Research Article - Journal of Translational Research (2022) Volume 6, Issue 2
Visualization analysis of the influence of brain aging on exosomes based on cite space knowledge graphs.
Liting Lva*, Jinmei Zhangb, Meng Lib, Qiaojing Gaoc and Lu Wang
Department of Sports Anatomy, Institute of Sports Medicine and Health, Chengdu Sport University, No.2, Tiyuan Road, Chengdu, Sichuan, 610041 China
- *Corresponding Author:
- Liting Lva
Department of Sports Anatomy
Institute of Sports Medicine and Health
Chengdu Sport University, No.2
Tiyuan Road, Chengdu, Sichuan, 610041 China
Tel: +861588214463
E-mail: 1148754758@qq.com
Received: 16-Feb-2022, Manuscript No. AATR-22-54634; Editor assigned: 18-Feb-2022, PreQC No. AATR-22-54634 (PQ); Reviewed: 4-Mar-2022, QC No. AATR-22-54634; Revised: 10-Mar-2022, Manuscript No. AATR-22-54634 (R); Published: 17-Mar-2022, DOI:10.35841/aatr -6.2.106
Abstract
To objectively analyze the literature about the influence of brain aging on the expression of exosomes at home and abroad in the past 12 years, and to summarize the research status, research hotspots and development trends in this field, in an attempt to provide ideas and basis for its development.
Keywords
Brain aging, Exosome, MicroRNA, Cite Space, Visualization analysis.
Introduction
Aging refers to a multifunctional and progressive loss of physiological, biochemical and immune functions, which would induce a decreased survival rate and an increased risk of chronic diseases and disabilities [1]. The elderly population is increasing worldwide every year. As is reported in the research report published by the Institute for Health Metrics and Evaluation (IHME) of the University of Washington in The Lancet, the elderly population worldwide has reached 1 billion in 2018 and is predicted to be 2.13 billion in 2050 [2]. Population aging would exert a significant impact on the disease burden worldwide, which induces an increased burden for most diseases [3]. Brain aging is one of the most distinct characteristics of aging. It involves a complex physiological process, which is usually related to the decline of sensory, motor and cognitive functions. In essence, brain aging is a normal physiological process. However, it is a risk factor for some common neurodegenerative diseases [4]. Brain aging is usually closely related to the changes in some structures, functions and proteins of the brain, especially the atrophy of prefrontal lobe and hippocampus, which play a crucial role in learning, memory and cognitive process [5,6]. Therefore, it is an urgent demand to explore approaches to achieve healthy aging, reduce the number of disabled elderly and prevent the occurrence of senile neurodegenerative diseases in the world. Further, researchers would make every endeavor to identify effective intervention methods or means to delay the aging of the body.
Brain aging is a process in which the quality control of the biological system gradually declines. This complex control system shall accurately regulate the expression of cells and tissue-specific genes [7]. It has been demonstrated in some studies that the quality control of DNA and protein is closely related to age-related diseases [8,9]. The destruction of DNA and protein homeostasis is also closely related to neurodegenerative diseases, such as Alzheimer's disease (AD) and Parkinson's disease (PD). The accumulation of misfolded proteins will cause DNA mutation and protein toxicity and affect the normal aging process [10]. However, there are relatively few studies on the RNA in brain aging, compared with DNA and protein. Besides, it has been suggested in some studies that non-coding RNAs (ncRNAs) play a significant role in normal and pathological aging, especially in the central nervous system (CNS) [11,12].
