Research Article - Journal of Primary Care and General Practice (2018) Volume 1, Issue 1
'Vitamin d-binding protein in somali women living in sweden was low and unaffected by treatment.'
Taye Demeke1*, Martin Gillstedt2, Amra Osmancevic2, Anne Lene Krogstad2, Håkan Sinclair3, Eva Angesjö4, Gamal Abd El-Gawad5, Kerstin Landin-Wilhelmsen6
1Angered Primary Health Care, Gothenburg
2Department of Dermatology, Sahlgrenska University Hospital, Gothenburg
3Department of Geriatric Medicine, Borås
4Brämhult Primary Health Care, Borås
5Cleopatra Medical Center, Gothenburg, Sweden
6Section for Endocrinology, Institution of Medicine, Sahlgrenska University Hospital, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
- *Corresponding Author:
- Taye Demeke
SE-42432 Gothenburg, Sweden
Tel: +46 317478313, +46 7008472931
E-mail: taye.demeke@vgregion.se
Accepted date: October 19, 2017
Abstract
Background: Vitamin D deficiency is common in immigrants. Whether vitamin D-binding protein (DBP) is genetically determined or influenced by external factors is not fully clarified. Objective: The aim was to study the influence of age, sex and ethnicity, and the effect of vitamin D treatment on serum DBP. Design: A randomized, placebo-controlled and a case-controlled study were performed. Somali women with skin type V (n = 113; <50 years), latitude 0-10°N, living in Sweden, latitude 57°N, were given 800 IU or 1600 IU cholecalciferol daily or UVB, and similar amounts of placebo (drops or Wood's lamp) during twelve weeks. Serum DBP and S-25(OH) D were monitored before and after treatment. A random population sample of men (n = 50) and women (n = 228; 65<50 years) with skin type II-III from the same region was used as controls. Results: Vitamin D deficiency was prevalent in 73 % of the Somali women and in 5% of the population of whom 0% in women <50 years. DBP was lower in Somali women than in Swedish women (both < 50 and ≥ 50 years; p<0.0001). DBP did not differ between men and women in the population and did not correlate with age, body weight or bone mineral density. DBP was positively correlated with S-25(OH) D in the control women (r=0.19; p=0.004). DBP did not increase with increasing S-25(OH) D levels during vitamin D treatment in Somali women. Conclusions: Vitamin D binding protein was related to vitamin D status but was independent of, or unaffected by, age, sex, body weight, bone status or vitamin D treatment.
Keywords
Vitamin d-binding protein, immigrants, treatment.
Abbreviations
DBP: Vitamin D-binding protein, S-PTH: serum parathyroid hormone, HRT: Estrogen hormone replacement therapy, BMI: Body mass index, CV: The coefficient of variation, BMD: Bone mineral density, DXA: Dual energy X-ray Absorptiometry, UVB: Ultraviolet B.
Introduction
Vitamin D-binding protein (DBP) is the main carrier of vitamin D and its metabolites in the circulation. The concentration of this protein is stable and the amount of DBP in serum exceeds by far the concentration of serum calcidiol (S-25 (OH) D) and no correlation has been established between them [1]. Most of the circulating S-25(OH) D and calcitriol (S-1, 25(OH)2 D) is strongly bound to DBP and 10-15% is loosely bound to albumin. Only a small amount (less than 1%) of the vitamin is found in unbound form [2]. Various studies suggest the existence of racial differences in DBP levels [3]. A small amount of loosely albumin-bound vitamin D and the fraction of the free form determine the bioavailable state of the vitamin, which is also biologically active according to “The free hormone hypothesis”[4]. The protein variant in dark-skinned people, with higher affinity for S-25(OH)D, is associated with low levels of vitamin D and DBP. On the contrary, the DBP in light-skinned people has lower affinity for S-25(OH)D and has been shown to be associated with high values of vitamin D [5,6]. The biological influence of S-25(OH)D on the level of serum parathyroid hormone (S-PTH) was not dependent on DBP [7]. Bioavailable vitamin D may be used for diagnosing vitamin D deficiency in order to exclude overestimated vitamin D deficiency in dark-skinned people. The aim of this study was to investigate the influence of age, gender, ethnicity and time, and the effect of vitamin D treatment on vitamin DBP. The relationship between DBP and bone status was also studied. The hypothesis was that DBP should be strongly associated with the S-25(OH) D concentration in the retrospective cross sectional and during the intervention studies.
