Research Article - Journal of Agricultural Science and Botany (2018) Volume 2, Issue 1
Levels of some selected trace and essential elements in honey from selected woredas of sidama zone, southern region, Ethiopia
Ashenafi Emiru Teka*
Department of Chemistry, Natural and Computational Sciences, Wolkite University, Wolkite, Ethiopia
- *Corresponding Author:
- Ashenafi Emiru Teka
Department of Chemistry
Natural and Computational Sciences
Wolkite University
Wolkite, Ethiopia
E-mail: ashenafi.emiru@gmail.com
Accepted on February 9, 2018
Citation: Teka AE. Levels of some selected trace and essential elements in honey from selected wore as of sidama zone, southern region, Ethiopia. J Agric Sci Bot 2018;2(1):12-18.
DOI: 10.35841/2591-7897.2.1.12-18
Visit for more related articles at Journal of Agricultural Science and BotanyAbstract
Honey is a natural sweet substance and is produced by honeybees from the nectar of blossoms, from secretion of living parts of plants. In the present study the levels of some selected trace and essential elements (Cu, Co, Mn and Zn) in honey sampled from selected Woredas of Sidama Zone (i.e. Aroresa, Bensa, chire, Arbegona and Hula Woredas), Southern Region, Ethiopia were analyzed. Known weight (1 gm) of honey samples were digested by microwave digester using 5 ml of HNO3 and 2 ml of H2O2. The validity of the digestion procedure was checked by spiking the samples with a standard of known concentration of the analyte metals mixture solutions and determining the percent recovery. The contents of the minerals in the digests were analyzed using flame atomic absorption spectrometer (FAAS). The following concentration ranges (mg/kg) were found in Honey: Cu (0.027-0.0696), Mn (0.0693- 0.815) and Zn (0.0620-0.3359). Whereas Co was not detected in all honey samples. The concentration of Mn was highest followed by Zn and Cu in the five honey samples (i.e. Mn>Zn>Cu). The concentration of each metal (i.e. Cu, Mn, Zn) is highest in the Hula Woreda honey sample (locally known as Dumo) and least in Aroresa Woreda honey sample (locally known as Getame).
Keywords
Honey, Copper, Cobalt, Manganese, Zinc, Essential element, Flame atomic absorption spectrometer, Microwave assisted digestion.
Introduction
As the only available natural sweetener honey was an important food for Homo sapiens from his very beginnings. Indeed, the relation between bees and man started as early as Stone Age [1]. In order to reach the sweet honey, man was ready to risk his life. The first written reference to honey, a Sumerian tablet writing, dating back to 2100-2000 BC, mentions honey’s use as a drug and an ointment [2]. In most ancient cultures honey has been used for both nutritional and medical purposes [2-5]. Honey is the natural sweet substance produced by honey bees from the nectar of plants, which bees collect, transform by combining with specific substances of either own, deposit, hydrate, store and leave in honeycomb to ripen and mature [6]. Honey possesses valuable nourishing, healing and prophylactic properties [7]. The various chemical components of honey include sugars which represent the largest portions of honey composition, about 82% [8], protein that include a number of enzymes (diastase, invertase, glucose oxidase, catalase, etc.), and free amino acids [9]. The composition of honey depends highly on the type of flowers utilized by bees, climatic conditions in which the plants grow and maturation [10,11]. Bee honey can be a good source of major and trace elements needed by humans, where it contains metals up to 0.17%. Metals such as Cr, Co, Cu, Fe, Mn and Zn are essential for humans, and they may play an important role in a number of biochemical processes [12,13]. Some of them are present at the trace level and being toxic if they exceed safety levels [14]. Honey is the results of a bio-accumulation process useful for the collection of information related to the environment where bees live. Since the forage area of the hive is more than 7 Km2 and the bees come in contact with air, soil and water, the concentration of metals in honey reflects their amount in the whole region. Therefore, honey has been recognized as a biological indicator of environmental pollution [15-19].