Exosomes are small membranous vesicles with a diameter of about 30-150 nm, which are first found in the supernatant of the sheep reticulocyte culture [13]. Exosomes contain many bioactive substances, such as proteins, mRNA, microRNA, cytokines and transcription factor receptors. They could carry such biological macromolecules as proteins, RNAs and lipids, and transmit the inclusions between maternal cells and target cells under physiological and pathological conditions; therefore, they are mediators of information transmission between cells [14]. MicroRNAs are non-coding ribonucleic acids (ncRNAs) with a length of 19-24 nucleotides. They are a kind of gene expression regulator as the post-transcriptional level extensively existing in plants, animals, viruses, fungi and human body. MicroRNAs are not only expressed in tissues, but also can leave tissue cells and enter the blood, stably existing in blood and become circulating microRNAs; besides, they extensively participate in such physiological and pathological processes as cell growth, proliferation, apoptosis and differentiation [15]. It has been indicated in a study that microRNAs can regulate up to 60% of mRNAs in the human body and participate in the pathogenesis of various neurodegenerative diseases; further, they are also the key regulatory factors for neuron development and function [16]. MicroRNAs can regulate many mechanisms related to brain aging, including synaptic plasticity, inflammation, neuroprotection, lipid metabolism, mitochondrial function and so forth [17]. Moreover, the studies on the influence of exosomes on brain aging emerge at the beginning of the 21st century. As a new carrier of information exchange between cells, exosomes can effectively regulate recipient cells, which has attracted great attention in the scientific community and gradually become one of the research hotspots [18].
Cite Space software is information visualization analysis software developed based on Java, and it enables researchers to evaluate published research results, influence and development trends through statistical and quantitative methods. CiteSpace supports multiple types of bibliometrics research, including collaborative network analysis, co-citation analysis and cooccurrence analysis, and can generate visual maps, such as geospatial visualization [20]. Collaborative network analysis can be employed to identify core countries/regions, institutions and authors in a specific field and their cooperative relationships. Besides, co-citation analysis and co-occurrence analysis can be employed to reflect the research basis and research hotspots in this field respectively. The development graphs of various fields can be plotted via extracting such data as countries, institutions, authors and keywords, thus visually presenting the information in this field [21]. Additionally, it can be employed to analyze the development pattern and evolution trend of disciplines, as well as the research hotspots and research frontiers of disciplines [22]. It has been continuously developed and extensively adopted in the medical field [23,24]. Therefore, based on the related literature collected in Web of Science database, CiteSpace 5.8R1 was adopted in this paper to comprehensively analyze the present situation, research hotspots and development trends of the relationship between brain aging and exosomes from 2009 to 2021, in an attempt to provide reference and application for future research in related fields.
Data Sources and Research Methods
Data sources
In this study, the core database of Web of Science was determined as the retrieval platform, with the literature retrieval formula expressed as (TS= (brain aging AND mRNA*) OR TS= (brain aging AND exosome*) OR TS= (brain aging AND microRNA*) OR TS= (brain aging AND miRNA*)), with the document type being "Article". The retrieval was performed on August 17, 2021, with the retrieval time span being "all years". A total of 3,761 pieces of literature were retrieved out, and 2,581 pieces were obtained after the duplicate removal with CiteSpace software and the manual screening. All pieces of literature were related to brain aging and exosomes.
Methods
The core database of Web of Science was adopted as the retrieval platform, and the related literature about the influence of brain aging on exosomes published since the establishment of the database was included. Besides, the countries, authors, institutions, co-citations and keywords were subjected to visualization analysis by CiteSpace, and the related visual scientific network graphs were generated
Research Methodology
The retrieval and analysis tool of CiteSpace 5.8R1 software for WOS was adopted to perform the statistical analysis on the time distribution, space distribution, literature and periodical distribution, author information, keyword and other data of studies on the interaction between brain aging and exosomes. The data analysis module of CiteSpace 5.8R1 software for WOS was adopted to perform the visualization analysis on the knowledge graphs of the Author, Country, Institution and Keyword of the retrieved literature, in an attempt to perform analysis and statistics of the research status, research hotspots and research trends in this field.
