Research Article - Biomedical Research (2017) Volume 28, Issue 19
Intensity-modulated radiation therapy in nasopharyngeal carcinoma: virtual touch tissue quantification of submandibular gland
Wei Han1, Junjie Liu2, Zhijie Liu1, Lei Zhou1 and Xiaodong Zhu1*
1Department of Radiotherapy, Affiliated Tumor Hospital of Guangxi Medical University, Nanning, Guangxi, P.R China
2Department of Ultrasonography, Affiliated Tumor Hospital of Guangxi Medical University, Nanning, Guangxi, PR China
- *Corresponding Author:
- Xiaodong Zhu
Department of Radiotherapy
Affiliated Tumor Hospital of Guangxi Medical University, PR China
Accepted date: August 30, 2017
Abstract
Objectives: The present study is to evaluate Virtual Touch Tissue Quantification (VTQ) technique in the prevention of submandibular gland damage in patients with Nasopharyngeal Carcinoma (NPC) after Intensity-Modulated Radiation Therapy (IMRT).
Material and Method: A total of 61 patients diagnosed with NPC were treated with IMRT. All the patients underwent Ultrasonography (US) while submandibular volume was determined and VQT examination while Shear Wave Velocity (SWV) was measured. For IMRT, the total cumulative dose of PGTVnx was 69.52-74.32 Gy/30-32f in 6-6.5 w. The follow-up lasted for 6 months. The doses of submandibular glands were analysed by Dose-Volume Histogram (DVH) on TPS system. The parameters were analysed by paired samples t-test, independent t-test, Pearson correlation, Receiver Operating Characteristics (ROC) curves and multiple logistic regression analysis using SPSS 22.0 software.
Results: The volumes of submandibular glands in the planning target volume were decreased at the beginning of radiotherapy (P<0.05). The mean volumes of submandibular glands decreased from 9.485 ± 1.622 cm3 to 6.987 ± 1.069 cm3 at the end of radiotherapy, and 5.986 ± 0.827 cm3 at the 6th month after radiotherapy (P<0.05). The SWV value was 1.68 ± 0.21 m/s before radiotherapy, 2.38 ± 0.19 at the end of radiotherapy, and 2.52 ± 0.47 m/s at the 6th month after radiotherapy, respectively (P<0.05). The doses of Dmax, Dmean and D50 were 70.08 ± 4.08 Gy, 58.24 ± 3.66 Gy and 52.87 ± 2.18 Gy, respectively. The incidence rates of xerostomia were 59.02% and 54.10% at the end of radiotherapy and the 6th month after radiotherapy, respectively. The threshold values of Dmean and D50 for diagnosing postradiation xerostomia were 40.42 Gy and 36.38 Gy, respectively.
Conclusions: The present study demonstrates that VQT technique can be used in the evaluation of the submandibular glands damages. It is effective in the prevention of postradiation xerostomia.
Keywords
Nasopharyngeal carcinoma, Submandibular gland, Intensity modulated radiotherapy, Virtual touch tissue quantification
Introduction
Nasopharyngeal Carcinoma (NPC), a malignancy that occurs at the top and lateral wall of nasopharynx, has a distinct geographic distribution in southern China. For example, Guangxi in southern China has high incidence rates of 20.6-39.94/100,000 each year [1]. For patients with NPC, radiation therapy is the main potential cure. Intensity- Modulated Radiation Therapy (IMRT) is radiation therapy techniques conform to the shape of the tumor target. It can precisely deliver high radiation doses to the tumor target, while minimizing the doses to surrounding normal tissues. IMRT is the first choice of radiotherapy for NPC. It has greatly improved local control, regional control, distant metastasis-free survival, tumor-free survival, disease-specific survival and 5 y overall survival rates to 89.8%, 95.2%, 74.1%, 69.6%, 83.2% and 77.1%, respectively [2]. Although IMRT reduces damages to nasopharyngeal normal tissues during radiotherapy, xerostomia following IMRT is common with a incidence rate of 100% in cervical lymph nodes metastases of NPC, because submandibular gland can receives high dose of radiation [3]. At present, the relationship between injury dose-volume and function of submandibular gland remains unclear.
The diagnosis of xerostomia after radiotherapy mainly depends on three methods: autonomic symptoms, salivary gland function test and saliva flow rate. Ultrasonography is the main method of detecting salivary gland function. Conventional ultrasound is one of the main methods of evaluation, and has been applied in parotid gland and submandibular gland. Bradus et al. [4] have reported parotid ultrasound finding of xerostomia in 1988. A technique that has been just introduced in clinical practice is VTQ technology. VTQ technology can produce shear waves in vivo with a propagation velocity of 1-10 m/s, which spread away from the region of interest, perpendicularly to the acoustic push pulse, generating a localized, and micron-scale displacement of the tissue. By recording the shear wave time difference between adjacent waves and the wavelength, Shear Wave Velocity (SWV) (m/s) can be quantified. SWV values can reflect a stiffer region in the tissue as it travels through this region. Therefore, SWV value is an intrinsic characteristic of the tissue [5]. The use of this technology is based on the fact that increased submandibular gland volume is associated with structural alteration [6].
