water for 1 minute. 9) Distilled water for 10 second. 10) Ethanol in upward concentration 50%, 70%, 80% for 10 second in each concentration. 11) Orange-G6 for 1 minute. 12) Ethanol in concentration 95% for 10 second. 13) Eosin stains for 2 minute. 14) Ethanol in concentration 95% then 100% for 10 second in each concentration. 15) Xylol plus alcohol for 10 second 50% concentration for each of it. 16) Xylin for10-20 minute. 17) After dryness of slides drop of DPX was put on each slide and cover it by cover slide.
The slides are sent to the cytologist to be examined blindly (no clinical information were provided to him). When the results appear, a follow up would be arranged for the patients by the gynaecologist.
Statistical Analysis
SPSS V. 15 (Statistical Package for Social Sciences) used for data input and analysis. Chi square test for independence use to verify the association between discrete variables. Screening tests were validated using validity measure (sensitivity, specificity, predictive values) with their 95% confidence interval. Findings with p value <0.05 were statistically significant.
Results
The total number of women included in this study was 156.
Table 3 shows characteristics of women under study. They had an age spectrum between 25 and 65 years. The number of women in the reproductive age was 135 (86.5%) while the number of postmenopause was 21 (13.5%). The majority of patients were house wife 125 (80.1%), married 136 (87.2%), non-smoker 133 (85.3%), grand multiparous 75 (48.1%) with low education level (literacy and primary school) 97 (62.2%) and most of them 109 (69.9%) did not use oral contraceptive pills.
Table 3. Patient characteristics.
| Age of patients | No. | % |
|---|---|---|
| 25-35 year | 73 | 46.80% |
| 36-45 year | 58 | 37.20% |
| 46-55 year | 15 | 9.60% |
| 56-65 year | 10 | 6.40% |
| Marital status | ||
| Married | 136 | 87.20% |
| Widow | 14 | 9% |
| Divorce | 6 | 3.80% |
| Duration of marriage | ||
| 1-5 year | 25 | 16.10% |
| 6-10 year | 34 | 21.80% |
| 11-20 year | 57 | 36.50% |
| >20 year | 40 | 25.60% |
| Education level | ||
| Literacy | 24 | 15.40% |
| Primary school | 73 | 46.80% |
| Secondary school | 54 | 34.60% |
| University | 5 | 3.20% |
| Parity | ||
| Nulliparous | 22 | 14.10% |
| 01-Feb | 10 | 6.40% |
| 03-Apr | 49 | 31.40% |
| >4 | 75 | 48.10% |
| Occupations | ||
| House wife | 125 | 80.10% |
| Teacher | 17 | 10.90% |
| Official | 12 | 12.70% |
| College student | 2 | 1.30% |
| Smoking | ||
| Non smoker | 133 | 85.30% |
| Smoker | 23 | 14.70% |
| Oral contraceptive pills | ||
| Non user | 109 | 69.90% |
| User | 47 | 30.10% |
Table 4 shows the result of Pap smear for all women under study. 31(19.8%) of women were Pap smear test positive of them 8 (5.2%) had HSIL, 21 (13.4%) had LSIL and 2 (1.2%) had cancer. The rest 125 (80.2%) of women were Pap smear test negative, 88 (56.4%) of them were normal and 37 (23.8%) had acute inflammation.
Table 4. Pap smear results.
| Pap smear | Number | Percentage |
|---|---|---|
| LSIL(CIN1) | 21 | 13.40% |
| HSIL(CIN2/CIN3) | 8 | 5.20% |
| Cancer | 2 | 1.20% |
| Normal | 88 | 56.40% |
| Inflammation | 37 | 23.80% |
| Total | 156 | 100% |
Table 5 shows VIA test results for all 156 women under study. 85 (54.5%) women had acetowhite positive areas, 31 (19.9%) of them had high threshold positive and 54 (34.6%) had low threshold positive. The remaining 71 (45.5%) had negative VIA test.