Subjects and Methods
Premenopausal Somali women with skin type V (n=113) from latitude 0-10º N, age 18-54 years, living in Gothenburg and Borås, latitude 57º N, for at least two years, were recruited on a voluntary basis. Flyers in the Somali language to Somali community shops and advertisements at health care units, a nearby pharmacy and a supermarket were used for the recruitment. One of the women was postmenopausal. None had vitamin D supplementation at baseline. The inclusion criteria were: no history of serious disease, no medication that might interfere with or affect bone metabolism, no pregnancies and no lactations.
Intervention
A randomized, double-blind, placebo-controlled study was performed in Somali women in Gothenburg (n=98), with either oral cholecalciferol 800 or 1600 IU/day, or placebo for 12 weeks [8]. Sunflower oil drops containing vitamin D (80 IE/ drop) provided by Unimedic (Stockholm, Sweden) was used. Hybrid sunflower oil was used as placebo (APL, Pharma Specials, Huddinge, Sweden). The patients were asked to return their bottles after each six-week period for visual inspection and weighing of the remaining content. Compliance was calculated by counting the remaining content in the bottles.
The Somali women in Borås (n=12) underwent a placebocontrolled trial with UVB radiation (TL12, Corona IV-UVB TL100W/1, Tranås, Sweden) or placebo (Wood’s lamp) on two different skin areas: the upper body and the face and hands, respectively. All women were followed until 24 months, i.e. 12 weeks after the treatments were terminated [8].
Controls
A random population sample of men (n=50) and women (n=228) with skin type II-III, aged 25-64 years, was recruited from the third population screening by the World Health Organization (WHO) MONItoring of trends and determinants in CArdiovascular disease (MONICA) study, Gothenburg, Sweden [9]. Blood samples for hormones were analyzed in every 4th participating subject in age-groups 25-34, 35-44, 45-54 and 55-64 years [10]. Women <50 years were considered premenopausal according to their menstrual history and gonadotropin levels and women >50 as postmenopausal. Estrogen hormone replacement (HRT) was used by 31% of the postmenopausal women. Calcium/vitamin D supplementation and bone-specific agents were used by 0.5%. The cohort was re-examined 13 years later in order to study the intra-individual change in DBP with time. At that time 8% of the postmenopausal women had HRT and 10% had calcium/ vitamin D agents. This originally randomly selected cohort from the city census was used in order to study the natural course of metabolic biochemistry data and hormones in the population which were used for reference data at the Laboratory for Clinical Chemistry of the university hospital.
Measurements
Weight (kg) was measured to the nearest 0.1 kg without shoes. Height (cm) was measured without shoes using a wall-mounted stadiometer. Body mass index (BMI) was calculated as body weight divided by height squared (kg/m2).
Fasting blood samples were drawn in the morning between 8 and 10 am. In menstruating women, samples were taken on cycle day 7-9. All investigations were performed between September and May in order to avoid the influence of exposure to external sunlight. The samples were kept at -80ºC and analyzed at the same time in 2014.
Analysis of serum (S) vitamin D-binding protein (DBP) was performed with an enzyme-linked immunosorbent assay (ELISA; Cat. No DVDBP0, R&D Systems, Minneapolis, MN USA) according to the manufacturer’s instructions. The assay was performed on duplicate samples after dilution 1:4000. The coefficient of variation (CV) between assays was 7.4% and the intra-assay CV was 6.2%. S-25(OH)D was measured by an immunoassay (DiaSorin, Stillwater, MN, USA) and S-PTH (Abbot GmbH & Co., Wiesbaden, Germany). S-25(OH)D <25 nmol/l was considered as deficiency, >25 <50 nmol/l as insufficiency and >50 nmol/l as sufficient levels, according to the IOM [11]. S-Calcium was analysed according to the routine method at the accredited laboratory at the Sahlgrenska university hospital, reference range 2.15-2.49 mmol/l. Serum intact parathyroid hormone (S-PTH) was measured by immunoradiometric assay (Roche Cobas, Rotkreuz, Switzerland), reference range 1.6-6.9 pmol/l. S-estradiol, serum-free estradiol and s-sex hormone binding globuline (SHBG) were analysed in controls only.
Bone mineral density (BMD) was measured with Dual energy X-ray Absorptiometry (DXA, Lunar Prodigy enCORETM, GE Healthcare, LU44663, Madison, WI, USA) in Gothenburg, and with Hologic Discovery A, nr 71022 (Mariborough, MA, USA) in Borås. Calcaneal bone measurements were performed with quantitative ultrasound (LUNAR, Achilles Madison, WI, USA) in the men and women of the population [12]. Speed of sounds (SOS), broadband ultrasound attenuation (BUA) and a combined stiffness index were recorded. This device has been shown to correlate well with BMD both at the lumbar spine and the neck of the femur [13].