Honey production in Ethiopia
Africa is blessed with numerous types of wild honeybee [20]. Ethiopia is one of the countries of the continent which own big honey production potential. Owing to its varied ecological and climatic conditions, Ethiopia is home to some of the most diverse flora and fauna in Africa. Its forests and woodlands contain diverse plant species that provide surplus nectar and pollen to foraging bees. Most of the honey produced worldwide is sold with just the designation honey. Generally this means that the honey contains nectar and honeydew contributions from several plant species and therefore is a blend of different kinds of honey. It is thus called polyfloral or multifloral honey. Honeys that originate predominantly from a single botanical source are called unifloral honeys [21]. Ethiopia is the largest honey producer in Africa and 10th largest honey producer all over the world. Also considerable amount of wax is produced in the country. On a world level, Ethiopia is fourth in beeswax and tenth in honey production [22]. The total honey production of Ethiopia is estimated up to 24000 metric tons; only a small amount of this is marketed. Besides poor marketing conditions the main reason is that about 80% of the total Ethiopian honey production goes in to the local Tej-preparation, a honey wine, which consumed as national drink in large quantities [23]. Therefore this study was conducted to collect information on concentration of trace essential elements in honey produced from some selected Woredas of Sidama Zone in the mid rift valley of Ethiopia.
Flame atomic absorption spectrometry
Flame atomic absorption spectrometry (FAAS) is a quantitative analytical method based on measuring the light absorption of free, ground state atoms. The ground state atoms are excited by electromagnetic radiation (light), while absorbing photons having equivalent wavelength with the excitation energy. The absorption spectrum of atoms (similarly to emission spectrum) is line spectrum. The lines are present at exactly determined wavelengths and they have a very small, approximately 0,001 nm FWHM (full width at half maximum). This type of absorption spectrum of atoms gives the high selectivity of atomic absorption spectrometry. At the best line of a given element the probability of absorption of other elements is very low thus complex systems containing several elements can be analyzed without the separation of elements. This procedure has great advantage to molecule absorption spectrometry methods where there is a higher probability of optical interfering effect due to the band absorption and usually the analysis of complex systems is possible only after the application of separation techniques. With atomic absorption we measure the concentration of free atoms in the atomizing unit.
Objectives
General objective
• To determine the concentration of some selected trace and essential elements in honey from five different Woredas of Sidama Zone in the Southern region.
Specific objectives
• To determine the concentration of trace and essential elements(Cu, Co, Mn, Zn) of honey by FAAS collected from Aroresa, Chire, Arbegona, Bensa and Hula Woredas.
• To compare the levels of these metals in honey between Woredas.
• To compare the obtained results with other researcher works.
Methodology
Description of the study area
This study was conducted in selected Woredas of Sidama Zone in the Southern region of Ethiopia which produce honey. These are Aroresa, Bensa, Chire, Arbegona and Hula Woredas. Sidama zone covers 6972.1 square kilometer and lies between 6.14 to 7.18° latitude and 37.92 to 39.19° longitudes, with an elevation ranging 501-3000 meters above sea level (Figure 1).
Sampling
Five different honey samples were collected from five different Woreda towns. Six samples were taken in each Woreda and composited into one sample to represent the Woreda honey sample. These honey samples were believed to be representative of each Woreda because they were come from all corners of the Woredas. The samples were preserved in covered plastic containers and kept at 4°C until analysis.
Equipments and reagents
Equipments: A digital analytical balance (Mettler Toledo, Model AG204, Switzerland) with ± 0.0001 g precision was used to weigh honey samples. A refrigerator (Hitachi, Tokyo, Japan) was used to keep the sample and the digested sample till analysis. BUCK SCIENTIFIC BMS-1 (East Norwalk, USA) Microwave Oven digester was used to digest the honey samples. BUCK SCIENTIFIC MODEL 210 VGP (East Norwalk, USA) atomic absorption spectrophotometer equipped with deuterium ark back ground correctors was used for analysis of the analyte metals (Co, Cu, Mn, Zn) using airacetylene (C2H2) flame.
Reagents and chemicals: All Reagents that were used in the analysis were analytical grade. HNO3 (69% Spectrosol, BDH, England) and H2O2 (30% Merch, Germany) were used for the digestion of honey samples. Stock standard solutions containing 1000 mg/L of the metals Co, Cu, Mn, Zn (BUCK SCIENTIFIC PURO-GRAPHICtm) were used for preparation of calibration standards and in the spiking experiments. Deionized water was used throughout the experiment for sample preparation, dilution and rinsing apparatus prior to analysis.
Procedures
Cleaning apparatus: Apparatus such as digestion vessels, measuring cylinders and volumetric flasks were washed with detergents and tap water, rinsed with deionized water and kept in dust free place until analysis begins.