In the present study, we investigate the alteration in submandibular gland volume in NPC patients who undergo IMRT, and evaluate SWV values of injured submandibular glands after radiotherapy using VTQ technique. The Dose- Volume Histograms (DVHs) of the submandibular glands following radiotherapy are also analysed and the thresholds of mean dose for protecting the normal submandibular glands are identified.
Materials and Methods
Patient characteristics
From July 2015 to March 2016, primary nasopharyngeal carcinoma patients were diagnosed at the Affiliated Tumor Hospital of Guangxi Medical University using full IMRT. The inclusion criteria were as follows: i) the primary focus of NPC was pathologically diagnosed as nasopharyngeal carcinoma; ii) no neck surgery history; iii) patients received full IMRT; iv) patients at the Affiliated Tumor Hospital of Guangxi Medical University received radiotherapy in follow-up after treatment; v) all subjects were conscious at the same time, with normal communication, awareness of purpose, significance and process of the inspection.
The present study was approved by the ethics committee of the International Center for Clinical Trial Registration of the Affiliated Tumor Hospital of Guangxi Medical University with a registration number of ChiCTR-OOC-16008853. The patients were examined by ultrasonography, with data collection and xerostomia investigation. The follow-up was conducted by telephone interview and patient review. During the course of the study, normal treatment of the patients was not interfered and no treatment intervention was performed on the patients. All inspection and follow-up of the study were free of charge, and the patients do not assume any normal treatment costs. The patients had informed consent and the right to withdraw at any time of the treatment process. The study guaranteed the privacy of patients, and personal data were confidential. Research data were only used in this research and its related articles, and their publication will not disclose patients’ identity information.
A follow-up study of 6 months after radiotherapy was carried out. By September 30, 2016, a total of 61 patients with complete follow-up were included in the study. There were 44 males and 17 females. The oldest patient was 72 y old and the youngest one was 17 y old. The mean age was 44.475 + 12.477 y, and the median age was 46 y. According to the seventh edition of AJCC, 4 cases were at stage II, 11 cases were at stage III, and 46 cases were at stage IV. There were 54 cases of undifferentiated carcinomas, 6 cases of differentiated nonkeratinized carcinomas and 1 case of keratinized carcinomas. Among the 61 patients, bilateral submandibular glands in 28 cases were located in the clinical target area or in the lymph node prophylactic irradiation target (CTV), and those in 33 cases were unilateral in the CTV. The 122 submandibular glands were divided into two groups according to whether they were located in the target. In CTV of group A, there were 89 submandibular glands, In CTV of group B, there were 33 outer submandibular glands.
Radiation therapy
IMRT were performed on a 6MV-X linear accelerator (Varian, USA). Thermoplastic mask system extending from vertex of scalp to shoulder aids in immobilization of the patient in supine position. CT scan was performed on a 2.5 mm section thickness range from the skull to the subclavian 2 cm zone. CT images were transmitted to MIM (USA) 3D treatment planning system via image fusion and matching. Accordance with the International Radiation Units and Surveying Committee (ICRU) principles outlined the target volume and endangers the organs. GTVnx included primary tumor and posterior pharyngeal lymph nodes were seen by imaging and clinical examination. GTVnd involved cervical lymph nodes that met the diagnostic criteria of metastases. CTV1 plans involved high risk structures of spread from NPC such as nasopharynx, skull base, posterior pharyngeal space, parapharyngeal space, sphenoid sinus, pterygopalatine fossa, nasal cavity, maxillary sinus 1/3 and high risk lymphatic drainage area, including 5 mm beyond GTVnx+GTVrpn and the entire nasopharynx mucosa and submucosal 5 mm. CTV2 included posterior nasal cavity, posterior maxillary sinus, posterior ethmoid sinus, partial cervical vertebrae, clivus, and CTV1 at a higher risk of spread from the gross tumor. CTVnd included GTVnd and lymph node drainage area. PTV was the corresponding external CTV 5 mm in 3D direction and modified according to the adjacent anatomy. According to the Radiation Therapy Oncology Group (RTOG) 0225 requirements, adequate doses of PRV were delivered. IMRT plans were performed with 9 field uniform angle coplanar irradiation, multi-leaf collimator static intensity modulated radiotherapy, and 6MV-X ray equipped with a bed angle 0º. PTV prescription doses were PGTVnx 69.52-74.32Gy/32f and PGTVnd 68.25-72.64Gy/32f. PGTVnd was divided into two groups: PGTVnd-L and PGTVnd-R. Adequate doses of PCTV1 and PCTV2 were 60-65 Gy/30f, 50.68-57.60 Gy/30f, respectively. Segmentation times were 30-32. Radiotherapy times were 5 times a week for 6 w.
Dosimetric analysis
The 61 patients with first-pass IMRT plan Pinnacle data including maximum dose (Dmax), mean dose (Dmean), 50% volume dose (D50), 30% volume dose (D30) and DVH were analysed using the Treatment Planning System (TPS) for submandibular glands at the doctor's workstation.