Research Results and Analysis
Distribution of Publication Volume
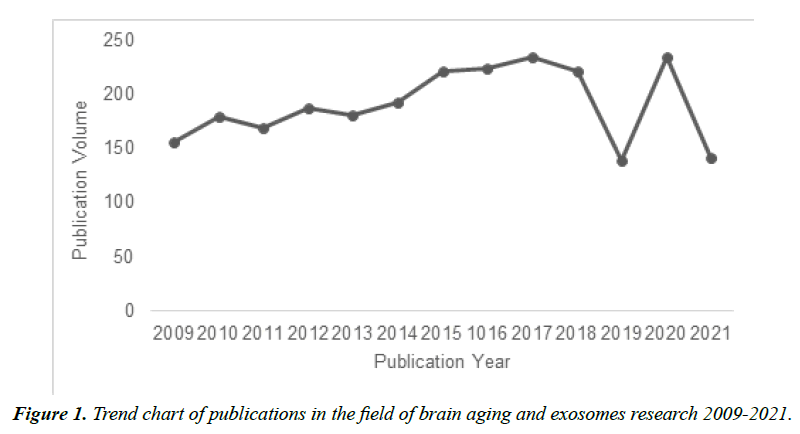
The first research literature about brain aging and exosomes was published in Alzheimer Disease & Associated Disorder in 2009. The focus of this literature is placed on the difference in the mRNA expression of apoptosis-related proteins in patients with Alzheimer's disease [25]. During the exploring period in this field, the overall publication volume showed an increasing trend, but with some fluctuations. As shown in Figure 1. The publication volume in this field presents a stable trend from 2009 to 2014, and a slightly increasing trend from 2015 to 2017, which indicates that there is no significant breakthrough in this field during this period; The publication volume decreases sharply in 2019 due to COVID-19, and subsequently increases significantly in 2020, reaching 235 articles. These trends indicate that the research on brain aging and exosomes has attracted accumulating attention, and more studies are being conducted. Besides, the data of the publication volume in 2021 is the statistics of the first eight months of this year.
Country or Region Analysis
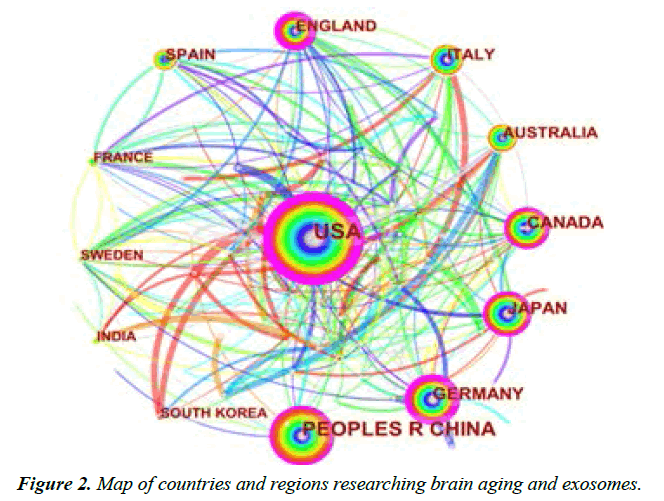
The objective to analyze the distribution of countries and regions is to understand the research situation in this field in each country. After the literature was imported into CiteSpace software, Country was selected as the node, with the time zone slice=1 and the threshold topN=50. The network knowledge graph related to countries was plotted. The size of the node circle represents the number of articles published by the country in this field, and the number of lines and the thickness of them are directly proportional to the degree of cooperation between countries. The node with prunosus outer circle represents that the country has a high centrality, and the centrality represents that the node is important in this network knowledge graph, which reflects that the country has an important influence in this field [19]. Figure 2 presents the distribution diagram of countries/regions with published relevant literature in this field. It can be seen from the figure that the node circle in the United States is the largest, which indicates that the research in this field in the United States has a larger influence. The United States is the first country to put forward the "Human Brain Project (HBP)" [26]. Which is implemented to explore the relationship between circuits and cognitive and behavioral abilities, with the focus placed on the diagnosis and treatment effects of post-traumatic stress, brain injury and memory loss of the brain [27]. Table 1 lists the top 10 countries/regions with the largest publication volume, and the total publication volume in these 10 countries is as high as 1,937, accounting for about 75.1% of the total publication volume. The United States has 711 cooperative articles, with a centrality of 0.48, which indicates a significant influence in this field. China ranks second in the publication volume in this field, including 411 cooperative articles in total, with a centrality of 0.10, which indicates that China has certain cooperation with other countries in this field, but the degree of cooperation is not outstanding.
