Research Article - Journal of Parasitic Diseases: Diagnosis and Therapy (2017) Volume 2, Issue 1
Antiprotozoal activity of flavonoids from Eupatorium arnottianum.
María Clavin1, Silvia Cazorla2,3, Renata Spina4, Miguel A. Sosa4, Emilio Malchiodi2, Virginia Martino1,Fernanda Frank2,3,†, Liliana Muschietti1,†,*
1Universidad de Buenos Aires. Facultad de Farmacia y Bioquímica. Cátedra de Farmacognosia. IQUIMEFA (UBACONICET), Junín 956 2° F (1113), Buenos Aires, Argentina.
2Universidad de Buenos Aires. Facultad de Farmacia y Bioquímica. Cátedra de Inmunología. IDEHU (UBACONICET), Junín 956 4° F (1113), Buenos Aires, Argentina.
3Universidad de Buenos Aires. CONICET. Instituto de Microbiología y Parasitología Medica, IMPaM. Paraguay 2155 13° F (1211), Buenos Aires, Argentina.
4Universidad Nacional de Cuyo-CONICET. Instituto de Histología y Embriología "Dr. Mario H. Burgos", Facultad de Ciencias Médicas, CC 56 (5500), Mendoza, Argentina.
- *Corresponding Author:
- Dr. Liliana Victoria Muschietti
Faculty of Pharmacy and Biochemistry
Pharmacognosy Chair, University of Buenos Aires
IQUIMEFA (UBA-CONICET), Buenos Aires, Argentina
Tel: +54 11 52874282
E-mail: lmusch@ffyb.uba.ar
Accepted December 04, 2016
Citation: Clavin M, Cazorla S, Spina R, et al. Antiprotozoal activity of flavonoids from Eupatorium arnottianum. J Parasit Dis Diagn Ther. 2016;2(1):1-6
Abstract
Dichloromethane and ethanol extracts of Eupatorium arnottianum were evaluated for invitro leishmanicidal activity. The dichloromethane extract produced 80% and 85% of growth inhibition on both Leishmania mexicana and Leishmania amazonensis promastigotes respectively, at a concentration of 100 μg/mL. At the same concentration, the ethanol extract inhibited the growth of L. mexicana and L. amazonensis promastigotes in 62% and 65% respectively. Bioassayguided fractionation of these extracts led to the isolation and identification of flavonoids nepetin, eupatilin, jaceosidin, eriodictyol and hyperoside as bioactive compounds against both species of Leishmania with IC50 values <10 μg/mL, with nepetin being the most active compound against both Leishmania species (IC50 values <2 μg/mL). On Trypanosoma cruzi epimastigotes, jaceosidin, eupatilin and nepetin were found to be the most active flavonoids, with IC50 values 0.06, 0.89 and 2.94 μg/mL respectively. It was observed by transmission electron microscopy that eupatilin and jaceosidin induced intense cytoplasmic vacuolization on L. mexicana promastigotes, followed by parasite destruction. Nepetin induced a mild vacuolization, although the morphology of parasites was preserved. On T. cruzi epimastigotes, eupatilin and jaceosidin induced a moderate vacuolization and some kinetoplast swelling while nepetin did not induce any significant effects on parasites. At the ultrastructural level, the differential effects of eupatilin and jaceosidin on both trypanosomatids may indicate that these molecules affect selectively certain targets on Leishmania spp. We conclude that plants from the Eupatorium genus could be an important source of compounds (flavonoids) with leishmanicidal and trypanocidal activity, thus constituting an interesting option for the treatment of parasitic diseases.
Keywords
Eupatorium arnottianum, Flavonoids, Leishmanicidal activity, Trypanocidal activity, In vitro assays.