SWE inspection method
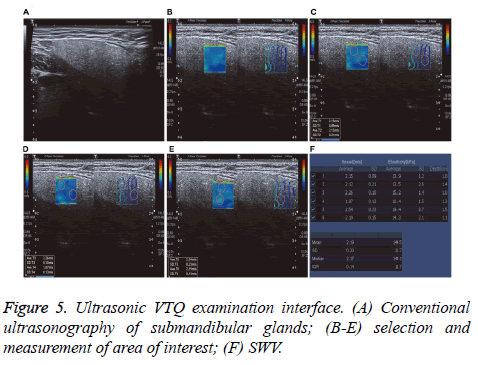
Two experienced examiners performed GE E9 color Doppler ultrasound examinations equipped with a 9L4 high-frequency probe, frequency of 4-9 MHz and configuration ARFI imaging software. Examination techniques included conventional US techniques and VQT. The patients were placed in decubitus with the head slightly extended. During the examination, the patients breathed normally and avoided swallowing. Scanning range included bilateral submandibular gland and surrounding tissues. SWV automatically measurements using VQT techniques were performed by placing the predefined measurement sample in the gland substance and to avoid gland blood vessels. Every gland was measured 5 times in different ROIs, and mean SWV value was calculated while the maximum and minimum values were excluded.
Follow up after radiotherapy xerostomia
All postradiation xerostomia cases had met the EORTC QLQC30 V3.0 criteria [7]. Assessment criteria were divided into 5 grades [8]: Grade 0, no symptoms of xerostomia; Grade 1, mild dry mouth at night or wakefulness; Grade 2, mild dry mouth without affecting eating; Grade 3, drinking when eating or speaking; Grade 4, serious dry mouth, burning sensation of the mouth, difficulty in swallowing and chewing. More than grade 2 was diagnosed as postradiation xerostomia.
Statistical analysis
Statistical analysis was performed with SPSS version 22.0 statistical software. Results were expressed as means ± standard deviation (͞x ± standard deviations). The alterations of submandibular gland volume and SWV values were analysed by paired samples t-test, independent t-test and the Pearson correlation. The Hosmer-Lemeshow test was performed to assess the goodness of fit of the logistic regression model. Receiver Operator Characteristic (ROC) curve analyses were used to determine the optimal cut-off value. A p-value of less than 0.05 was considered as statistically significant.
Results
The volume change of submandibular gland
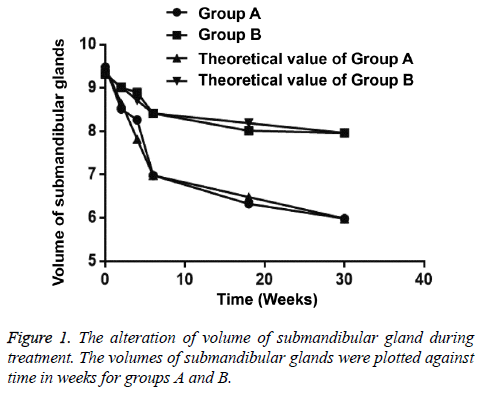
The volume of submandibular gland showed a decreasing tendency during radiotherapy. The volume of 89 submaxillary glands was 7.863-10.682 cm3 (mean, 9.485 cm3) in group A and the volume of 33 submandibular gland 8.473-10.057 cm3 (mean, 9.327 cm3) in group B before radiotherapy. At the 6th month after radiotherapy, the average reduction was 36.89% in group A and that was 14.61% in group B compared with preradiotherapy volumes of submaxillary glands (Tables 1 and 2).
| Time (w) | Volumes of submandibular glands (cm3, x ± s) | t | P |
|---|---|---|---|
| 0 | 9.485 ± 1.622 | ||
| 2nd | 8.513 ± 0.829 | 5.034 | 0 |
| 4th | 8.265 ± 1.428 | 5.326 | 0 |
| 6th | 6.987 ± 1.069 | 12.131 | 0 |
| 18th | 6.328 ± 0.741 | 16.702 | 0 |
| 30th | 5.986 ± 0.827 | 18.13 | 0 |
Table 1. The volume alteration of submandibular glands in group A during radiotherapy (n=89).
| Time (w) | Volumes of submandibular glands(cm3, x ± s) | t | P |
|---|---|---|---|
| 0 | 9.327 ± 0.854 | ||
| 2nd | 9.016 ± 0.928 | 1.417 | 0.08 |
| 4th | 8.897 ± 1.014 | 1.863 | 0.034 |
| 6th | 8.415 ± 1.213 | 3.532 | 0 |
| 18th | 8.103 ± 0.588 | 6.781 | 0 |
| 30th | 7.964 ± 0.679 | 7.177 | 0 |
Table 2. The volume alteration of submandibular glands in B group during radiotherapy (n=33).
In group A, the volume of submandibular gland was significantly reduced during radiotherapy (p<0.05). The frequency of volume shrinkage at the 4th w of radiotherapy was statistically significantly less than that at the 2nd w. The frequency of volume shrinkage was minimum at the 6th w of radiotherapy.