Digestion of honey samples: One gram of honey was accurately weighed in to a 100 ml Teflon® microwave digestion vessel, in triplicate. In to the microwave digestion vessel 2 ml of H2O2 (30% Merch, Germany) and 5 ml of HNO3 (69% Spectrosol, BDH, England) were added and left to digest. The samples were then digested in a microwave oven digester (East Norwalk, USA) according to the program in Table 1. After digestion the samples were quantitatively transferred to a 25 ml volumetric flask and made up to the mark with deionized water. The digested samples were kept in the refrigerator, until the level of all the metals in the sample solutions were determined by FAAS.
| Step | Temperature (°C) | Run time (min.) | ||
|---|---|---|---|---|
| Start | Finish | Start | Finish | |
| 1 | Low(127) | 145 | 0 | 10 |
| 2 | 145 | 160 | 10 | 20 |
| 3 | 160 | 190 | 20 | 40 |
Table 1. Microwave digestion operating conditions.
Determinations of trace metals: Secondary standard solutions containing 10 mg/L were prepared from the atomic absorption spectroscopy standard stock solutions that contained 1000 mg/L. These secondary standards were diluted with deionized water to obtain four working standards for each metal of interest. Co, Cu, Mn and Zn were analyzed with FAAS (Buck Scientific Model 210VGP) equipped with deuterium arc background corrector and standard air-acetylene flame. Three replicate determinations were carried out on each sample.
Results and Discussion
Instrument calibration
The qualities of results obtained for trace metals analysis using AAS are seriously affected by the calibration and standard solution preparations procedures. The instrument was calibrated using four series of working standards. The working standard solutions of each metal were prepared freshly by diluting the intermediated standard solutions mentioned under section of Determinations of trace metals. Concentrations of the intermediate standards, working standards and value of correlation coefficient of the calibration graph for each of the metals are listed in Table 2.
| Metal | Concentration of intermediate standard (mg/L) | Concentration of standards, in mg/L | Correlation coefficient of calibration curves (R) |
|---|---|---|---|
| Co | 10 | 0.05, 0.08, 0.1, 0.2 | 0.9997 |
| Cu | 10 | 0.02, 0.1, 0.3, 0.6 | 0.9998 |
| Mn | 10 | 0.04, 0.1, 0.3, 0.5 | 0.9997 |
| Zn | 10 | 0.05, 0.1, 0.5, 1.0 | 0.9999 |
Table 2. Working standards and correlation coefficients of the calibration curves for determinations of metals using flame atomic absorption spectrophotometer.
Determination of trace metals in different honey samples
The concentration of four metals (Co, Cu, Mn, Zn) in honey samples were determined by FAAS using four point external calibration curve. Among the identified elements except Cobalt (Co) which is below the method detection limit, all have been identified and shown in Table 3. The results showed that the samples had variable composition of each analyte metals with slight concentration range.
| Element | Concentration of metals (mg/kg) | ||||
|---|---|---|---|---|---|
| aAR | bBS | cCR | dAG | eHU | |
| Co | ND | ND | ND | ND | ND |
| Cu | 0.027 ± 0.0017 | 0.0334 ± 0.0037 | 0.0420 ± 0.0037 | 0.0377 ± 0.0034 | 0.0696 ± 0.0028 |
| Mn | 0.0693 ± 0.0025 | 0.2159 ± 0.007 | 0.2262 ± 0.0184 | 0.2119 ± 0.0128 | 0.815 ± 0.0882 |
| Zn | 0.0620 ± 0.0049 | 0.1916 ± 0.0053 | 0.0640 ± 0.0045 | 0.1299 ± 0.0034 | 0.3359 ± 0.0098 |
ND = Below the method detection limit.
aAR = Honey sample from Aroresa.
bBS = Honey sample from Bensa.
cCR = Honey sample from Chire.
dAG = Honey sample from Arbegona.
eHU = Honey sample from Hula.
Table 3. Mean concentration (mean ± SD mg/kg) of triplicate analyses of trace metals in honey samples.