Introduction
Parasitic diseases caused by Leishmania and Trypanosoma species, known as neglected tropical diseases, are responsible for high mortality and morbidity rates in developing countries. Leishmaniasis is caused by more than 20 species of the kinetoplastid protozoan parasite Leishmania, which is transmitted to humans by species of phlebotomine sand flies. It occurs in 98 countries with 350 million people living at risk. According to the varied manifestations of the disease, leishmaniasis is classified into four clinical forms: visceral, mucocutaneous, diffuse or disseminated cutaneous, and cutaneous. It is estimated that each year there are 200,000- 400,000 new cases of visceral leishmaniasis, 700,000- 1,200,000 new cases of cutaneous leishmaniasis, and about 20,000-30,000 deaths. Chemotherapy of leishmaniasis has been based on the use of pentavalent antimonials as first-line drugs. Other therapies include amphotericin-B (deoxycholate and liposomal), miltefosine and paromomycin. However, the latter present serious limitations since they are toxic, have low therapeutic efficacy against cutaneous leishmaniasis chronic manifestations, require parenteral treatment, and are of high cost [1,2].
American trypanosomiasis, also known as Chagas disease, is a potentially life-threatening illness caused by the protozoan parasite Trypanosoma cruzi and transmitted by a triatomine vector. About 5.7 million people are estimated to be infected mostly in Latin America where Chagas disease is endemic, and 70 million individuals worldwide are at risk, mainly due to population migration. With an annual incidence of 56,000 new cases in the Americas, it is estimated that this disease causes an average of 7,000 deaths per year. Chagas disease presents three different clinical stages: acute, indeterminate and chronic [3]. The chronic stage results in significant disability with great social and economic impact. Current treatments are based on the nitro-derivatives benznidazole and nifurtimox, which were developed more than four decades ago, and are mainly effective against the acute stage of infection. However, drawbacks include long treatment periods, dose-dependent toxicity, and a high dropout rate of patients due to side effects [1]. Thus, there is an urging need for new leishmanicidal and trypanocidal drugs.
A potential source for antiprotozoal agents comprises the secondary metabolites isolated from plants [4,5]. An interesting characteristic of natural products is the one associated with their structural complexity, because of the presence of multiple chiral centers, heterocyclic substituents, and polycyclic structures which cannot be easily synthesized in the laboratory [6].
Many species of the genus Eupatorium have been used as antiparasitic therapies [7]. Among them, one of the representative species of the genus is E. perfoliatum. This plant was used by the Native Americans for the treatment of feverish diseases like influenza, common cold and malaria [8]. The use of this plant against malaria was very popular, and E. perfoliatum was propagated as an “indigenous substitute for quinine” in the treatment of this disease [9].
Fifteen medicinal species of the genus Eupatorium grow in Argentina. One of them, the Eupatorium arnottianum Griseb (“clavel”, “tuji”), grows in northeastern and central Argentina and in southern Bolivia. This plant is used by “kallawaya” healers from the Bolivian Altiplano against colds, bronchitis, and asthma. Besides, it is used topically in plasters to treat bone fractures and luxation [10].
In a previous study, we described the anti-inflammatory effect of the dichloromethane extract of E. arnottianum and the isolation of its bioactive compounds [11].
In this work, we report the antiprotozoal activity of dichloromethane and ethanolic extracts of E. arnottianum against Leishmania mexicana and L. amazonensis promastigotes, the causal agents of cutaneous and mucocutaneous leishmaniasis respectively. The bioassayguided fractionation of both extracts led to the isolation and identification of bioactive flavonoids. In addition, the in vitro activity of these compounds against Trypanosoma cruzi epimastigotes and the effects on the parasite ultrastructure were studied.
Materials and Methods
Plant material
E. arnottianum was collected in the surroundings of Paraná, Entre Ríos province (Argentina), in February 2014. A voucher specimen is kept at the Herbarium of the Museo de Farmacobotánica J. A. Dominguez, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires (BAF 1337).
Parasites
Leishmania mexicana (MHOM/BR/75/M2903 strain), L. amazonensis (MHOM/BR/75/M2269 strain) promastigotes and T. cruzi epimastigotes (RA strain) were grown in liver infusion tryptose (LIT) medium. Cultures were maintained by weekly passages at 26 ºC and 28 ºC. Parasites were passaged 24 or 48 h before the experiments.