In group B, the volume of submandibular gland was significantly reduced at the 4th w of radiotherapy (p=0.034), while that was not statistically reduced at the 2nd w (p=0.080). The alterations of submandibular gland volume were maximum at the 6th w of radiotherapy. The frequency of volume shrinkage in group B was statistically significant higher than that in group A at the 6th w of radiotherapy and at the 6th month after radiotherapy (p<0.05) (Table 3 and Figure 1).
| Group | 6th | 30th | t | P |
|---|---|---|---|---|
| A (n=89) | 6.987 ± 1.069 | 5.986 ± 0.827 | 6.987 | 0 |
| B(n=33) | 8.415 ± 1.213 | 7.964 ± 0.679 | 1.864 | 0.033 |
| t | -6.316 | -12.281 | ||
| P | 0 | 0 |
Table 3. The volumes of submandibular glands at the 6th w of radiotherapy and at the 6th month after radiotherapy.
The value of SWV
The ultrasound findings of submandibular gland showed homogeneous low echo, no capsule, visible duct echo, and visible margins of the gland. In group A, the echoes of submandibular glands were changed into heterogeneous during radiotherapy. The linear high echoes could be found at the 4th w of radiotherapy. Some patients showed diffuse hypoechoic nodules following radiotherapy.
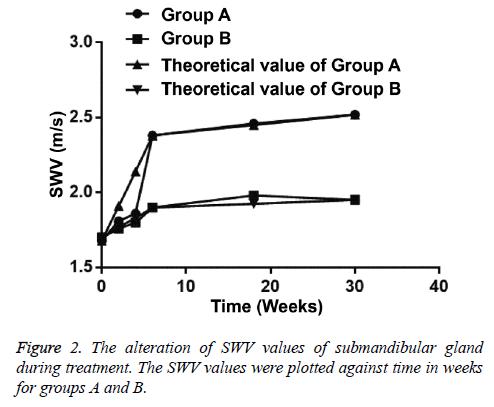
In group A, the SWV value of 89 submandibular glands was 1.68 ± 0.21 m/s before radiotherapy and that was 1.81 ± 0.30 m/s at the 2nd w (p<0.05). The SWV value of 89 submandibular glands was increased continuously at the 4th week of radiotherapy and that was maximum at 6th w. SWV value of 89 submandibular glands was still increased at the 3rd and 6th month during follow-up after radiotherapy (Table 4).
| Time (w) | SWV value (m/s, x ± s) | t | P |
|---|---|---|---|
| 0 | 1.68 ± 0.21 | ||
| 2nd | 1.81 ± 0.30 | 3.349 | 0 |
| 4th | 1.86 ± 0.28 | 4.852 | 0 |
| 6th | 2.38 ± 0.19 | 23.319 | 0 |
| 18th | 2.46 ± 0.41 | 15.974 | 0 |
| 30th | 2.52 ± 0.47 | 15.394 | 0 |
Table 4. The SWV values of submandibular glands in group A during radiotherapy (n=89).
In group B, SWV value of submandibular glands was significantly increased at the 4th w of radiotherapy (p=0.006), while that was not statistically increased at the 2nd w (p=0.088) (Table 5). SWV value of submandibular glands in group A was statistically significantly higher than that in group B at the 6th w of radiotherapy and at the 6th month after radiotherapy (p<0.05) (Table 6). In group B, after radiotherapy, the SWV value of submandibular glands at the 6th month was higher than the 3rd month (Figure 2).
| Time (w) | SWV value (m/s, x ± s) | t | P |
|---|---|---|---|
| 0 | 1.70 ± 0.14 | ||
| 2nd | 1.76 ± 0.21 | 1.366 | 0.088 |
| 4th | 1.80 ± 0.17 | 2.608 | 0.006 |
| 6th | 1.90 ± 0.23 | 4.267 | 0 |
| 18th | 1.98 ± 0.42 | 3.633 | 0 |
| 30th | 1.95 ± 0.15 | 6.999 | 0 |
Table 5. The SWV values of submandibular glands in group B during radiotherapy (n=33).
| Group | 6th | 30th | t | P |
|---|---|---|---|---|
| A (n=89) | 2.38 ± 0.09 | 2.52 ± 0.47 | 2.76 | 0.003 |
| B (n=33) | 1.90 ± 0.23 | 1.95 ± 0.15 | 1.046 | 0.15 |
| t | 16.634 | 6.823 | ||
| P | 0 | 0 |
Table 6. The SWV values of submandibular glands at the 6th w of radiotherapy and at the 6th month after radiotherapy.