| Ranking | Country | Publications | Centrality |
|---|---|---|---|
| 1 | USA | 711 | 0.48 |
| 2 | Peoples R China | 411 | 0.10 |
| 3 | Germany | 155 | 0.23 |
| 4 | Japan | 145 | 0.18 |
| 5 | Canada | 112 | 0.11 |
| 6 | Australia | 92 | 0.06 |
| 7 | Italy | 89 | 0.07 |
| 8 | England | 85 | 0.2 |
| 9 | Spain | 72 | 0.04 |
| 10 | France | 65 | 0.09 |
Table 1. Top 10 prolific countries and regions researching brain aging and exosomes.
Visualization Analysis of Author Cooperation
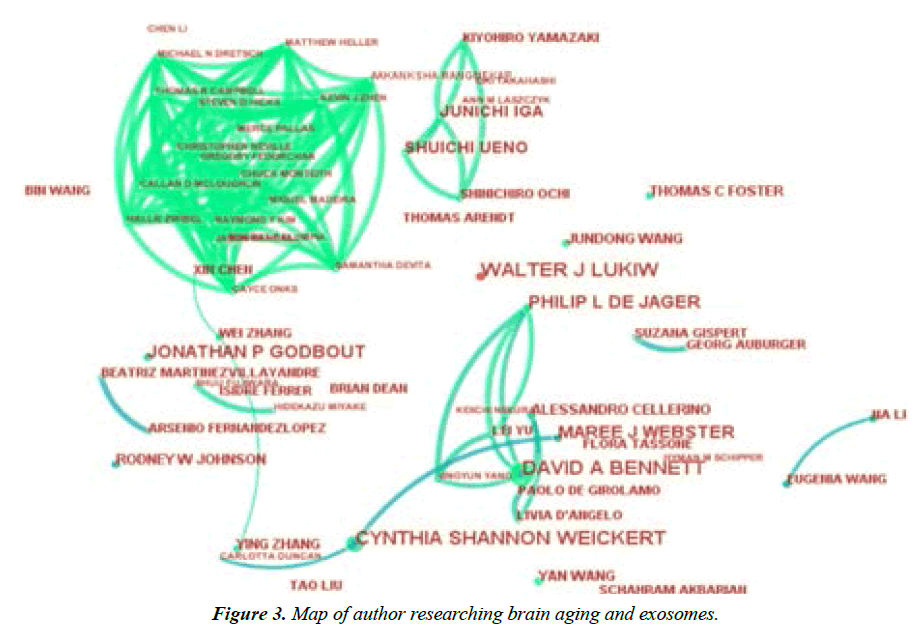
The visualization analysis of author cooperation can be employed to analyze the scientific community and its evolution in a certain field, explore the cooperative relationship between authors and discuss the current situation of investigation on the relationship between brain aging and exosomes at home and abroad [21]. With the adoption of CiteSpace software, Author was selected as the node, with the time zone slice=1 and the threshold top N=50. The network knowledge graph related to authors was plotted. After debugging and beautification, the visualization graph of author cooperation can be obtained, as shown in It can be seen that there is a distinct author population in the research field of brain aging and exosomes at home and abroad. Each node in the figure represents an author. The larger the node circle, the more publication volume the author has. The line between both nodes represents that there is cooperation between authors. The thicker the line, the closer the collaboration between the authors. According to the author cooperation graph generated by CiteSpace, the publication volume of authors was imported into Excel for statistical ranking, and a total of 1110 authors were included.