Table 5. VIA results.
| VIA | Number | Percentage |
|---|---|---|
| Low threshold | 54 | 34.60% |
| High threshold | 31 | 19.90% |
| Negative | 71 | 45.50% |
| Total | 156 | 100% |
Histopathological examination was the gold standard test for the diagnosis of CIN and cancer in this study, which include 105 cases. All cases (85) with positive acetowhite and (20) randomly selected women with normal screening test were subjected for cervical biopsy and histopathological examination. Table 6 shows histopathological examination result which include 31 (29.5%) of women were diagnosed as CIN1, 12 (11.4%) as CIN2, 7 (6.7%) as CIN3 and squamous cell carcinoma in 2 (1.9%) cases. The remaining 53 (50.5%) cases were histopathologicaly negative acute inflammation in 24 (22.9%) cases and normal examination in 29 (27.6%) cases.
Table 6. Histopathology results.
| Histopathology | Number | Percentage |
|---|---|---|
| CIN1 | 31 | 29.50% |
| CIN2 | 12 | 11.40% |
| CIN3 | 7 | 6.70% |
| Cancer | 2 | 1.90% |
| Normal | 29 | 27.60% |
| Acute inflammation | 24 | 22.90% |
| Total | 105 | 100% |
Table 7 shows the distribution of histopathological findings based on pap. Smear and VIA test results. In cases were both screening tests were positive 17 cases had CIN, 2 cases had cancer and 1 case had negative histopathology. In cases with positive Pap smear and negative VIA 5 cases had CIN and 5 cases had negative histopathological examination. In cases with positive VIA and negative Pap smear, 25 cases had CIN and 40 cases with negative histopathology. When both screening tests (pap. and VIA) were negative 3 cases had CIN1 and 7 cases had negative histopathological examination.
Table 7. Distribution of histopathological findings based on screening test findings of patients.
| Pap smear | VIA | Number | Histopathology positive | Histopathology negative | ||||
|---|---|---|---|---|---|---|---|---|
| CIN1 | CIN2 | CIN3 | Cancer | Normal | Acute inflammation | |||
| + | + | 20(19.1%) | 7(6.7%) | 4(3.9%) | 6(5.7%) | 2(1.9%) | - | 1(0.9%) |
| + | - | 10(9.5%) | 4(3.9%) | 1(0.9%) | - | - | 3(2.8%) | 2(1.9%) |
| - | + | 65(61.9%) | 17(16.3%) | 7(6.7%) | 1(0.9%) | - | 21(20%) | 19(18%) |
| - | - | 10(9.5%) | 3(2.8%) | - | - | - | 4(3.9%) | 3(2.8%) |
| Total | 105(100%) | 31(29.5%) | 12(11.4%) | 7(6.7%) | 2(1.9%) | 28(26.6%) | 25(23.9%) | |
Table 8 shows a comparison between the results of pap. smear (screening test) and histopathology (diagnostic test), Pap smear can picked up 24(22.8%) cases with positive histopathology for CIN and cancer(true positive); 6(5.7%) cases were misdiagnosed as positive by cervical smear but they were negative after Histopathological examination (false positive). Missing occurred in 28(26.6%) cases in whom Pap smear was negative but histopathology was positive (false negative). The remaining 47(44.9%) cases had negative both Pap smear and biopsy (true negative).
Table 8. Comparison between Pap smear results and histopathology results.
| Histopathology | positive | negative | Total |
|---|---|---|---|
| Pap smear | |||
| Positive | 24(22.8%) | 6(5.7%) | 30(28.5%) |
| Negative | 28(26.6%) | 47(44.9%) | 75(71.5%) |
| Total | 52(49.4%) | 53(50.6%) | 105(100%) |
Table 9 shows a comparison between the results of VIA (screening test) and histopathology (diagnostic test), 44(41.9%) cases had positive both tests (true positive), 12(11.5%) cases had negative both tests (true negative). Disagreement between the two tests occur in 41(39%) cases in whom VIA were positive but histopathology were negative (false positive) and 8(7.6%) cases were positive by histopathology but negative by VIA (false negative).