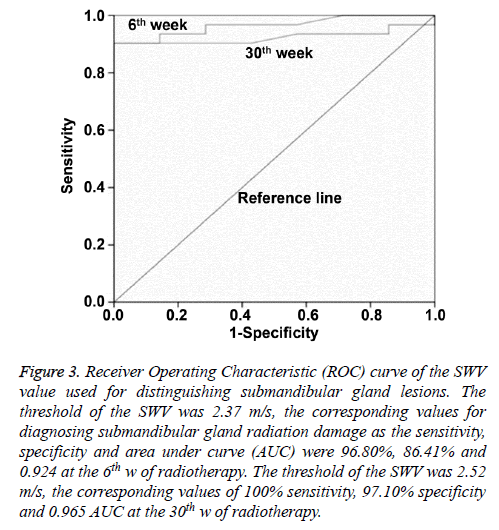
Based on Receiver Operating Characteristics (ROC) curve analysis, when the Youden index reached the highest point, the optimum threshold of SWV for diagnosing submandibular gland radiation damage was 2.37 m/s, and the corresponding sensitivity, specificity and Area Under the Curve (AUC) were 96.80%, 86.41% and 0.924, respectively, at the end of radiotherapy. The optimum threshold of SWV for diagnosing submandibular gland radiation damage was 2.52 m/s, and the optimum sensitivity, specificity and AUC were 100%, 97.10% and 0.965, respectively, at the 6th month after radiotherapy (Figure 3).
Figure 3: Receiver Operating Characteristic (ROC) curve of the SWV value used for distinguishing submandibular gland lesions. The threshold of the SWV was 2.37 m/s, the corresponding values for diagnosing submandibular gland radiation damage as the sensitivity, specificity and area under curve (AUC) were 96.80%, 86.41% and 0.924 at the 6th w of radiotherapy. The threshold of the SWV was 2.52 m/s, the corresponding values of 100% sensitivity, 97.10% specificity and 0.965 AUC at the 30th w of radiotherapy.
The actual dose changes of submandibular gland
In the 61 patients, the maximal and mean doses of submandibular glands were 70.08 ± 4.08 Gy and 58.24 ± 3.66 Gy, respectively. The irradiation dose of 30% and 50% submandibular gland volume were 57.13 ± 2.96 Gy and 52.87 ± 2.18 Gy, respectively.
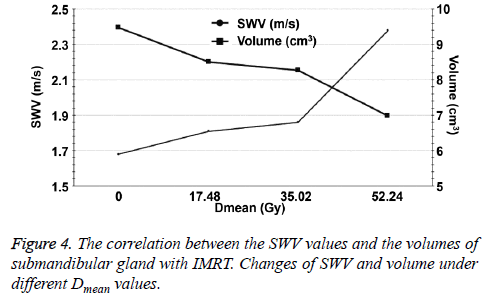
For radiotherapy, the volume of the submandibular gland showed a decreasing tendency and the decrease was reduced at the 2nd w. Then, the decrease became fast at the 4th w when Dmean was 35.02 Gy. The tendency of SWV was similar to that of volume (Figures 4 and 5).
Postradiation xerostomia
We followed up 61 patients for xerostomia during radiotherapy and at the 3rd and 6th month after radiotherapy. The incidence of xerostomia in 28 patients out of 61 was 78.57% at the end of radiotherapy and that was 82.14% at the 3rd and 6th month after radiotherapy, and bilateral submandibular glands were located in the target volume. The incidence of xerostomia in 33 patients out of 61 was 42.42% at the end of radiotherapy, and unilateral submandibular glands were located in the target volume. Then, the incidence of xerostomia was reduced, being 30.30% at the 6th month after radiotherapy. Among the 61 cases, the Dmean of parotid glands was not significantly different between the 28-case group and the 33-case group, because parotid glands were protected (p>0.05, Table 7).
| All (n=61) | Bilateral (n=28) | Unilateral (n=33) | |
|---|---|---|---|
| 2nd | 8 (13.11%) | 7 (25.00%) | 1 (3.03%) |
| 4th | 14 (22.95%) | 11 (39.29%) | 3 (9.09%) |
| 6th | 36 (59.02%) | 22 (78.57%) | 14 (42.42%) |
| 18th | 36 (59.02%) | 23 (82.14%) | 13 (39.39%) |
| 30th | 33 (54.10%) | 23 (82.14%) | 10 (30.30%) |
Table 7. The incidence of xerostomia during radiotherapy and after radiotherapy.