Table 2 lists the top 10 authors with the largest publication volume in this field. It can be seen that among many research populations in this field, the researchers from the United States account for the majority, and the internal research institutions have close cooperation within this country, while the external cooperation is relatively scattered and scarce. The author walter j lukiw has the largest publication volume, mainly published in 2017- 2021. The research focus of this author mainly includes the physiological and biochemical mechanism of neurodegenerative diseases, especially in the physiological and pathological mechanism of Alzheimer's disease. In recent years, microRNA has been mainly explored as a candidate biomarker for diagnosis, prediction, prognosis and treatment of Alzheimer's disease [28,29]. The research framework provides a systematic theoretical basis for researchers. Cynthia shannon weickert has the second largest publication volume. The research focus of this author mainly includes schizophrenia and brain aging, especially the relationship between brain aging and schizophrenia, as well as the molecular mechanism of pro-inflammatory factors and schizophrenia [30,31]. Which provides data support and research ideas for researchers. Figure 3. It can be found from the above findings that, the line between research authors is not dense from the overall perspective, which indicates that the cooperation between authors is less and scattered. Besides, due to the fact that the cooperation between authors is limited by geographical factors, most authors are limited to the cooperation among domestic authors, which is not conducive to the overall development of this field. Although the total publication volume in China ranks second, the publication volume of authors is scattered. It can be recommended that the prolific authors in this research field can continue to expand cooperative research, and scholars can also communicate with prolific authors and strive for more cooperation.
| Ranking | Author | Publications | Institution | Nationality |
|---|---|---|---|---|
| 1 | Walter J Lukiw | 11 | Louisiana State University | USA |
| 2 | Cynthia Shannon Weickert | 11 | New South Wales University | AUS |
| 3 | David A Bennett | 11 | Rush University | USA |
| 4 | Jonathan P Godbout | 7 | Ohio State University | USA |
| 5 | Philip L De Jager | 7 | Columbia University | USA |
| 6 | Maree J Webster | 7 | Stanley Laboratory of Brain Research | USA |
| 7 | Shuichi Ueno | 6 | Ehime University | JPN |
| 8 | Junichi Iga | 6 | Ehime University | JPN |
| 9 | Rodney W Johnson | 5 | Illinois University | USA |
| 10 | Yan Wang | 5 | Tianjin Dementia Institute | CHN |
Table 2. Top 10 active authors in brain aging and exosomes research.
Cooperation Analysis of Publishing Institutions
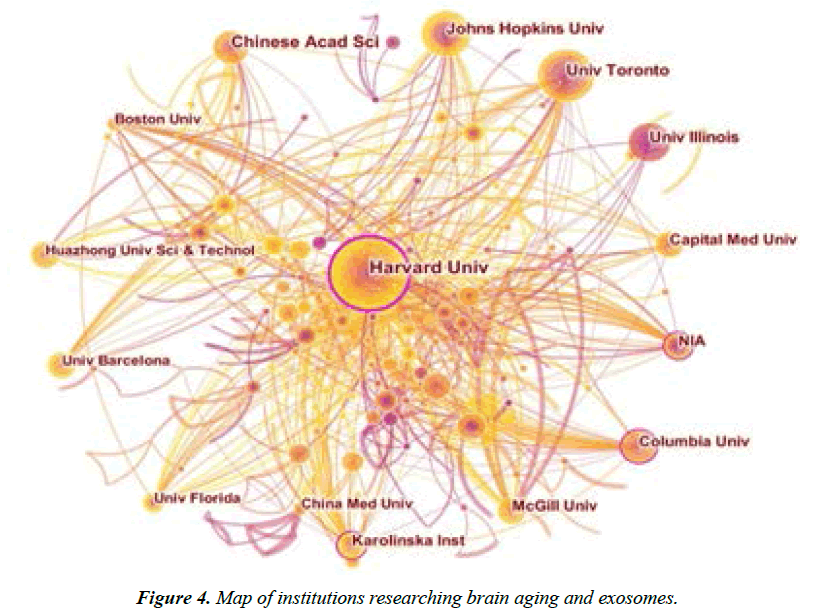
The research on brain aging and exosomes can be conducted from another perspective, namely the analysis of research institutions. Through analyzing institutions and authors, their main research directions can be understood, which contributes to providing a clearer understanding of the main research institutions in this field and their contributions [32]. With the adoption of CiteSpace software, Institution was selected as the node, with the time zone slice=1 and the threshold topN=50. The network knowledge graph related to institutions was plotted. Table 3 lists the top 10 institutions with the largest publication volume in the field of brain aging and exosomes. As can be seen from the table, the research institutions mainly include universities, and Harvard University in the United States ranks first (54 articles), with a centrality of 0.25, far ahead of other institutions. It can be seen that Harvard University has made significant contributions and exerted a significant influence in the research field of brain aging and exosomes. Chinese Academy of Sciences (CAS) ranks second (34 articles), with a centrality of 0.06; Johns Hopkins University (JHU) in the United States ranks third, including 31 articles, with a centrality of 0.09. It can be seen that these institutions are also representative institutions in this research field with a significant influence. Cooperative research can