Table 9. Comparison between VIA results and histopathology.
| Histopathology | Positive | Negative | Total |
|---|---|---|---|
| VIA | |||
| Positive | 44(41.9%) | 41(39%) | 85(80.9%) |
| Negative | 8(7.6%) | 12(11.5%) | 20(19.1%) |
| Total | 52(49.4%) | 53(50.6%) | 105(100%) |
Table 10 shows VIA test results were divided into low and high threshold and both were compared to the result of histopathological examination. The sensitivity of low threshold VIA was 80% (CI 64-96%) which was approximate to high threshold VIA 82% (CI 68-96%) and (p-value=0.852), so there was no statistically significant difference between the sensitivity of low and high threshold VIA. Pap smear had lower sensitivity of 46% (CI 33-59%) than both low and high threshold VIA and the difference was statistically highly significant (p=0.000).
Table 10. Validity of screening test.
| Screening test | Sensitivity 95%CI | Specificity 95%CI |
PPV 95%CI |
NPV 95%CI |
|---|---|---|---|---|
| Pap smear | 46% (33-59%) | 88% (79-97%) | 80% (65-95%) | 62% (52-72%) |
| VIA+ | 80% (64-96%) | 30% (17-43%) | 37% (24-49%) | 75% (56-94%) |
| VIA++ | 82% (68-96%) | 68% (55-94%) | 77% (62-92%) | 75% (56-93%) |
The specificity of low threshold VIA was (30% (CI 17-43%)) which was lower than those for high threshold (68% (CI 55- 94%)) and Pap smear (88(CI 79-97%)) and the differences were statistically highly significant (p=0.000). There was no statistically significant difference between the specificity of high threshold VIA and Pap smear (p=0.494).
The PPV of low threshold VIA was (37% (CI 24-49%)) significantly lower than those for high threshold VIA (77% (CI 62-92%)) and for pap smear (80% (CI 65-95%)) (p=0.006), while PPV of high threshold VIA and pap smear were close to each other with no significant differences (p=0.776).
The NPV were similar for both low and high threshold VIA (75% (CI 65-94%)) and both were higher than that of pap smear and the difference were statistically not significant (p=0.333).
Discussion
Cervical Screening Program is a method of preventing cancer by detecting and treating early abnormalities, which, if left untreated, could lead to cancer in a woman's cervix. The best known screening service is for carcinoma of the cervix; this should be a preventable disease for the following reasons; there is usually a phase of premalignancy, dysplasia or intraepithelial neoplasia, the cervix is a relatively accessible organ to examine.
Cells can easily be obtained in the pre malignant phase [15]. There are 3 bases for any screening program; there should be a long latent interval in which pre malignant changes or occult cancer can be detected, secondly there is an effective treatment for premalignant changes and cancer and finally the screening program should be cost effective. Screening for cervical cancer certainly satisfies the first 2 criteria. However, the cost effectiveness of cervical cancer screening is debatable, but this is more of a political issue. Cervical screening should be effective, an accurate, simple, low-cost, culturally acceptable, and safe screening test is essential [16].
Low coverage of the target group is one of the most common reasons for failure of cervical Screening coverage. Generally screening 80% of women once in their life time is considered acceptable. Programmes with lower coverage rates will be less successful at reducing cervical cancer incidence or mortality [5].
There are many advantages for any cervical screening program; reassurance for most who have no premalignant changes, reassurance to a few that any premalignant changes found are at a very early stage, avoidance of radical treatments if the condition is picked up early and finally this can all result in an increased life expectancy.
Saying that there are a few possible disadvantages: fear of finding cancer is one of them, the anxiety generated while waiting for the results and finally the fear that comes from false positive results [15]. The National Health Service Cervical Screening Programme (NHSCSP) has issued a series of guidelines governing the management of the programme, the most recent of which is an evidence based document covering all of the major aspects of screening, diagnosis, treatment and follow up. The programme has had a dramatic effect, with a major fall in the incidence of death from cervical cancer [17].