General experimental procedure
Ultraviolet (UV) and infrared spectra (FT-IR) were recorded on Shimadzu® UV2101PC and Nicolet® 380 FT-IR spectrophotometers respectively. Proton nuclear magnetic resonance (1H-NMR) experiments were recorded on a Bruker® MSL300 spectrometer set at 300 MHz and electron ionization-mass spectrometry (EI-MS) experiments were performed on a Shimadzu® QP 5000-GC 17A mass spectrometer. A semi preparative RP-18 [LiChrospher, 5 μm (125 × 4)] column was used for the HPLC analysis (Waters Corporation, Milford, MA). Standards of eriodictyol, hyperoside, caffeic and chlorogenic acids were purchased at Sigma, St. Louis, USA.
Preparation of plant extracts
Air-dried and powdered aerial parts of E. arnottianum (800 g) were successively extracted by maceration with dichloromethane and 50% aqueous ethanol. Extracts were filtered and concentrated in a vacuum to obtain dry dichloromethane (DCM) and ethanol (EtOH) extracts. Extracts were then tested for their in vitro activity against L. mexicana and L. amazonensis promastigotes.
Bioassay-guided fractionation of the DCM extract
The DCM extract was adsorbed on CeliteTM and eluted successively with hexane, acetone and methanol. Three main fractions (F1–F3) of 500 ml each were obtained and subsequently tested for in vitro leishmanicidal activity against L. mexicana and L. amazonensis promastigotes.
Purification of fractions F1–F3 was carried out by a combination of open column chromatography (CC) and semi-preparative high performance liquid chromatography (DAD-HPLC). This fractionation led to the isolation of three flavonoids which were identified by spectroscopic techniques (UV, FT-IR, 1HNMR and EI-MS) and by comparing literature data [11,12]. Purity of compounds was carried out by HPLC and calculated as the ratio of the peak area of the compound and the total area of all the peaks and expressed as percentage.
Bioassay-guided fractionation of the EtOH extract
The dried EtOH extract was suspended in distilled water and extracted in a liquid/liquid semi continuous extractor successively with diethyl ether (FD) and ethyl acetate (FE). These two fractions were analyzed for in vitro leishmanicidal activity.
FD was purified by CC over Sephadex LH-20 and eluted successively with dichloromethane, ethyl acetate and methanol to obtain 20 fractions of 100 mL each. According to their profile in thin-layer chromatography (TLC), these fractions were combined into 7 final fractions (FD1–FD7). From fraction FD5 two flavonoids were isolated by preparative TLC on silicagel 60 F254 (2 mm, KGaA Darmstadt, Germany) (Merck), employing chloroform-ethyl acetate-formic acid (5:4:1) as mobile phase. The detection of the spots was performed with UV light (366 and 254 nm) and by means of natural product reagent (NPR).
From fraction FD6, two hydroxycinnamic acids were isolated by semipreparative DAD-HPLC on a RP-18 column (250 × 4.6 mm, 5 μ, Phenomenex Luna, USA; 1.2 mL/min) with a gradient of methanol-2% acetic acid (15-50%) and water-2% acetic acid (85 to 50%) as the solvent system. Identification of the four compounds was performed by DAD-HPLC and by comparing their retention times (Rt) and UV data of peaks, in comparison with those of commercial standards.
All pure compounds were tested for in vitro antiprotozoal activity against L. mexicana and L. amazonensis promastigotes and T. cruzi epimastigotes.