Multi factor analysis
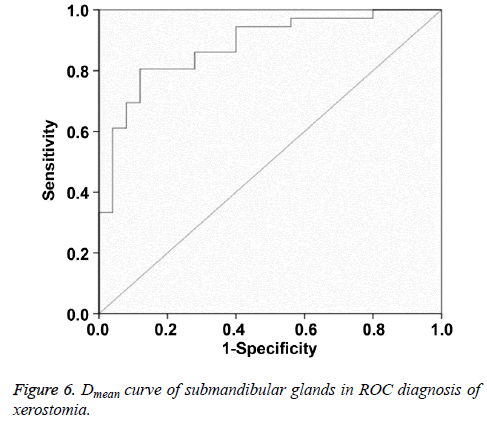
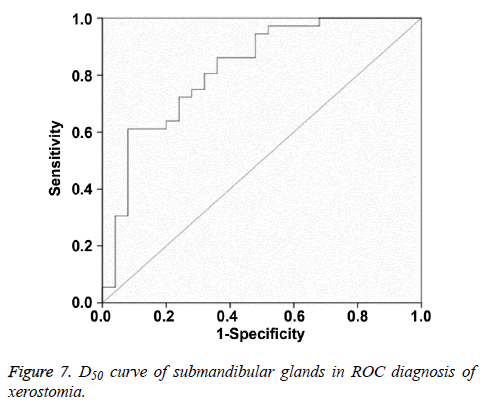
Multiple logistic regression analysis showed that Dmean and D50 were significantly correlated with postradiation xerostomia at the 6th month after radiotherapy. In ROC curve analysis, when the Youden index reached the highest point, the optimum threshold of Dmean and D50 for diagnosing postradiation xerostomia was 40.16 Gy and 36.38 Gy, respectively. The corresponding AUC and 95% confidence interval were 0.882, 0.797-0.967 and 0.821, 0.711-0.931, respectively (Figures 6 and 7 and Table 8).
| Factor | S.E. | Wald | df | P |
|---|---|---|---|---|
| Dmean | 0 | 18.714 | 1 | 0 |
| Dmax | 0 | 7.133 | 1 | 0.08 |
| D30 | 3.482 | 5.286 | 1 | 0.125 |
| D50 | 0.001 | 24.375 | 1 | 0.002 |
Table 8. The results of multiple logistic regression analysis at the 30th week after radiotherapy.
Discussion
Radiation therapy is the primary treatment of many head and neck tumors including NPC. Previous studies show that the degree of parotid injury was positively correlated with the doses received, but the parotid could be protected by the 3D conformal and IMRT because of the advantages of doses. In the present study, the Dmean of parotid glands is not significantly different between the 28-case group and the 33- case group, because parotid glands were protected. This is similar to above-mentioned conclusions. Submandibular gland is one of the most important salivary glands that contribute about 70% of the total volume of unstimulated saliva. Radiation therapy often causes submandibular gland fibrosis, atrophy and permanent sequela. Marks et al. [9] report that the incidence of xerostomia is nearly l00% after conventional radiotherapy. Xerostomia seriously affects the life quality of patients and usually accompany the patients during lifetime. The incidence of patients with mandibular osteomyelitis and osteonecrosis is increased because the secretion of salivary amylase and immunoglobulin A is decreased. At present, postradiation xerostomia has not become a valid prevention method.
For IMRT, the dose distribution is consistent with the 3D configuration of the tumor by computer optimization and control. It has been shown that the function of salivary gland even in the vicinity of the tumor target volume could be protected. In recent years, with the development and application of IMRT technology, the incidence rates of postradiation xerostomia are decreased, especially for longterm postradiation xerostomia. Lee et al. [10] report that 64% of the patients have grade 2 at the 3rd month after IMRT, and 2% of the patients have grade 2 at the 24th month after IMRT. Kam et al. [11] report that the radiotherapy dose for 63 patients with NPC is 66 Gy, the incidence rate of grades 2 and 3 is 57% at the 3rd month after IMRT, and the incidence reduces to 23% two years later. The results of this study show that the incidence rates of postradiation xerostomia at the end of IMRT, at the 3rd month and 6th month after IMRT were 59.02%, 59.02% and 54.10%, respectively. The incidence rates of xerostomia for unilateral submandibular gland in the target volume are lower than those for bilateral submandibular glands in the target volume, being consistent with a previous study [12]. In the future, a single submandibular gland transfer could be performed on the uninvolved neck on the side contralateral to the primary tumor before radiation therapy.
Submandibular gland after radiation therapy is currently damaged using observer-rated xerostomia grading. It is recognized that xerostomia evaluation criteria rely on an observer using RTOG/EORTC. LENT/SOMA is reported in 1995 and widely used since. Furthermore, the National Cancer Institute has the grading of Common Toxicity Criteria (CTC). However, the different grades are somewhat ambiguous, and the correlation between salivary flow rates and the symptoms is weak [13]. In the present study, the criteria used a grading system that is proposed by Wang et al. [8] based primarily on subjective and objective symptoms of xerostomia, and this criterion is less subjectively affected. The measurements of the total volume of saliva or salivary flow rates were considered to be the most objective xerostomia evaluation [14], but this method is invasive and has different results from different researchers [15].