Different programmes have different regimes of screening intervals; one of the most successful programs is the British one, the Table 11 shows the screening intervals followed in this program [17].
Table 11. Screening intervals.
| Age group (years) | Frequency of screening |
|---|---|
| 25 | First invitation |
| 25-49 | Three yearly |
| 50-64 | Five yearly |
| 65+ | Only screen those who have not been screened Since age50 or those who have had recent abnormal tests |
Women under 25 years are not invited because teenagers' bodies, particularly the cervix are still developing, which means young women may get an abnormal result when there is nothing wrong. This could lead to unnecessary treatment so screening young women might do more harm than good. Although lesions treated in very young women may prevent cancers from developing many years later the evidence suggests that screening could start at age 25 [18].
Types of visual detection
In the 1980s, the idea of looking at the cervix with the naked eye for early detection of disease (known as ‘down-staging’) in lowresource settings was promoted, studies used unaided visual inspection, which involved simply performing a speculum examination to look at the cervix with the unaided eye for any signs of early cancer, this approach has been found not sufficiently accurate in identifying precursor lesions and cancer [19,20].
Then there was the introduction of what is called Naked eye inspection after application of 3-5% acetic acid (VIA): sometimes referred to as DVI (direct visual inspection), this offers a lowtechnique, low-cost method of screening for dysplasia. The process involves a standard speculum examination followed by visual inspection of the cervix one minute after washing it with a 3-5% acetic acid solution. Based on the presence of acetowhite changes, the acetic acid coagulates protein of cytoplasm and nuclei and since abnormal epithelium is of a high nuclear density, this prevents light from passing through the epithelium, which thus appears white [2], the provider can recommend further treatment as needed. Because results of the screening are immediate, patients can sometimes be offered treatment immediately, removing the need for follow-up visit.
Recently there was the introduction of VIA with magnification (VIAM) uses a device such as the AviScope TM a low-power (4x) handheld visual inspection device with a built-in light source to examine the cervix after application of acetic acid [21]. There is also the Visual inspection with Lugol’s iodine (VILI), which involves visualizing the cervix after applying Lugol’s iodine to detect lesions. This technique is under evaluation as an independent primary screening test.
And finally there is E cervicography which Involves photographing the cervix after application of an acetic acid. The developed photographs, called cervigrams, are projected as slides and interpreted by specially trained colposcopists, Cervigrams can also be helpful as educational tools. Cervicography, however, is relatively expensive and requires a reliable logistics infrastructure [22]. VIA is a promising approach, many aspects of VIA make it an appealing approach for use in low-resource settings. In most cases, costs associated with launching and sustaining VIA screening are lower than those associated with other methods; VIA is a relatively simple, easy-to learn approach that is only somewhat reliant upon infrastructure for its adequate performance, assuming that sufficiently trained providers are available [23]. The approach does not require laboratory involvement and non-physicians can perform the procedure, as a result, VIA generally has the potential for greater population coverage than other available screening approaches [24]. The results of the procedure are available immediately, making it possible to provide further management, including an offer of immediate treatment of some suspected precancerous lesions during the same visit [25].
The slow natural history of cervical cancer lends itself well to a screening program that identifies dysplasia and prevents it from progressing to invasive carcinoma [26,27]. The Pap test has been successful in reducing the incidence and mortality of cervical cancer in developed countries with organized screening programs, but because of the limitation in cytology facilities in this country we evaluated VIA as a suitable alternative test to Pap smear and for the same reason only 156 cases were studied [28].
The sensitivity and specificity for cytology in the present study were 46% and 88% respectively, which are similar to that reported by Cohn et al. and Gaffikin et al. [22,29], which were 44.3% and 90.6% respectively and slightly differ from those reported by Samira et al. which were 52.6% and 72.1% respectively. The false negative rate for cervical cytology in this study was 26.6% which is within the range reported by different studies (6-45%).