Ethics
The projects were approved by the Ethical committee at the University of Gothenburg and the clinical trial by the Medical Products Agency (Uppsala, Sweden), EudraCT no. 2010- 021756-26. The study was performed in accordance with the Helsinki declaration and written consent was obtained from all participants.
Statistics
All data were analyzed using R version 3.0.3 (The R Foundation for Statistical Computing, Vienna, Austria).
Means, standard deviations and medians were calculated with conventional methods. Means are written as Mean ± Standard Deviation. Wilcoxon’s rank sum test was used for two-sample comparisons. Wilcoxon´s signed rank test was used for pairwise comparisons 13 years apart. Spearman’s correlation coefficient and Pearson’s correlation coefficient were used to test for correlations between two variables. Multiple linear regression was used to correlate DBP with 25(OH) D and BMI for both the Somali women and the WHO population. A mixed effects regression model was used to determine if the DBP levels were unaltered throughout the five visits in the study in the Somali women who received oral treatment; one model for each of the three oral treatment groups (placebo, 800 IU/day and 1600 IU/ day). The drop-out rate was high and described in detail in the main study [8]. The present data on DBP were calculated on women who completed the entire study period.
All tests were two-sided and P<0.05 was considered statistically significant.
Results
Anthropometric and background data are given in Table 1. Vitamin D deficiency, i.e. S-25(OH)D <25 nmol/l, was prevalent in 73% of Somali women and in 5% of all, and in 0% of women <50 years, in the population.
| Somali women <50 years N=113 |
Controls Women < 50 years N=65 |
Controls women >50 years N=163 |
Controls Men N=50 |
|
|---|---|---|---|---|
| Age, years | 34+9 CI: (32-36) |
42+8 CI: (40 - 44) |
56+4 CI: (56 - 57) |
45+11 CI: (42-48) |
| Body weight, kg | 74+14 CI: (71 - 77) |
65+9 CI: (63 - 68) |
70 +12 CI: (68 - 72) |
85+18 CI: (80 - 90) |
| Height, cm | 164+6 CI: (163–165) |
167+7 CI: (166 - 169) |
164+6 CI: (163 - 165) |
179+8 CI: (177 - 182) |
| Body mass index, kg/m2 | 28+5 CI: (27 - 29) |
23+3 CI: (23 - 24) |
26+4 CI: (25 - 27) |
26+6 CI: (25 - 28) |
| S-Calcium, mmol/l | 2.34+0.09 *** CI:(2.32–2.35) |
2.47+0.14 CI:(2.42-2.54) |
2.48 +0.18 CI: (2.39 – 2.72) |
2.43+0.10 CI: (2.40-2.46) |
| S-PTH, pmol/l | 6.0+2.3 *** CI: (5.5 – 6.4) |
3.4+1.2 CI: (2.9 – 3.9) |
4.3+1.2 CI: (3.3 – 5.3) |
3.7+1.2 CI: (3.4 – 4.1) |
| S-25(OH)D, nmol/l | 23+13 *** CI: (20 - 25) |
58+15 CI: (48 - 55) |
49+16 CI: (45 - 50) |
46+18 CI: (41 - 51) |
| DBP, ng/ml | 205+86 *** CI: (189 - 221) |
276+07 CI: (249 - 302) |
281+91 CI: (267 - 295) |
301+102 CI: (272 - 330) |
| 13-year, follow-up | ||||
| Age, years | 54+8 CI: (52 - 56) |
68+4 CI: (68 - 69) |
57+11 CI: (54 - 60) |
|
| Body weight, kg | 70+9 CI: (67 - 73) |
71+14 CI: (68 - 73) |
88+14 CI: (83 - 94) |
|
| Height, cm | 166+7 CI: (164 - 169) |
162+6 CI: (161 - 163) |
180+8 CI: (176 - 183) |
|
| Body mass index, kg/m2 | 25+4 CI: (24 - 27) |
27+5 CI: (26 - 28) |
27+4 CI: (26 - 29) |
|
| S-Calcium, mmol/l | 2.34+0.07 CI: (2.31-2.36) |
2.37+0.09 CI: (2.35 – 2.38) |
2.34+0.07 CI:(2.31–2.37) |
|
| S-PTH, pmol/l | 4.7+2.0 CI: (4.0- 5.4) |
5.3+1.9 CI: (5.0- 5.6) |
5.1+1.2 CI: (4.6 – 5.6) |
|
| S-25(OH)D, nmol/l | 63+22 CI: (55 - 70) |
68+26 CI: (63 - 73) |
60+17 CI: (49 - 62) |
|
| DBP, ng/ml, 13 years´ follow-up | 256+99 CI: (222 - 290) Ns versus start |
268+77 CI: (254 - 282), P=0.03 |
264+86 CI: (230 - 298) p<0.001 |
Table 1: Characteristics of the Somali women (n=113) living in Sweden and native Swedish men (n=50) and women as controls (n=228). Means+SD and 95% confidence limits (CI) are given. *** =p<0.001 for comparison between Somali women <50 years and Controls, women <50 years. Ns=not significant
DBP was lower in the Somali women, 205 ± 86 ng/ml, compared with native Swedish women of similar age, 276 ± 107 ng/ml (p<0.00001). There were no differences in DBP between post- and premenopausal women in the control group. Postmenopausal women in the population sample on HRT had similar DBP levels as women without HRT.