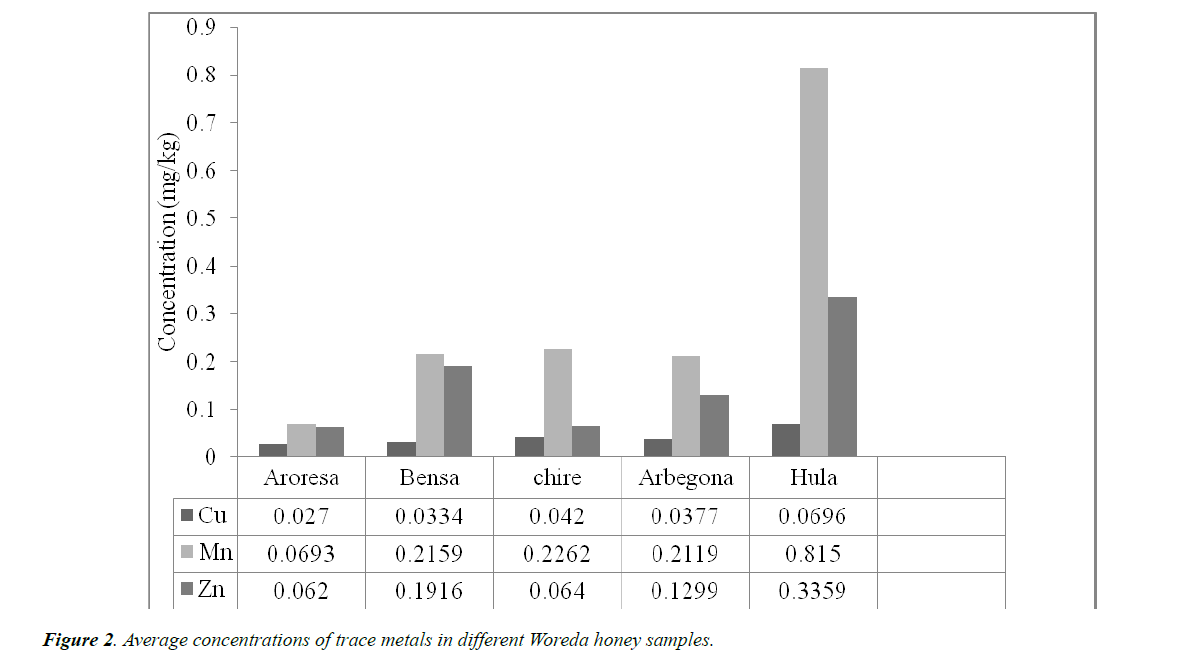
As can be seen from Table 3, Mn (0.0693 ± 0.0025 mg/kg) was observed to be of the highest concentration followed by Zn (0.0620 ± 0.0049) and Cu (0.027 ± 0.0017) was the least of all the studied metals in AR. The level of metals in this honey sample decreases in the order Mn > Zn > Cu.
Mn (0.2159 ± 0.007 mg/kg) was observed to contain the highest concentration of all the metals studied in BS followed by Zn (0.1916 ± 0.0053). As in the previous cases Cu (0.0334 ± 0.0037 mg/kg) was the one with the least concentration in this honey sample. The order of metal concentration follows Mn > Zn > Cu.
As in the above two Woredas; the concentrations of metals in CR, AG and HU Woredas the order of metals concentration is Mn (0.2262 ± 0.0184, 0.2119 ± 0.0128, 0.815 ± 0.0882) > Zn (0.0640 ± 0.0045, 0.1299 ± 0.0034, 0.3359 ± 0.0098) > Cu (0.0420 ± 0.0037, 0.0377 ± 0.0034, 0.0696 ± 0.0028) respectively.
Generally, all the results from the five Woredas showed a similar order of magnitude in the level of the concentration of metal. The order in the five samples of trace metals is Mn > Zn > Cu as shown in Figure 2.
Comparison of the concentration of trace metals in honey samples between wore as
• Cobalt (Co)
Table 3 shows the concentrations of Co in all samples were below the method detection limit (0.06 mg/g). Though Co concentrations were below the method detection limit it does not means that the honey samples were free of Co!