Regarding VIA; the sensitivity reported by this study was 84.6% which significantly higher than that for pap smear (46%) a finding similar to that reported by Ghaemmaghami et al. [30], were the sensitivity of VIA and pap smear 74.3% and 37.1% respectively, and also by Cohn et al. were the sensitivity of VIA was 76.7% which is higher than sensitivity of pap smear 4403%. Also by Rana et al. [31] were the sensitivity for VIA was 93% which was significantly higher than that for pap smear (83%).
In this study VIA results were divided into two thresholds (low and high) in order to see whether its specificity can be further improved without loss in sensitivity. There was no statistically significant difference between the sensitivity of low and high threshold VIA, a finding similar to that reported by Samira et al. while the specificity of high threshold VIA (68%) was significantly higher than that for low threshold VIA (30%) this is also similar to that reported by Samira et al (specificity values were 20% and 72.7% for low and high threshold respectively) so by depending on high threshold VIA only the specificity was increased without losing the sensitivity.
We reported no statistically significant difference between the specificity of high threshold VIA and cervical cytology which is similar to that reported by Samira et al. and Sankaranarayanan et al. [14].
With respect to the PPV, there was no statistically significant difference between high threshold VIA and Pap smear and both have significantly higher PPV as compare to low threshold VIA while in Samira et al study high threshold VIA has a higher PPV (72.7%) than that for Pap smear (45.5%) and both were higher than that for low threshold VIA. We reported no statistically significant difference regarding the NPV between the low threshold and high threshold VIA, and Pap smear. So that the use of VIA as a primary screening test means that women assessed as test negative will be reassured that most probably they don’t have CIN or cancer. This finding is consistent with that reported by Samira et al where the NPV for low and high threshold VIA were the same (80%) and for Pap smear was (77.5%). After reviewing the above results high threshold VIA has a significantly higher sensitivity than Pap smear with no significant difference in the specificity, PPV, NPV in addition to that, it is a simple objective test, the result of the procedure is immediately available, it requires only acetic acid, a speculum, and a light source.
Conclusion
High threshold VIA is a suitable substitute to Pap smear as a screening test for premalignant and malignant disease of the cervix. The only limitation for VIA was the high false positive rate which may over loud the referral system and result in unnecessary treatment of women.
References
- Shafi M. Premalignant and malignant disease of the cervix. In DewHurst's Textbook of Obstetrics and Gynecology (7th edn). Wiley-Blackwell, London. 2008;614-24.
- Holschneider CH. Premalignant & malignant disorders of the uterine cervix. In: Decherney AH, Nathan L, Goodwin TM (eds.), Current diagnosis & treatment: Obstetrics & Gynecology (10th edn). McGraw-Hill, New York. 2007;833-54.
- Addis I, Hatch D, Berek J. Intraepithelial disease of the cervix, vagina and vulva. In: Jonathan Berek (ed.), Berek and Novak's Gynecology (14th edn). Lippincott Williams and Wilkins, USA. 2007;561-600.
- Almeida-Parra Z, Penalver M, Mendez L. Cervical Carcinoma. Clin Gynecol J. 2006;5:651-63.
- Khanna J. Incidence and natural history of cervical cancer. Prog Reprod Health Res. 2004;65:2-8.
- Parkin DM, Bray F. The burden of HPV-related cancers. Vaccine. 2006;S3:11-15.
- Bidus M, Elkas J. Cervical and Vaginal cancer In: Berek and Novak's Gynecology (14th edn). Lippincott Williams and Wilkins, USA. 2007;1403-57.
- Vaccarella S, Bruni L, Seoud M. Burden of human papillomavirus infections and related diseases in the extended Middle East and North Africa region. Vaccine. 2013;32-44
- Redman C. Management guidelines for the treatment of cervical precancerous lesions. Laryngoscope. 2002;112(4):700.