In vitro leishmanicidal and trypanocidal activity assay
Growth inhibition of Leishmania spp. promastigotes and T. cruzi epimastigotes was evaluated by a [3H] thymidine uptake assay. DCM and EtOH extracts, fractions (F1, F2, F3, FD and FE) and pure compounds were dissolved at concentrations of 7.5 μg/mL in absolute ethanol or ethanol-water 1:1 with 2% DMSO (maximum concentrations of DMSO and ethanol in the microwell were 0.03% and 0.7% respectively). Cell density was adjusted to 1.5 × 106 μg/mL and cultured in the presence of extracts, fractions or compounds isolated for 72 h. Extracts and fractions were tested at 100 μg/mL, and pure compounds at concentrations ranging from 0.1 to 100 μg/ mL. Amphotericin B (0.27–1.6 mM ICN) and Benznidazole (5 to 20 mM; Elea) were used as positive controls for Leishmania spp. and T. cruzi respectively (data not shown). The percentage of inhibition was calculated at 48 h as 100 – [(Number of treated parasites)/(Number of untreated parasites)] × 100, and 50% inhibitory concentration (IC50) values were estimated by linear regression.
Transmission electron microscopy (TEM)
L. mexicana promastigotes were grown for 48 h in either absence or presence of 2.5 μg/mL of eupatilin or jaceosidin and 5 μg/mL of nepetin. T. cruzi epimastigotes were grown for 48 h in either absence or presence of 10 μg/mL of eupatilin, or jaceosidin. For electron microscopy, parasites were washed twice with phosphate buffered saline (PBS), centrifuged at 1000 × g for 5 min and pellets were fixed overnight with 3% glutaraldehyde in cacodylate buffer (pH 7.4). Subsequently, samples were washed three times with PBS and post-fixed with 1% OsO4 overnight. After washing twice with PBS, samples were dehydrated with acetone (50, 75, 90 and 100%) and embedded using the Spurr low viscosity kit (TED PELLA INC) at 60°C overnight. Ultrathin sections (made with an automatic Leica ultracut R ultramicrotome) were stained with saturated uranyl acetate for 6 min, washed once with PBS and post-stained for 4 min with 5% lead citrate. Sections were observed with a Siemens Elmiskop 1.
Statistical analysis
To calculate the IC50 values, percentage inhibition values were plotted against the log of drug concentration (μM) and fitted with a straight line, determined by a linear regression (GraphPad Prism 5.0, 2007). Results presented here are representative of three to four independent experiments.
Results and Discussion
Leishmanicidal and trypanocidal activities of E. arnottianum extracts, fractions and compounds have been tested by a sensitive technique, previously reported by our group, which takes advantage of the [3H] thymidine uptake assay for quantification of the parasiticidal effect of natural products [13].
The DCM extract of E. arnottianum, at a concentration of 100 μg/mL, displayed 80% and 85% of growth inhibition on L. mexicana and L. amazonensis promastigotes respectively. At the same concentration, the EtOH extract was less active and showed 62% of growth inhibition against L. mexicana and 65% against L. amazonensis promastigotes.
Both extracts were subjected to a bioassay-guided fractionation to isolate the active compounds responsible for the leishmanicidal activity. Amongst the tested fractions, F1, F2 and F3 (from the DCM extract) and FD (from the EtOH extract) showed the highest in-vitro inhibitory effect against L. mexicana promastigotes with a range of 60-80% of growth inhibition. Fractions F1, F3 and FD showed the highest activity against L. amazonensis (70-80%). The results are displayed in Table 1.