DBP did not differ between men, 301+102 ng/ml, and women in the population. DBP was unaltered after a 13-year follow-up period in women <50 years but declined in older women in spite of more calcium/vitamin D use, and men in the population (Table 1).
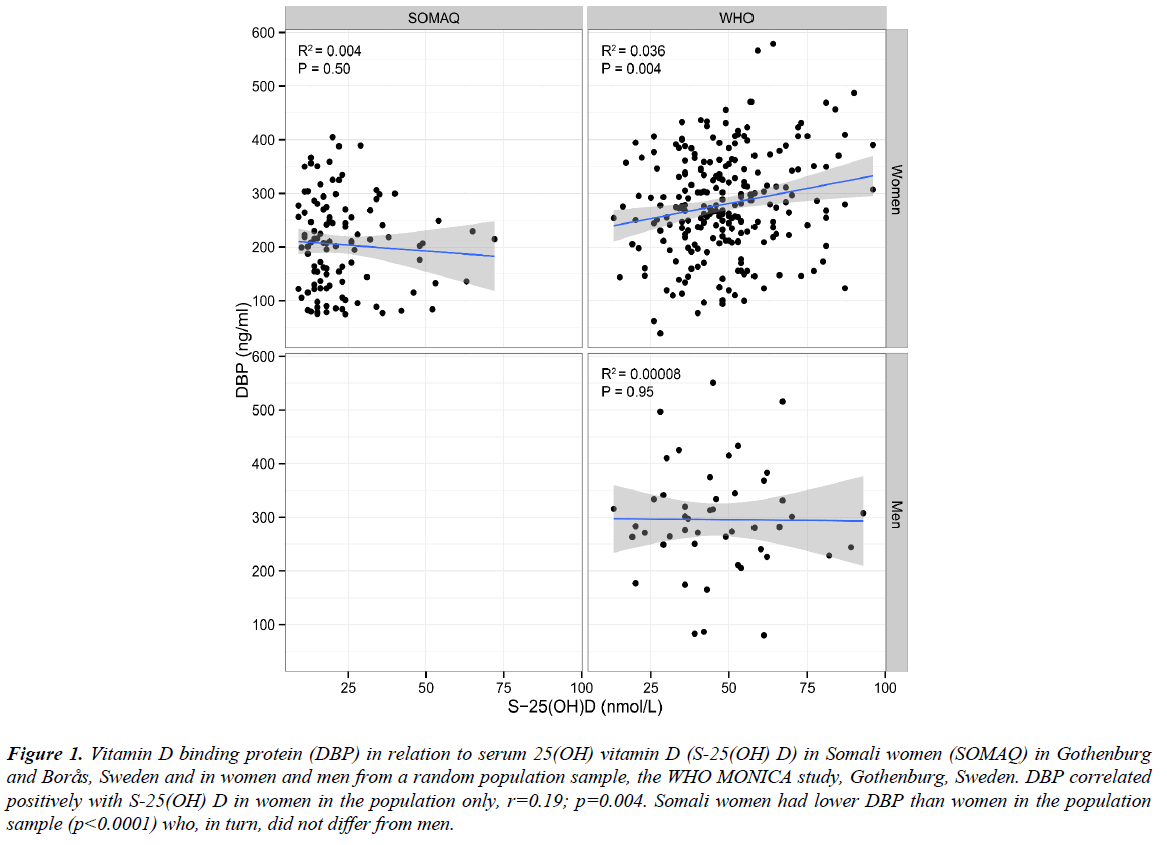
DBP did not correlate with age or BMI in any group. DBP correlated positively with S-25(OH)D in the women in the population sample only (r=0.19; p=0.004) (Figure 1). There was no intra-individual correlation in the change in DBP and change in S-25(OH)D during 13 years of follow-up, neither in the total native population sample nor in the women separately.