- Walraven G. Prevention of cervical cancer in Africa. Afr J Reprod Health. 2003;7:7-12.
- Callahan T, Caughey A. Cervical neoplasia and cervical cancer blueprints obstetrics and gynecology (5th edn). 2009;298-316.
- Lazo A. The molecular genetics of cervical carcinoma. Brit J Cancer. 1999;80:2008-18.
- Davey S, Fox E. Primary and preventive care. In: Brandon J, Bankowski Md, E Amy, et al. (eds). The Johns Hopkins Manual of Gynecology and Obstetrics. 2002;1-7.
- Rock JA, Howard WJ III. Cervical cancer precursors and their management. In: Te Linde's Operative Gynecology (9th edn). Lippincott Williams & Wilkins, USA. 2008;1208-1226.
- Symonds D. Improving the prognosis in cervical cancer. In: Studd J (ed.), Progress in Obstetrics and Gynecology. Churchill Livingstone, UK. 2003;317-32.
- Sankaranarayanan R, Wesley S. A Practical Manual on Visual Screening for Cervical Neoplasia. World Health Organization, Geneva. 2003;46-52.
- Sasieni PJ, Adams J, Cuzick J. Benefits of cervical screening at different ages: evidence from the UK audit of screening histories. Brit J Cancer. 2003;89:88-93.
- Hacker N. Cervical dysplasia and cancer. In: Hacker & Moore's Essentials of Obstetrics and Gynecology. Elsevier. 2010;429-39.
- Chi D, Rustum N, Plante M, et al. Cancer of the Cervix. In: Linde's Operative Gynecology (10th edn). Lippincott Williams & Wilkins, USA. 2011;1227-90.
- Wesley R, Sankaranarayanan R, Mathew B, et al. Evaluation of visual inspection as a screening test for cervical cancer. Brit J Cancer. 1997;75:436-40.
- Gaffikin L, Ahmed S, Chen Q, et al. Risk factors as the basis for triage in low-resource cervical cancer screening programs. Int J Gynecol Obstet. 2003;80:41-7.
- Cohn E, Herzog J. New innovations in cervical cancer screening. Clin Obstet Gynecol. 2001;44:538-49.
- Rengaswamy S, Wesley R, Thara S, et al. Test characteristics of visual inspection with 4% acetic acid (VIA) and Lugol’s Iodine (VILI) in cervical cancer screening in Kerala, India. Int J Cancer. 2003;106:404-08.
- Sankaranarayanan R, Budukh AM, Rajkumar R. Effective screening programmes for cervical cancer in low-and middle-income developing countries. Bull World Health Organ. 2001;79(10):954-62.
- Gaffikin L, Lauterbach M, Blumenthal PD. Performance of visual inspection with acetic acid for cervical cancer screening: a qualitative summary of evidence to date. Obstet Gynecol Surv. 2003;58(8):543-50.
- Eileen M. Human papilloma virus and cervical cancer. Clin Microbiol Rev. 2003;16:1-17.
- Holowaty P, Miller B, Rohan T. Natural history of dysplasia of the uterine cervix. J Nat Cancer Inst. 1999;91:252-58.
- Gage C, Ferreccio C, Gonzales M, et al. Follow-up care of women with an abnormal cytology in a low resource setting. Cancer Detect Prev. 2003;27:466-71.
- University of Zimbabwe. Visual inspection with acetic acid for cervical-cancer screening: test qualities in a primary-care setting. Lancet. 1999;353:869-73.
- Ghaemmaghami F, Behtash N, Modares M, et al. Visual inspection with acetic acid as a feasible screening test for cervical neoplasia in Iran. Int J Gynecol Cancer. 2004;14:465-69.
- Rana T, Zia A, Sher S, et al. Comparative evaluation of Pap smear and visual inspection of acetic acid (VIA) in cervical cancer screening program in Lady Willingdon Hospital, Lahore. Annals of King Edward Medical University. 2010;16.