• Copper (Cu)
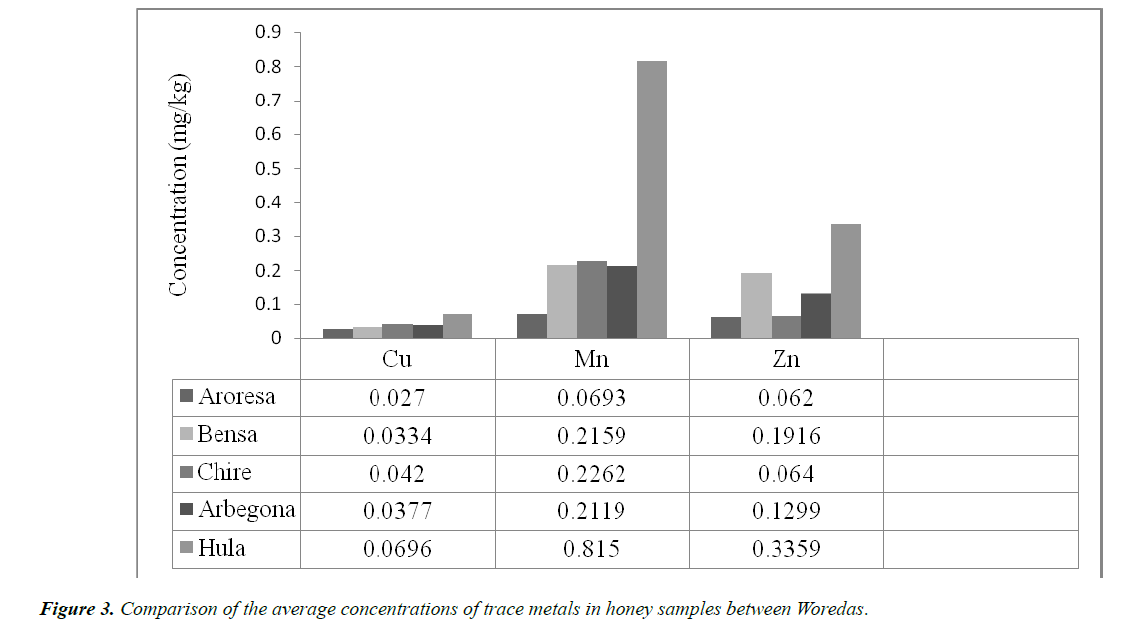
The lowest and highest levels of Cu ranged between 0.027 to 0.0696 mg/kg. The values of the Cu concentrations are not varied too much from sample to sample with the lowest concentration obtained from sample AR (0.027 mg/kg).
The highest level of Cu concentration was obtained from sample HU, which was found to be 0.0696 mg/kg. As we can see from Table 3, all other sampling sites Cu concentration was in these extreme values.
• Manganese (Mn)
The highest concentration of Mn was measured in HU (0.815 mg/kg). This concentration of Mn was obtained by diluting the sample of Hula honey five times. Because, this concentration is out of the calibration point series; thus, to include in the calibration point series, the sample was diluted with five times and then the obtained result also multiplied by five. The lowest concentration of Mn was obtained from AR with a value of 0.0693 mg/kg. Thus all the others Mn concentrations in the other samples were found between these highest and lowest values (i.e. from 0.0693-0.815 mg/kg).
• Zinc (Zn)
As Cu and Mn, the concentration of Zinc was measured highest in Hula (HU) and lowest in Aroresa (AR) Woredas honey samples. Therefore, as we can see from Table 3 above the highest concentration of Zn was recorded in HU honey sample with a value of 0.3359 mg/kg and the lowest concentration of Zn was found in AR (0.0620 mg/kg).
Generally, what we have said above for Cu, Mn and Zn concentration (shown in Table 4) in the five sites of honey samples can be generalized by the help of the following bar graph as shown below (Figure 3).
| Metal | Range of concentration (mg/kg) |
|---|---|
| Cu | 0.027-0.0696 |
| Mn | 0.0693-0.815 |
| Zn | 0.0620-0.3359 |
Table 4. Range of concentrations of each analyte metal in all samples.
Comparison of trace metal levels in honey with other countries reported values
Camina JM et al. [24] have determined the metal concentrations using ICP-OES of natural honey samples collected from different places of the centre of Argentina. They observed that the honey samples contain; Cu: 0.05-0.68 μg/g, Mn: 0.07-0.68 μg/g and Zn: 0.14-3.87 μg/g. The results revealed that there is a good comparability among the metals Cu, Mn, and Zn, with the present study.