Figure 1: Vitamin D binding protein (DBP) in relation to serum 25(OH) vitamin D (S-25(OH) D) in Somali women (SOMAQ) in Gothenburg and Borås, Sweden and in women and men from a random population sample, the WHO MONICA study, Gothenburg, Sweden. DBP correlated positively with S-25(OH) D in women in the population only, r=0.19; p=0.004. Somali women had lower DBP than women in the population sample (p<0.0001) who, in turn, did not differ from men.
DBP correlated positively with S-estradiol (r=0.27), S-free estradiol (r=0.22) and S-SHBG (r=0.35; p=0.01 for all) in women<50 years of age in the population. These correlations disappeared 13 years later. DBP did not correlate with BMD in the Somali women, neither at the lumbar spine nor in the femur neck regions. DBP did not correlate with calcaneal bone measurements SOS, BUA or stiffness in men or women in the population, respectively.
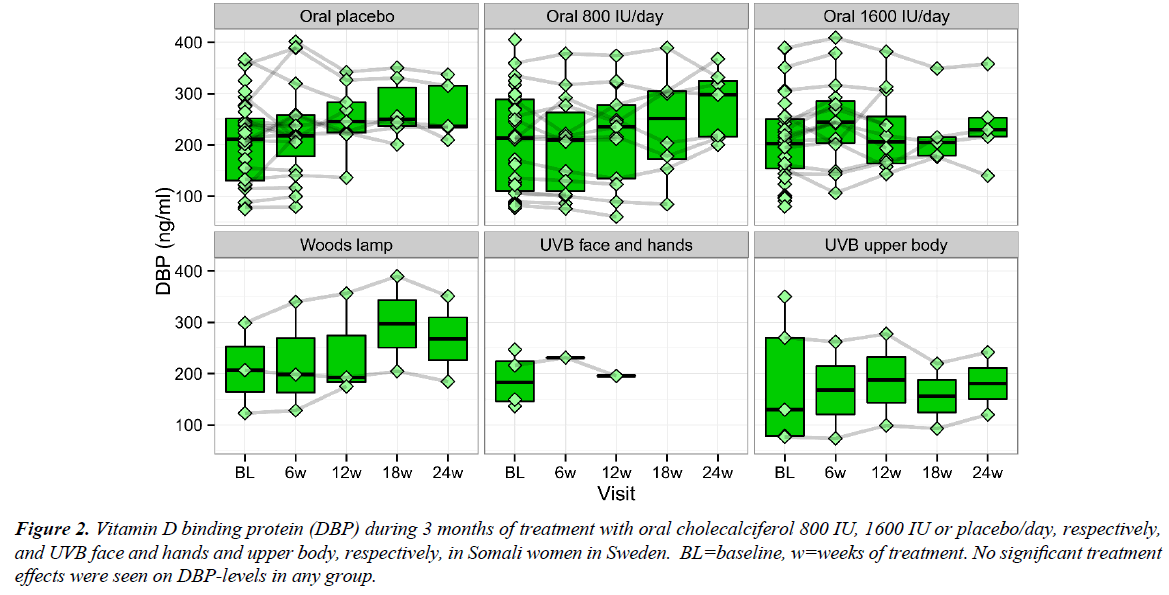
In the intervention studies, S-25(OH)D increased on 800 and 1600 IU/day cholecalciferol and UVB but the majority of the Somali women still had S-25(OH)D below the sufficient level; i.e., 50 nmol/l. The DBP levels were unaltered in the Somali women treated with placebo (p=0.53), 800 IU/day (p=0.31) and 1600 IU/day (p=0.62) or UVB, respectively, throughout all the five visits (Figure 2).
Figure 2: Vitamin D binding protein (DBP) during 3 months of treatment with oral cholecalciferol 800 IU, 1600 IU or placebo/day, respectively, and UVB face and hands and upper body, respectively, in Somali women in Sweden. BL=baseline, w=weeks of treatment. No significant treatment effects were seen on DBP-levels in any group.