| Extract/Fraction (100 mg/ml) |
(% growth inhibition) L. mexicana L. amazonensis |
|
|---|---|---|
| DCM | 80 | 85 |
| EtOH | 62 | 65 |
| F1 | 60 | 75 |
| F2 | 65 | 35 |
| F3 | 80 | 80 |
| FD | 70 | 70 |
| FE | 45 | 55 |
Table 1. In vitro leishmanicidal activity of extracts and fractions from E. arnottianum on promastigotes of L. mexicana and L. amazonensis
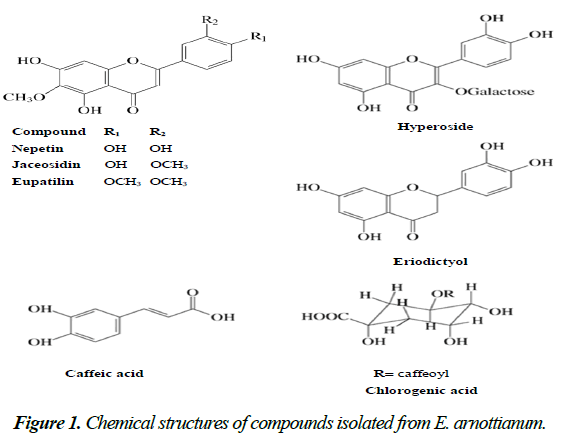
The bioassay-guided fractionation of DMC led to the isolation of three flavonoids which were identified by spectroscopic techniques as eupatilin (from fraction F1), jaceosidin (from fraction F2) and nepetin or eupafolin (from fraction F3). Fractionation of the EtOH extract allowed the isolation of other two flavonoids identified as eriodyctiol and hyperoside (from fraction FD5) and hydroxycinnamic, caffeic and chlorogenic acids (from fraction FD6). These compounds were identified by DAD-HPLC, by comparing their UV spectra and Rt with those of commercial standards (Fig. 1).
Flavonoids are a class of polyphenolic compounds widely distributed in the plant kingdom and represent one of the largest and the most diverse class of plant secondary metabolites. They are naturally present in vegetables, fruits, and beverages (wine and tea) and thus, they are important components of the daily western diet. These compounds display a remarkable array of biochemical and pharmacological effects, suggesting that certain members of this group may affect the function of various mammalian cellular systems. Flavonoids have shown to exhibit anti-inflammatory, antithrombogenic, antidiabetic, anticancer, neuroprotective and antiparasitic properties through different mechanisms of action. These biological effects appear to be related to their ability to modulate several cell-signaling cascades [14].
The activity of the isolated compounds against both species of Leishmania is shown in Table 2. All tested flavonoids showed 50% inhibitory concentration (IC50) values <10 μg/mL. The best leishmanicidal activity for L. mexicana was exerted by eriodictyol (IC50=1.15 μg/mL) followed by nepetin (IC50=1.91 μg/mL). Against L. amazonensis, nepetin (IC50=1.44 μg/mL) and eupatilin (IC50=1.56 μg/mL) were the most active compounds. Caffeic and chlorogenic acids were less active against both parasites (Table 2)./p>
| Compound | IC50(mg/ml)a L. mexicana L. amazonenis |
|
|---|---|---|
| Eupatilin | 5.31±1.59 | 1.56±1.11 |
| Jaceosidin | 5.26±1.31 | 2.33±0.75 |
| Nepetin | 1.91±0.32 | 1.44±0.28 |
| Eriodictyol | 1.15±0.17 | 6.97±1.83 |
| Hyperoside | 3.03±0.69 | 7.95±1.41 |
| Caffeic acid Cafeico |
9.95±1.28 | 13.11±1.34 |
| Chlorogenic acid | 8.48±2.01 | 10.44 ±1.98 |
aIC50 : Concentration required to give 50% inhibition, calculated by linear regression analysis at the concentrations tested (0.1-100 µg/mL) at 72 h culture.