Ultrasonography (US) with noninvasive, real-time, convenient features can be used as the preferred examination for salivary gland. Many studies show that US examinations are performed for the parotid and submandibular gland. Salaffi et al. [16] show that US of salivary glands is the most useful method in distinguishing glandular structural changes in patients with primary Sjogren's syndrome, being consistent with the results by Takagi et al. [17]. Conventional US is a semi-quantitative method to diagnose advanced radiation injury of salivary glands and it is susceptible to the operator's experience and subjective factors. VTQ technique produces shear waves that spread away from the region of interest, perpendicularly to the acoustic push pulse, and generates a localized, micron-scale displacement of the tissue. By recording the shear wave at several locations and correlating these measurements with the elapsed time, SWV can be quantified. Generally, the stiffer a region in the glands is, the greater SWV is, as it travels through this region. Badea et al. [6] results demonstrate that the mean value of SWV of normal left submandibular gland is 1.68 ± 0.46 m/s in the center of the gland and 1.88 ± 0.4 m/s in the periphery, and the SWV of the normal right submandibular gland is 1.74 ± 0.35 m/s in the center and 1.84 ± 0.43 m/s in the periphery. After radiation therapy, the values of SWV are increased, implying structural transformation. SWV values can be used in the evaluation of salivary gland pathology. In this study, SWV values of submandibular glands at the 2nd, 4th, 6th w of IMRT and the 3rd, 6th month after IMRT were 1.68 ± 0.21, 1.81 ± 0.30, 1.86 ± 0.28, 2.38 ± 0.19, 2.46 ± 0.41, 2.52 ± 0.47, respectively, being similar to previous studies [18]. Moreover, the results of this study demonstrate that the threshold of SWV is 2.37 m/s, and the corresponding values for diagnosing submandibular gland radiation damage as the sensitivity and specificity were 96.80% and 86.41% at the 6th w of radiotherapy. The threshold of SWV is 2.52 m/s, with 100% sensitivity and 97.10% specificity at the 30th week of radiotherapy. VTQ technology can be used in the evaluation of submandibular gland radiation damage.
Radiation-induced damages of salivary glands are dosedependent. The more the doses in the salivary glands are, the greater the structural transformation is, eventually leading to permanent damages. Dry mouth of patients will appear when the total cumulative dose of irradiation reaches up to 10 Gy [19]. However, in this study, SWV value is not a positively correlated with the radiation. The results show that the rate of increase of SWV is mild in the first 2 w and slower in the second 2 w. As the total cumulative dose is more than 35.02 Gy, the rate of increase of the SWV becomes fast. It is supposed that the submandibular gland cells are well differentiated with a slow cell division cycle, acinar cells are believed to be more radiosensitive than ductal cells. The damages of submandibular gland after radiation therapy, followed by a recovery phase play an important role in mesenchymal cells and epithelial cells [19]. Although the recovery of submandibular glands in the target volume is continuous, it could not resist the damage caused by radiation therapy. Therefore, the increase rate of SWV is gradually slowed down. When the dose reaches the threshold of submandibular gland injury, the self-repair mechanism is destroyed, and the increase rate of SWV becomes fast. A similar compensatory mechanism has been activated: a recovery of the submandibular gland of the uninvolved target. That was why the symptoms of postradiation xerostomia are eased at the 6th month after radiotherapy. We also find that the larger the submandibular gland volume is, the better the selfrepair function is. Saarilahti et al. [20] report that thirty-six patients are treated with IMRT and have at least one parotid gland excluded from the target volume. The contralateral parotid gland in 18 out of 36 patients could be excluded from the planning target volume, and the contralateral submandibular gland could be spared. The mean total cumulative dose of the spared parotid glands is 23.2 Gy and that of the spared submandibular glands is 25.9 Gy. Mean unstimulated saliva flow is 60% of the baseline value among patients who have one submandibular gland spared and 25% among those who do not at the twelve months following IMRT. The contralateral submandibular gland protection is effective in the prevention of postradiation xerostomia. These results were similar to our study.
Multivariate regression analysis was performed to assess Dmax, D30, and D50 of the submandibular gland and the results of Dmean and D50 are significant. Dmean and D50 values are further analysed with ROC curve. When Youden index reaches the highest point, the thresholds of Dmean and D50 for predicting postradiation xerostomia are 40.16 Gy and 36.38 Gy, respectively. Therefore, we propose that the threshold range of Dmean for protecting submandibular gland is 35-40 Gy.
Conclusions
In conclusion, the present study demonstrates that VQT technique is a valid examination in the evaluation of the submandibular gland following IMRT. After radiation therapy, the values of the SWV are increased, indicating a change in the consistency of the gland. The thresholds of SWV for diagnosing submandibular gland radiation damage at the end of radiotherapy and at the 6th month after radiotherapy are 2.37 m/s and 2.52 m/s, respectively. Submandibular gland sparing with IMRT is safe in selected patients treated for head and neck cancer. The Dmean range of 35-40 Gy is effective in the prevention of postradiation xerostomia.
Acknowledgements
We express our gratitude to Ms. Ling Li and all colleagues in Radiotherapy Department for their support in enrolling the patients. The present study has been registered at the International Clinical Trial Registration Platform with a registration number of ChiCTR-OOC-16008853.
References
- Su WH, Chiu CC, Yao Shugart Y. Heterogeneity revealed through meta-analysis might link geographical differences with nasopharyngeal carcinoma incidence in Han Chinese populations. BMC Cancer 2015; 15: 598.
- Mei F, Zi-xuan F, Jie L, Peng Z, Tao L, Hao W, Jie W, Jian W, Ji-chuan W, Wei-dong W, Jin-yi L. Long-term results and prognostic factors in 582 nasopharyngeal carcinoma treated by intensitymodulated radiotherapy. Chinese J Rad Oncol 2011; 20: 369-373.