Discussion
Vitamin D deficiency was common among the Somali women living in Sweden in the present study, 73%, and the DBP levels were lower in this dark skinned group compared with lightskinned women in Sweden. The effect of oral vitamin D or UVB treatment in the Somali women did not affect the DBP levels. This speaks against the ELISA method being a confounder, or underestimating the DBP results in the Somali women, of this study.
DBP levels did not differ between men and women in the population sample. Hence, our hypothesis was confirmed that DBP was influenced by the vitamin D status (at sufficient levels) but not by age, body weight or sex. The influence of ethnicity could not be answered as none in the control group of women <50 years had vitamin D deficiency. Hence, it cannot be precluded whether the DBP levels were mainly affected by S-25(OH)D levels or by ethnicity per se. No relation was found with bone status or vitamin D treatment effects.
Vitamin D deficiency is highly prevalent worldwide [14,15], but the definition of what constitutes an adequate or optimal level is still elusive [11,16] and discussion is ongoing. Darkskinned people have consistently lower levels of vitamin D than light-skinned people, irrespective of migration [17].
Studies suggest the existence of ethnical differences in DBP levels [18]. DBP is the primary transporter of vitamin D and its metabolites in the circulation. Most of the circulating S-25(OH) D and S-1,25(OH)2D, 85-90%, is strongly bound to DBP and only 10-15% is loosely bound to albumin. Less than 1% of the vitamin is found in unbound form [4]. The protein variant in dark-skinned people, with higher affinity for S-25(OH)D, has been associated with low levels of vitamin D and DBP. On the contrary, the DBP in light-skinned people has lower affinity for 25(OH)D and was associated with high values of vitamin D and DBP [19,20]. Bioavailable 25(OH)D was similar in dark-skinned and light-skinned people, according to a previous cohort study [7]. The lower DBP in Somali women might be explained by the lower S-25(OH)D levels. The DBP might act as a reservoir and trigger a compensatory mechanism to make the free hormone available.
Free and bioavailable vitamin D has been shown to correlate with BMD in the young students [21]. BMD was lower in Somali women living in Sweden >2 years, most of whom were vitamin D-insufficient, compared with both the African- American and American referents, according to the DXA device [22]. However, there was no correlation between S-25(OH) D and BMD in these women. No correlation was found between DBP and BMD in spite of the concomitantly lower vitamin D and DBP levels found in the Somali women. The lack of an association between DBP and BMD is also in contrast to the finding by Powe et al. in African-American subjects 30-64 years of age, where the BMD was higher in the African-Americans in spite of lower S-25(OH)D and DBP [21]. Walsh et al. did not find any association between DBP and bone status or body weight, respectively, and this was reviewed by Jassil et al [23,24].
It has been suggested that the levels of bioavailable vitamin D; i. e., DBP, might be used as an additional tool in evaluating vitamin D deficiency. This could contribute to prevent overestimation of vitamin D deficiency in dark-skinned individuals, which is based on total S-25(OH)D [19,20]. However, it should be pointed out that the method used for DBP in the present study, ELISA from the R&D Systems, has recently been shown to underestimate the GC1f genotype in African descents [19,20].
The present study demonstrated that treatment with vitamin D supplements in Somali women did not significantly increase the level of DBP, while the S-25(OH)D levels did increase; however, still within a very low range [8]. The baseline levels of S-25(OH) D were undetectable in most cases; <10 nmol/l. Hence, the increase, or change, in S-25(OH) D, and thereby in DBP, in individuals on treatment was fairly small and the women only reached S-25(OH)D levels within the insufficient range, as seen in Figure 2. All the Somali women had lower S-25(OH)D levels than the population controls, both at baseline and after treatment. If sufficient levels of S-25(OH)D had been reached, this might have influenced the DBP levels as well. In another study, DBP levels increased after longer treatment (one year) with vitamin D supplements [5]. A third survey demonstrated that, the DBP levels remained stable in both darkskinned and light-skinned individuals after higher doses of vitamin D supplementation, with 50000 IU cholecalciferol daily during two months [25]. The authors concluded that measuring DBP as an evaluation of the treatment was not useful.
There was a strong positive correlation between DBP and S-25(OH)D in the control women, (Figure 1), speaking in favor of vitamin D status as a predictor of DBP, at least at sufficient vitamin D levels. This could be explained by the physiological interaction between S-25(OH)D at higher levels and S-PTH, where S-PTH decreases concomitantly with increasing vitamin D. The positive link between DBP and S-25(OH)D was also found in subjects with primary hyperparathyroidism with low vitamin D concentrations in addition to low DBP levels [7].