Table 2. In vitro leishmanicidal activity of isolated compounds from E. arnottianum on L. mexicana and L. amazonensis promastigotes
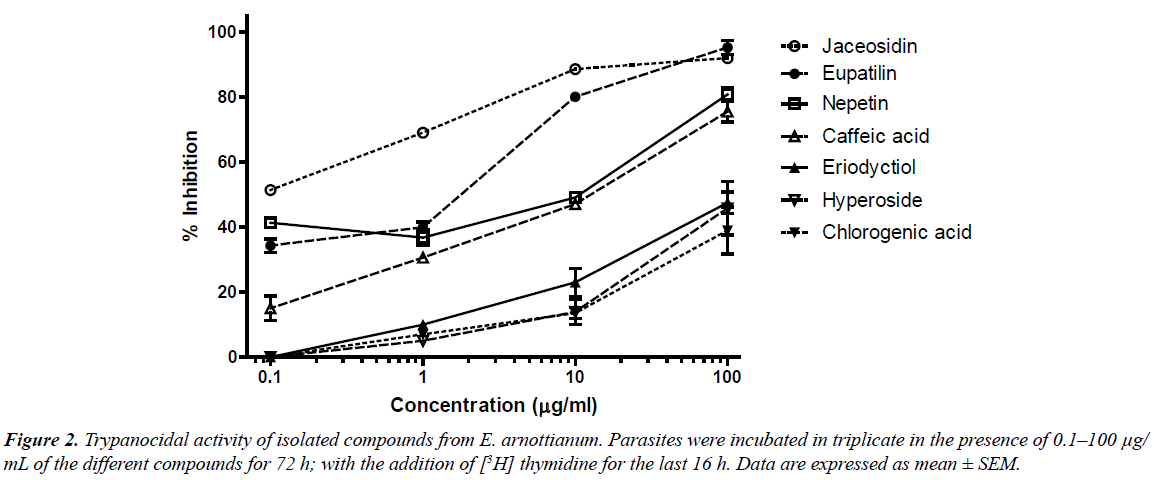
When compounds were tested against T. cruzi epimastigotes, eupatilin, jaceosidin and nepetin were found to be the most active flavonoids with IC50 values of 0.89, 0.06 and 2.94 μg/mL respectively (Fig. 2). For the other compounds, the activity was moderate.
The antiprotozoal activity of several flavonoids has been reported previously [15, 16]. Tasdemir et al. [17] have already evaluated the trypanocidal and leishmanicidal activity of a series of flavonoids and their analogues. In this work, the authors found IC50 values for eriodictyol and hyperoside of 14.5 μg/mL and >30 μg/mL respectively against T. cruzi. The discrepancy with our results may be due to the different parasite stages used (trypomastigote). Eupafolin (nepetin), isolated from E. perfoliatum was shown to possess antileishmanial (L. donovani axenic amastigotes) and antiplasmodial activity [8]. We have already reported the in vitro activity of two 6-methoxylated flavonoids, hispidulin from Ambrosia tenuifolia and santin from Eupatorium buniifolium against T. cruzi epimastigotes and trypomastigotes as well as L. mexicana promastigotes [18].
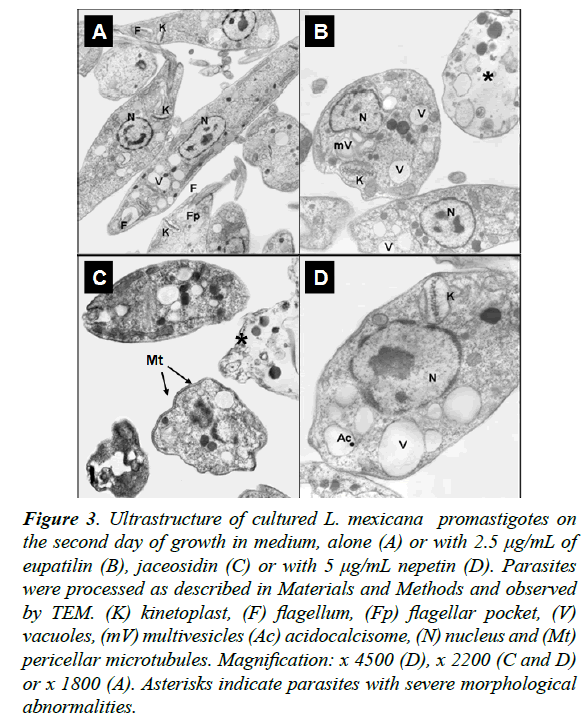
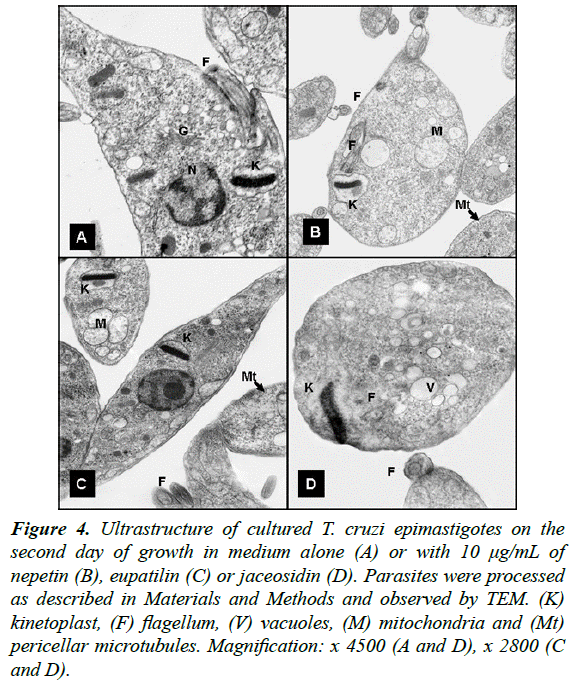
Moreover, to study morphological alterations and target organelles we focused on the effects of eupatilin, jaceosidin and nepetin on L. mexicana and T. cruzi ultrastructure by TEM. At 48 h of incubation, eupatilin and jaceosidin induced intense cytoplasmic vacuolization on L. mexicana promastigotes, followed by a destruction of around 60% of parasites (Fig. 3). Instead, nepetin induced a mild vacuolization and the shape of the parasites remained unaltered (Figure 3D). On T. cruzi epimastigotes, eupatilin and jaceosidin induced a moderate vacuolization and some kinetoplast swelling (Fig. 4), while nepetin did not induce any significant effects on the parasites (Fig. 4B).