- Lee N, Puri DR, Blanco AI, Chao KS. Intensity-modulated radiation therapy in head and neck cancers: an update. Head Neck 2007; 29: 387-400.
- Bradus RJ, Hybarger P, Gooding GA. Parotid gland: US findings in Sjogren syndrome. Work in progress. Radiology 1988; 169: 749-751.
- Tanter M, Bercoff J, Athanasiou A, Deffieux T, Gennisson JL, Montaldo G, Muller M, Tardivon A, Fink M. Quantitative assessment of breast lesion viscoelasticity: initial clinical results using supersonic shear imaging. Ultrasound Med Biol 2008; 34: 1373-1386.
- Badea AF, Tamas Szora A, Ciuleanu E, Chioreanu I, Baciut G, Lupsor Platon M, Badea R. ARFI quantitative elastography of the submandibular glands. Normal measurements and the diagnosis value of the method in radiation submaxillitis. Med Ultrason 2013; 15: 173-179.
- OuYang PY, Shi D, Sun R, Zhu YJ, Xiao Y, Zhang LN, Zhang XH, Chen ZY, Lan XW, Tang J, Gao YH, Ma J, Deng W, Xie FY. Effect of intensity-modulated radiotherapy versus two-dimensional conventional radiotherapy alone in nasopharyngeal carcinoma. Oncotarget 2016; 7: 33408-33417.
- Zhong-He W, Gao G. Reduction of postradiation xerostomia in patients with head and neck cancer by superior technology using 3D-RTPS. J Pract Stomatol 2002; 18: 488-490.
- Marks JE, Davis CC, Gottsman VL, Purdy JE, Lee F. The effects of radiation of parotid salivary function. Int J Radiat Oncol Biol Phys 1981; 7: 1013-1019.
- Lee N, Xia P, Quivey JM, Sultanem K, Poon I, Akazawa C, Akazawa P, Weinberg V, Fu KK. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys 2002; 53: 12-22.
- Kam MK, Teo PM, Chau RM, Cheung KY, Choi PH, Kwan WH, Leung SF, Zee B, Chan AT. Treatment of nasopharyngeal carcinoma with intensity-modulated radiotherapy: the Hong Kong experience. Int J Radiat Oncol Biol Phys 2004; 60: 1440-1450.
- Seikaly H, Jha N, Harris JR, Barnaby P, Liu R, Williams D, McGaw T, Rieger J, Wolfaardt J, Hanson J. Long-term outcomes of submandibular gland transfer for prevention of postradiation xerostomia. Arch Otolaryngol Head Neck Surg 2004; 130: 956-961.
- Eisbruch A, Rhodus N, Rosenthal D, Murphy B, Rasch C, Sonis S, Scarantino C, Brizel D. How should we measure and report radiotherapy-induced xerostomia? Semin Radiat Oncol 2003; 13: 226-234.
- Roesink JM, Schipper M, Busschers W, Raaijmakers CP, Terhaard CH. A comparison of mean parotid gland dose with measures of parotid gland function after radiotherapy for head-and-neck cancer: implications for future trials. Int J Radiat Oncol Biol Phys 2005; 63: 1006-1009.
- Meirovitz A, Murdoch-Kinch CA, Schipper M, Pan C, Eisbruch A. Grading xerostomia by physicians or by patients after intensity-modulated radiotherapy of head-and-neck cancer. Int J Radiat Oncol Biol Phys 2006; 66: 445-453.
- Salaffi F, Carotti M, Iagnocco A, Luccioli F, Ramonda R, Sabatini E, De Nicola M, Maggi M, Priori R, Valesini G, Gerli R, Punzi L, Giuseppetti GM, Salvolini U, Grassi W. Ultrasonography of salivary glands in primary Sjogrens syndrome: a comparison with contrast sialography and scintigraphy. Rheumatology (Oxford). 2008; 47: 1244-1249.
- Takagi Y, Kimura Y, Nakamura H, Sasaki M, Eguchi K, Nakamura T. Salivary gland ultrasonography: can it be an alternative to sialography as an imaging modality for Sjogrens syndrome? Ann Rheum Dis 2010; 69: 1321-1324.
- Knopf A, Hofauer B, Thurmel K, Meier R, Stock K, Bas M, Manour N. Diagnostic utility of Acoustic Radiation Force Impulse (ARFI) imaging in primary Sjoegren`s syndrome. Eur Radiol 2015; 25: 3027-3034.
- Jensen SB, Pedersen AM, Reibel J, Nauntofte B. Xerostomia and hypofunction of the salivary glands in cancer therapy. Support Care Cancer 2003; 11: 207-225.
- Saarilahti K, Kouri M, Collan J, Kangasmaki A, Atula T, Joensuu H, Tenhunen M. Sparing of the submandibular glands by intensity modulated radiotherapy in the treatment of head and neck cancer. Radiother Oncol 2006; 78: 270-275.