In the present study, no significant relation could be seen between DBP and age, weight, bone status or sex, respectively. Low DBP levels have been found in obesity [26,27]. However, very few subjects in the present random population sample were obese. DBP did not correlate with body weight in any of the studied group in the present study in line with previous findings [23,24].
The age independence of DBP was also found in a previous study of postmenopausal women [28,29]. Premenopausal women had higher DBP levels in a study by Pop et al. [30] and women on oral contraceptives and/or HRT have been shown to have higher DBP levels [24]. This could not be corroborated in the present study. However, there was a positive correlation between DBP and S-estradiol in the premenopausal control women in this study, in line with Pop et al. [30]. The intraindividual change in DBP declined by time in older women in the present study in spite of more use of calcium/vitamin D agents. .According to a Danish study on Caucasian women of childbearing age, the use of contraceptives was associated with an elevated DBP concentration and concomitant higher S-25(OH)D and 1,25(OH)2D levels, while the concentrations of free-25(OH)D and free-1,25(OH)2D did not differ between users and non-users of contraceptives. The studied women were healthy and all had S-25(OH)D over 25 nmol/L [31]. None of the Somali women in our study used contraceptives.
A limitation of the study was that the monoclonal ELISA method for DBP underestimates the 1f allele of DBP commonly found in subjects of African ancestry [19,20]. However, this would not have influenced the intervention study within the Somali women in the present study. Another limitation was that serum albumin was not analyzed. Furthermore, it would have been of interest to study DBP in a cohort of Somali women with sufficient 25(OH) D levels, or with longer treatment duration. One strength of the study was the homogeneity of the ethnic group of Somali women. Another strength was that a random population sample, which is considered as the optimal reference group, from the native population in Sweden was used as control group.
In conclusion, the DBP concentration was related to the vitamin D status but was independent of, or unaffected by, age, sex, body weight, bone measurement or treatment dose. DBP could be used together with S-25(OH) D in evaluating vitamin D deficiency in clinical practice, but was less sensitive to the effects of vitamin D treatment.
Grants
Grants were received from the ALF agreement at the Sahlgrenska University Hospital, the Swedish Heart Lung Foundation, the Swedish Council for Working Life and Social Research, the Swedish Medical Association and the Gothenburg Medical Association.
Acknowledgements
The laboratory help from Professor Agneta Holmäng, M.D., and Robert Jakubowicz, and the assistance with coordinating the Somali women by BMA Stella Nakate and Tarja Stenius is gratefully acknowledged.
Conflict of Interest Statement
The authors, Taye Demeke, Martin Gillstedt, Amra Osmancevic, Anne Lene Krogstad, Håkan Sinclair, Eva Angesjö, Gamal Abd El-Gawad, Kerstin Landin-Wilhelmsen, have no conflicts of interest
Authors´ Contribution
Taye Demeke: Designed research, recruitment, conducted the oral treatment clinical trial and writing and had primary responsibility for final content.
Martin Gillstedt: Analyzed data and performed statistical calculation, discussion and writing.
Amra Osmancevic: Designed research, recruitment, principal investigator for the oral treatment clinical trial and writing and had primary responsibility for final content.
Anne Lene Krogstad: Designed research, planning, discussion and writing.
Håkan Sinclair: Execution of the DXA in Borås and planning of the study.
Eva Angesjö: Principal investigator in the UVB trial in Borås and planning of the study.
Gamal Abd El-Gawad: Designed research, planning, discussion and writing.
Kerstin Landin-Wilhelmsen: Principal investigator in the WHO MONICA study designed research, planning, supervision, calculation, writing and had primary responsibility for final content.
All authors read and approved the final manuscript.
References
- Leong A, Rehman W, Dastani Z, et al. Causal Effect of Vitamin D Binding Protein (DBP) Levels on Calcemic and Cardiometabolic Diseases: A Mendelian Randomization Study. PLoS Med. 2014;11.
- Bikle DD, Gee E, Halloran B, et al. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63:954-9.
- Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369:1991-2000.
- Chun RF, Peercy BE, Orwoll ES, et al. Vitamin D and DBP: The free hormone hypothesis revisited. J Steroid Biochem Mol Biol. 2014;144:132-7.
- Fu L, Yun F, Oczak M, et al. Common genetic variants of the vitamin D binding protein (DBP) predict differences in response of serum 25-hydroxyvitamin D [25(OH)D] to vitamin D supplementation. Clin Biochem. 2009;42:1174-7.
- Levin GP, Robinson-Cohen C, de Boer IH, et al. Genetic variants and associations of 25-hydroxyvitamin D concentrations with major clinical outcomes. JAMA. 2012;308:1898-1905.