often produce a win-win effect, which can improve the achievements of scientific research and increase the output of scientific research literature. Presents the visualization graph of research institutions in the field of brain aging and exosomes, showing the cooperative network structure of domestic and foreign scholars in this field. Each node in the graph represents a research institution. The more circles in the node, the larger publication volume the institution has. The line between nodes represents the cooperation between the institutions. The thicker the line, the closer the cooperation between the two institutions. The outer circles of nodes of Harvard University, Columbia University, National Institute of Geriatrics of the United States and Karolinska Institute in Sweden are prunosus, which indicates that these institutions have a high centrality and a larger publication volume, and they are the core institutions in this field and have a significant influence in this field. On the whole, the cooperation among various institutions is relatively close, especially that Harvard University has the closest cooperation with other institutions in the United States, such as Boston University, University of Illinois and National Institute of Geriatrics. The cooperation between them fully demonstrates the characteristics of regional advantages; while, there is little cooperation between them and foreign institutions. However, there are few cooperative studies between China and foreign institutions. Chinese Academy of Sciences mainly cooperates with Chinese Academy of Medical Sciences and Peking University Third Hospital, followed by Case Western Reserve University in the United States. Besides, this institute does not cooperate closely with the core institutions in this field.
| Ranking | Institutions | Publications | Country | Centrality |
|---|---|---|---|---|
| 1 | Harvard University | 54 | USA | 0.25 |
| 2 | Chinese Academy of Science | 34 | CHN | 0.06 |
| 3 | Johns Hopkins University | 31 | USA | 0.09 |
| 4 | Toronto University | 28 | CAN | 0.04 |
| 5 | Illinois University | 28 | USA | 0 |
| 6 | Capital Medical University | 26 | CHN | 0.05 |
| 7 | NIA | 26 | USA | 0.11 |
| 8 | Columbia University | 26 | USA | 0.12 |
| 9 | McGill University | 26 | CAN | 0.04 |
| 10 | Karolinska Institute | 23 | SWE | 0.13 |
Table 3. Top 10 prolific institutions researching brain aging and exosomes.
Co-citation Analysis of Literature
The co-citation analysis of literature is another analysis method in literature metrological analysis. The higher the citation frequency of the literature, the higher the academic value of it [33]. The research subjects with a high influence in this field can be revealed by analyzing the research contents of highly cited literature. With the adoption of CiteSpace software, Reference was selected as the node, with the time zone slice=1 and the threshold topN=50. The network knowledge graph related to co-citations was plotted. The larger the node, the more times the literature is cited. The purple outer circle of the node indicates that the literature has a high centrality, and the red dot in the node indicates that the literature has a high emergent intensity. Table 4 lists the statistical results of the top five articles with the highest citation frequency in this field. It can be seen that these five articles were published from 2010 to 2015, which shows that the initial research results provide a direction for the later research and these findings are of great significance.
| Ranking | Author | Cited reference | Cocitation counts | Centrality | Year |
|---|---|---|---|---|---|
| 1 | Somel M | MicroRNA, mRNA, and protein expression link development and aging in human and macaque brain | 20 | 0.15 | 2010 |
| 2 | Lopez-Otin C | The hallmarks of aging | 19 | 0.03 | 2013 |
| 3 | Lau P | Alteration of the microRNA network during the progression of Alzheimer's disease | 14 | 0.13 | 2013 |
| 4 | Colantuoni C | Temporal dynamics and genetic control of transcription in the human prefrontal cortex |
13 | 0.06 | 2011 |
| 5 | You XT | Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity | 12 | 0.01 | 2015 |
Table 4. Top five cocited references related to brain aging and exosomes research.