Figure 3: Ultrastructure of cultured L. mexicana promastigotes on the second day of growth in medium, alone (A) or with 2.5 μg/mL of eupatilin (B), jaceosidin (C) or with 5 μg/mL nepetin (D). Parasites were processed as described in Materials and Methods and observed by TEM. (K) kinetoplast, (F) flagellum, (Fp) flagellar pocket, (V) vacuoles, (mV) multivesicles (Ac) acidocalcisome, (N) nucleus and (Mt) pericellar microtubules. Magnification: x 4500 (D), x 2200 (C and D) or x 1800 (A). Asterisks indicate parasites with severe morphological abnormalities.
Figure 4: Ultrastructure of cultured T. cruzi epimastigotes on the second day of growth in medium alone (A) or with 10 μg/mL of nepetin (B), eupatilin (C) or jaceosidin (D). Parasites were processed as described in Materials and Methods and observed by TEM. (K) kinetoplast, (F) flagellum, (V) vacuoles, (M) mitochondria and (Mt) pericellar microtubules. Magnification: x 4500 (A and D), x 2800 (C and D).
At the ultrastructural level, the differential effects of nepetin compared to jaceosidin and eupatilin on both trypanosomatids may indicate that these molecules affect selectively certain targets of the parasites. Further studies should be carried out to confirm this hypothesis and to identify such targets.
In conclusion, and to the best of our knowledge, this is the first study on leishmanicidal and trypanocidal activity of Eupatorium arnottianum and the flavonoids eupatilin, jaceosidin and nepetin isolated from the DCM extract. This plant contains some flavonoids with potent antiprotozoal activity which deserves further investigation as templates for novel antileishmanial and trypanocidal compounds.
References
- http://www.dndi.org/
- http://www.who.int/mediacentre/factsheets/fs375/en/
- WHO, Weekly Epidemiological Record (WER).Chagas disease in Latin America: an epidemiological update based on 2010 estimates (No. 6).2015; 90: 33-44.
- Schmidt TJ, Khalid SA, Romanha AJ, Alves TM, Biavatti MW, Brun R, Da Costa FB, de Castro SL, Ferreira VF, de Lacerda MV, Lago JH, Leon LL, Lopes NP, das NevesAmorim RC, Niehues M, Ogungbe IV, Pohlit AM, Scotti MT, Setzer WN, de N C Soeiro M, Steindel M, Tempone AG. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases-part I. Curr Med Chem 2012;19: 2128-2175.