- Yousefzadeh P, Shapses SA, Wang X. Vitamin D binding protein impact on 25-hydroxyvitamin D levels under different physiologic and pathologic conditions. Int J Endocrinol. 2014.
- Osmancevic A, Demeke T, Gillstedt M, et al. Vitamin D Treatment in Somali Women Living in Sweden - Two randomised, placebo-controlled studies. Clin Endocrinol (Oxf). 2016.
- Wilhelmsen L, Johansson S, Rosengren A, et al. Risk factors for cardiovascular disease during the period 1985-1995 in Goteborg, Sweden. The GOT-MONICA Project. J Intern Med. 1997;242:199-211.
- Trimpou P, Lindahl A, Lindstedt G, et al. Secular trends in sex hormones and fractures in men and women. Eur J Endocrinol 2012;166:887-95.
- IOM I of M (US). Dietary Reference Intakes for Calcium and Vitamin D. 2011;130.
- Landin-Wilhelmsen K, Johansson S, Rosengren A, et al. calcaneal ultrasound measurements are determined by age and physical activity. Studies in two Swedish random population samples. J Intern Med. 2000;247:269-78.
- Trimpou P, Bosaeus I, Bengtsson BA, et al. High correlation between quantitative ultrasound and DXA during 7 years of follow-up. Eur J Radiol. 2010;73:360-4.
- Prentice A. Vitamin D deficiency: A global perspective. In: Nutrition Reviews. 2008;66.
- Pludowski P, Holick MF, Pilz S, et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-A review of recent evidence. Autoimmun Rev. 2013;12:976-89.
- Dawson-Hughes B, Heaney RP, Holick MF, et al. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713-6.
- Forrest KYZ, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48-54.
- Engelman CD, Fingerlin TE, Langefeld CD, et al. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J Clin Endocrinol Metab. 2008;93:3381-8.
- Nielson CM, Jones KS, Chun RF, et al. Role of Assay Type in Determining Free 25-Hydroxyvitamin D Levels in Diverse Populations. N Engl J Med. 2016.
- Nielson CM, Jones KS, Chun RF, et al. Free 25-hydroxyvitamin D: Impact of vitamin D binding protein assays on racial-genotypic associations. J Clin Endocrinol Metab. 2016;101:2226-34.
- Powe CE, Ricciardi C, Berg AH, et al. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J Bone Miner Res. 2011;26:1609-16.
- Demeke T, El-Gawad GA, Osmancevic A, et al. Lower bone mineral density in Somali women living in Sweden compared with African-Americans. Arch Osteoporos. 2015;10:208.
- Walsh JS, Evans AL, Bowles S, et al. Free 25-hydroxyvitamin D is low in obesity, but there are no adverse associations with bone health. Am J Clin Nutr. 2016;103:1465-71.
- Jassil NK, Sharma A, Bikle D, et al. Vitamin D Binding Protein and 25-Hydroxyvitamin D Levels: Emerging Clinical Applications. Endocr Pract. 2017;23:605-613.
- Ponda MP, McGee D, Breslow JL. Vitamin D Binding Protein Levels Do Not Influence The Effect of Vitamin D Repletion on Serum PTH and Calcium: Data From a Randomized, Controlled Trial. J Clin Endocrinol Metab. 2014:jc20141181.
- Ding C, Gao D, Wilding J, et al. Vitamin D signalling in adipose tissue. Br J Nutr. 2012;108:1915-23.
- Karlsson T, Andersson L, Hussain A, et al. Lower vitamin D status in obese compared with normal-weight women despite higher vitamin D intake in early pregnancy. Clin Nutr. 2015;34:892-8.
- Bolland MJ, Grey AB, Ames RW, et al. Age-, gender-, and weight-related effects on levels of 25-hydroxyvitamin D are not mediated by vitamin D binding protein. Clin Endocrinol (Oxf). 2007;67:259-64.
- Gallagher JC. Vitamin D and Aging. Endocrinol Metab Clin North Am. 2013;42:319-32.
- Pop LC, Shapses SA, Chang B. Vitamin D-binding protein in healthy pre- and postmenopausal women: relationship with estradiol concentrations. Endocr Pract. 2016;21:936-42.
- Møller UK, Streym Streym S, Jensen L, et al. Increased plasma concentrations of vitamin D metabolites and vitamin D binding protein in women using hormonal contraceptives: a cross-sectional study. Nutrients. 2013;5:3470-80.