As shown in MicroRNA Figure 4, MRNA, and Protein Expression Link Development and Aging in Human and Macaque Brain presented by the largest node is published by Some M from Shanghai Institutes for Biological Sciences on Cold Spring Harbor Laboratory Press in 2010, with the highest citation frequency being 20, a centrality being 0.15 and an emergent intensity being 7.28. In this article, 23 cognitively healthy humans and 24 healthy macaques are selected as the research objects. The expression levels of microRNA, mRNA and protein in the prefrontal cortex are detected. It can be found that the expression of microRNA in these two species is highly affected by age. Among the 373 microRNAs from human participants, 115 microRNAs have significant expression changes with the increase of age, and the expression changes of microRNA related to age are similar to those of mRNA, which demonstrates that microRNA plays an important role in regulating the expression of mRNAs during human aging [34]. Although the regulation mechanism of specific microRNAs in the aging process is not explored in this article, it provides data support and experimental ideas for later research.
The Hallmarks of Aging has the second largest node, and it is published by Lopez-Otin C from University of Oviedo on Cell in 2013, with a citation frequency being 19, a centrality being 0.03 and an emergent intensity being 6.58. This article is a review that mainly describes the markers of aging, among which the change in the intercellular communication is one of the markers of aging. MicroRNA can be used as a new carrier for information exchange between cells. MicroRNA has biological characteristics and targeting specificity, and can be used as a molecular diagnostic marker for tumors, neurodegenerative diseases and major mental diseases [35]. Aging is related to increased transcription and abnormal production of many mRNAs. The key transcriptional changes related to aging, such as inflammation, mitochondrial and lysosomal degradation, would affect non-coding RNA, including miRNA related to aging, which could affect the aging process by targeting stem cells [36].
Alteration of the MicroRNA Network during the Progression of Alzheimer's Disease has the third largest node, and it is published by Lau P from Catholic University of Leuven on EMBO Molecular Medicine in 2013, with a citation frequency being 14, a centrality being 0.13 and an emergent intensity being 6.12. The focus of this article is placed on the mechanism of miRNAs expression changes in the brain of AD patients. In that study, the hippocampus of 41 patients with delayed AD and 23 controls are selected, and the prefrontal cortex of 49 patients with AD is also selected for miRNAs analysis. It can be found that miR-132-3p has significant changes in the two brain regions. miR-132-3p mainly occurs in neurons with hyper phosphorylation of Tau protein in the brain of AD patients. It has been demonstrated through evidence that miR-132-3p may induce the pathogenesis of AD through the abnormal regulation of Tau protein mRNA [37]. The study of miRNAs, such as miR-132-3p, deepens our understanding of the molecular mechanism of neurodegenerative diseases, which provides data support and theoretical ideas for further research.