- Schmidt TJ, Khalid SA, Romanha AJ, Alves TM, Biavatti MW, Brun R, Da Costa FB, de Castro SL, Ferreira VF, de Lacerda MV, Lago JH, Leon LL, Lopes NP, das NevesAmorim RC, Niehues M, Ogungbe IV, Pohlit AM, Scotti MT, Setzer WN, de N C Soeiro M, Steindel M, Tempone AG. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases-part II. Curr Med Chem 2012; 19: 2176-2228.
- MuschiettiLV, Sülsen VP, Martino VS. Bioprospection of potential trypanocidal drugs: a scientific literature survey over the period 2000–2010. In: Atta-ur-Rahman (ed.) Studies in Natural Products Chemistry Oxford: Elsevier 2013; 297-336.
- Woerdenbag HJ. Eupatorium species. In: P.A.G.M De Smet, K. Keller, R. Hänsel& R.F. Chandler (eds.) Adverse Effects of Herbal Drugs. Berlin: Springer Verlag 1993; 172-193.
- MaasMA, Hensel F, Batista da Costa R, Kaiser BM, Schmidt TJ. An unusual dimericguaianolide with antiprotozoal activity and further sesquiterpene lactones from Eupatorium perfoliatum. Phytochemistry, 2011; 72: 635-644.
- Hall TB. Eupatorium perfoliatum. A plant with history. Missouri Med1974; 71: 527-528.
- Kallawaya GL. Curanderos Itinerantes de los Andes.UNICEF. OPS. OMS. La Paz, 1987; 226.
- Clavin M, Gorzalzany S, Macho A, Muñoz E, Ferraro G, Acevedo C,Martino V. Anti-inflammatory activity of flavonoids from Eupatorium arnottianum. J Ethnopharmacol2007;112: 585-589.
- Yuan H, Lu X, Ma Q, Li D, Xu G,Piao G. Flavonoids from Artemisia sacrorumLedeb. and their cytotoxic activities against human cancer cell lines. ExpTherap Med 2016; 12,1873-1878.
- Sülsen V, Frank FM, Cazorla CA,Anesini EL,Freixa MB, Vila R, Muschietti LV, Martino VS. Trypanocidal and Leishmanicidal Activities of Sesquiterpene Lactones from Ambrosia tenuifoliaSprengel (Asteraceae). Antimicrob Agents Chemother, 2008; 52, 2415-2419.
- MuschiettiL,Martino V. Actividades biológicas de los flavonoides naturales. In:YunesRA and FilhoVC (eds.) Química de ProdutosNaturais, Novos Medicamentos e a Moderna Farmacognosia, Itajaí:Univali2014; 207-250.
- Marín C, Ramírez-Macías I, López-Céspedes A, Olmo F, Villegas N, Díaz JG, Rosales MJ, Gutiérrez-Sánchez R, Sánchez-Moreno M. In vitro and in vivo trypanocidal activity of flavonoids from Delphinium staphisagria against Chagas Disease. J Natl Prod, 2011; 74, 744-750.
- Beer MF, Frank FM, Elso G, Bivona E, Cerny N, Giberti G, Malchiodi E, Martino V, Alonso MR, Sülsen V,Cazorla S. Trypanocidal and leishmanicidal activities of flavonoids isolated from Stevia satureiifolia var. satureiifolia. PharmaceutBiol2016; 54, 2188-2195.
- Tasdemir D, Kaiser M, Brun R, Yardley V, Schmidt T, TosunF, RuediP. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: in vitro, in vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrobial Agents and Chemotherapy, 2006;50, 1352-1364.
- Sülsen VP, Cazorla SI, Frank FM, Redko FC, Anesini CA, Coussio JD, Malchiodi EL, MartinoVSMuschietti LV. Trypanocidal and Leishmanicidal Activities of Flavonoids from Argentine Medicinal Plants. Am J Trop Med Hyg2007; 77, 654-659.