Sadia Bibi et al. [25] have also determined the metal content in 7 commercially obtained honey samples from Austria, Pakistan, Canada, Germany, Australia, Saudi Arabia and America using AAS. They obtained a concentration range of Co: 0.001-0.018 ppm, Cu: 0.023-0.498 ppm, Mn: 0.064-0.341 ppm and Zn: 0.1368-0.4717 ppm. The results were comparable with the present study except in the case of Co which is not detected in the present case Table 5.
| Metal | Reported values | |||
|---|---|---|---|---|
| Argentina (84) (μg/g) | Pakistan (85) (ppm) | Morocco (86) (mg/kg) | This study (mg/kg) | |
| Co | - | 0.001-0.018 | - | ND |
| Cu | 0.05-0.68 | 0.023-0.498 | 0.51-4.75 | 0.027-0.0696 |
| Mn | 0.07-0.68 | 0.064-0.341 | 0.080-9.76 | 0.0693-0.815 |
| Zn | 0.14-3.87 | 0.1368-0.4717 | 0.04-2.74 | 0.0620-0.3359 |
ND = Below the method detection limit
Table 5. Comparison of the concentration of trace elements in the honey sample with earlier reported values.
Hasna Belouali, et al. [26] determined the concentration of metals with ICP-OES from honey samples collected in ten localities in the east of Morocco. They obtained a concentration range of Cu: 0.51-4.75 mg/kg, Mn: 0.080-9.76 mg/kg and Zn: 0.04-2.74 mg/kg. These results were comparable with the present study except in the case of Cu which is lower in the present study.
Among the selected Woredas, the Hulla honey (locally known as Dumo) is the richest in all minerals and Bensa honey (locally known as Gerebicho) is the second one. The least concentration of the elements were recorded in the Aroresa honey (locally known as Getame).
Thus the result of this work indicates that the determined elements have no health impacts. Because there is no health problem is reported in the reported values which are in a good agreement to the work of this paper. Therefore, these studied elements have significance for the maintenance of normal metabolic and physiological functions.
Statistical analysis
In this study, a one way ANOVA and SPSS (SPSS 15.0 for windows, The Apache software foundation, 2000) software was used to test the difference between different samples.
For Cu, Mn and Zn, there is significance difference (p< 0.05) at 95% confidence interval between the mean concentrations of the five honey samples (i.e. Aroresa, Bensa, Chire, Arbegona and Hula). Thus, the one way ANOVA shows a difference in concentration between different samples for metals. This difference could be attributed to the soil type where the plant grow, the geographical location, difference in botanical origin, difference in flowering period of the plant, difference in harvesting process of honey, difference from type of container and way of transportation to the market place.
Conclusion
Trace metals are natural components of the Earth's crust. They cannot be degraded or destroyed. To a small extent they enter our bodies via food, drinking water and air as trace elements, some trace metals (e.g., copper, manganese, zinc) are essential to maintain the metabolism of the human body. However, when present at higher concentrations can lead to poisoning.
In this study trace metal content of honey samples from selected Woredas of Sidama Zone in SNNPR, Ethiopia has been investigated. These trace metals were analyzed and measured by atomic absorption spectrophotometer. The results of this study indicate that manganese had highest concentration followed by zinc and copper in all honey samples but these concentrations were not very high. This means that; when comparing the present study results with other reported values almost they are comparable. There is no side effect at the observed level of concentrations.
In general, the results obtained in the present work are in good agreement with those obtained and reported for honeys from other countries.
As observed from this work results, the concentration of each metal (i.e. Cu, Mn, Zn) is highest in the Hula Woreda honey sample (locally known as Dumo). Generally, this implies that; the darker honeys are richer in mineral contents than lighter honey. The least mineral contents were recorded and reported in Aroresa Woreda honey sample (locally known as Getame) which has a white color appearance. The other honey samples i.e. Bensa honey (locally known as Gerebicho), Chire honey (locally known as Mikicho) and Arbegona honey (locally known as Mikicho) concentration lies between these highest and lowest values. The cobalt was not detected in all honey samples.
Therefore, the results (i.e. Co, Cu, Mn, Zn) obtained in this study showed that the honey produced in selected Woredas of Sidama zone were suitable for health and can be used as a baseline data for further investigation.
Acknowledgements
First of all I would like to thank the almighty God who led me through all the rough and difficult times and gave me strength and encouragement to complete this study.
I extend my acknowledgement to the NORAD (Norwegian Agency for Development Cooperation) Project and the coordinator (Andargachew Degebo (PhD), Hawassa University) that supports me by providing money to accomplish this study. Finally, I am also thankful to all persons that have direct or indirect contribution for the accomplishment of this study.
References
- Crane E. The archaeology of beekeeping. London: Gerald Duckworth & Co. 1983;677-89.
- Crane E. History of honey, In Crane E (Ed): Honey, a comprehensive survey. London: William Heinemann. 1975;439-88.
- Jones R. Honey and healing through the ages, In Munn P, Jones R (Ed): Honey and healing. Cardiff: International Bee Research Association (IBRA). 2001;1-4.
- Crane E. The world history of beekeeping and honey hunting, London: Gerald Duckworth & Co. 1999;439-88.
- Allsop KA, Miller JB. Honey revisited: A reappraisal of honey in pre-industrial diets. Br J Nutr. 1996;(75):513-20.
- Council EU. Council Directive 2001/110/EC of 20 December 2001 relating to honey. Official J Eur Communities L. 2002;10:47-52.
- Pereira PCM, Barraviera B, Burini RC, et al. Use of honey as nutritional and therapeutic supplement in the treatment of infectious diseases. J Venom Anim Toxins. 1998;(1):1-2.
- Hak-Gil C, Myung-Kyoo H, Jae-Gil K. The Chemical composition of Korean Honey. Korean J Food Sci Techn. 1988;20:631-36.
- White JW. Composition of American honeys. US Dept. of Agriculture; 1962:124-25.
- Abou-Tarboush HM, Al-Kahtani HA, El-Sarrage MS. Floral type identification and quality evaluation of some honey types. Food Chem. 1993;46(1):13-7.
- Anklam E. A review of the analytical methods to determine the geographical and botanical origin of honey.Food Chem. 1998;63(4):549-62.
- Falco G, Gomez-Catalan J, Llobet JM, et al. Contribution of medicinal plants to the dietary intake of various toxic elements in Catalonia, Spain. Trace Elem Electrolyt. 2003;20:120-24.
- Garcia JCR, Garcia JB, Latorre CH, et al. Comparison of palladium-magnesium nitrate and ammonium dihydrogen phosphate modifiers for lead determination in honey by electrothermal atomic absorption spectrometry. Food Chem. 2005;91:435-39.
- Codex Alimentaris Commission. Standard for Honey, Ref. no. CL 1993/14, SH, Codex Alimentarius Commission. F.A.O./W.H.O., Rome.1993:54-6.
- Przybylowski P, Wilczynska A. Honey as an environmental marker. Food Chem. 2001;74:289-91.
- Celli G, Maccagnani B. Honey bees as bio indicators of environmental pollution. Bull Insectol. 2003;56(1):137-39.
- Atrouse OM, Oran SA, Al-Abbadi SY. Chemical analysis and identification of pollen grains from different Jordanian honey samples. Inter J Food Sci. Techn. 2004;39:413-17.
- Rashad MN, Soltan ME. Major and trace elements in different types of Egyptian mono-floral and non-floral bee honeys. J Food Comp Anal. 2004;17:725-35.
- Muñoz E, Palmero S. Determination of heavy metals in honey potentiometric stripping analysis and using a continuous flow methodology. Food Chem. 2006;94:478-83.
- Adjare SO. Beekeeping in Africa. Agricultural Services Bulletin 68/6 Food and Agriculture Organisation of the United Nations Rome. 1990.
- Seeley TD. Allocation of labor among forage sites. In The wisdom of the hive: the social physiology of honey bee colonies; Harvard University Press: Cambridge/Massachusetts. 1995;84-121.
- Deffar G. Non-wood forest products in Ethiopia. Addis Ababa, Ethiopia. 1998.
- Hartmann I. The management of resources and marginalization in beekeeping Societies of South West Ethiopia. Paper submitted to the conference: Bridge Scales and Epistemologies, Alexandria. 2004;1-3.
- Camiña JM, Boeris MS, Martinez LD, et al. Minor, trace elements and chemical composition of honey. Chem Anal (Warsaw). 2004;49:717-21.
- Bibi S, Husain SZ, Malik RN. Pollen analysis and heavy metals detection in honey samples from seven selected countries. Pak J Bot. 2008; 40(2):507-16.
- Belouali H, Bouaka M, Hakkou A. Determination of some major and minor elements in the east of Morocco honey through Inductively Coupled Plasma Optical Emission spectrometry. Apiacta. 2008;43:17-24.