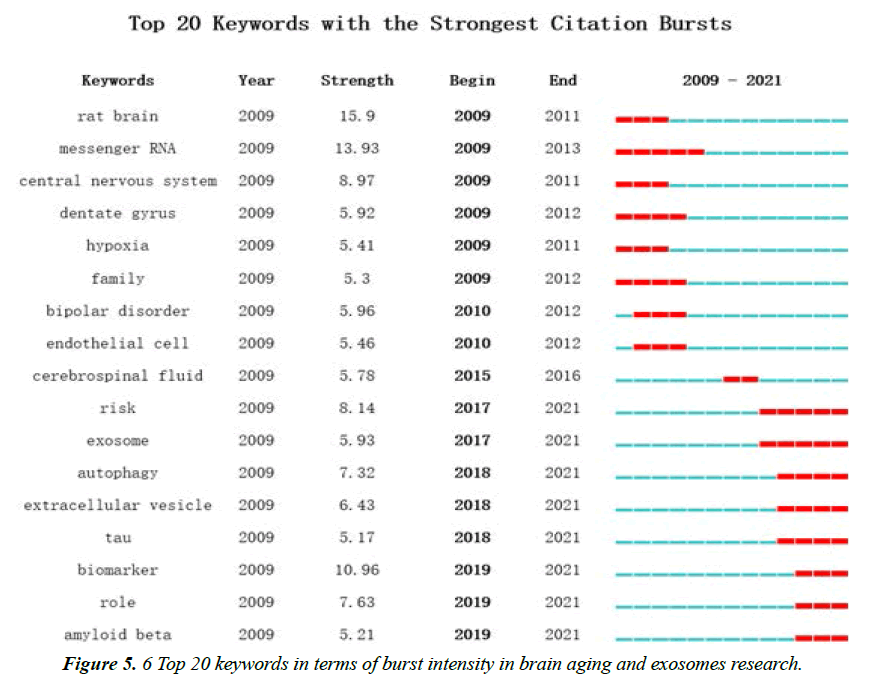
Analysis of Keyword Emergence
Keyword emergence refers to these keywords that suddenly appear in a short time or are cited with a high frequency in a period, and they could reveal the evolution of research topics in different periods [38]. The top 20 keywords were analyzed based on the articles screened out from the core database of Web of Science, with the results listed in Figure 5. The keywords with a larger emergent intensity in this field include rat brain, messenger RNA, biomarker, central nervous system and so forth. Among these articles, the basic research takes the dominant place. Through the study of the central nervous system of rat brains, messenger RNA and exosome are adopted as biomarkers to develop new biomarkers for the diagnosis and treatment of diseases related to the central nervous system, which is expected to overcome the complicated problems about central nervous system diseases in clinical practice.
In a recent study on the microRNA expression profile of whole blood in leukoaraiosis, microRNAs with significant changes in clinical subjects are verified by real-time quantitative polymerase chain reaction (RT-qPCR). It can be found that has-miR-1972 is significantly down-regulated in the early stage of leukoaraiosis, and has-miR-1972 mimic could significantly down-regulate the expression of leukoaraiosis-dependent BAIAP3. Besides, has-miR-1972 inhibitor could significantly up-regulate the expression of endogenous BAIAP3 in vitro. It can be predicted that has-miR-1972 can be adopted as a biomarker to detect the change of leukoaraiosis with time, and BAIAP3 may be a new target for has-miR-1972 in the treatment of leukoaraiosis [39]. According to the research of XiYang YB et al., ScN2B is involved in maintaining the normal physiological functions of prefrontal cortex and hippocampus, and it is related to accelerating the aging and memory decline of prefrontal cortex in aging mice. The downregulation of SCN2B gene by 60.68% could significantly improve the spatial cognitive memory of hippocampus in aged transgenic mice, and increase the synaptic excitability of hippocampus by increasing the number of spinous processes in hippocampal neurons [40]. In a recent study of YA-XIN TAN et al., MiRNA microarray analysis is employed to compare miRNA expression profiles in prefrontal cortex and hippocampus of SAMP8 mice aged 4 months and 12 months. It can be found that there are significant differences in miRRanking 449a. It can be demonstrated through the gene knockout and overexpression experiments that ScN2B is an effective target of miR-449a, and as a biomarker, miR-449a plays an important role in neuron growth and related aging process by targeting ScN2B [41].
Table 5 lists the classification of key keywords based on different topics, including research objects, research symptoms and research contents. Among them, the research objects mainly include the aging models of rats and mice. Basic research takes the dominant place; while, applicationoriented research is relatively less. The research symptoms cover almost all neurodegenerative diseases. There are many research methods in this field, such as basic animal experiments, randomized controlled trials, meta-analysis, clinical trials, questionnaires and so forth. The research contents mainly include molecular biological indexes, neurobiological indexes and other indexes. Among them, the molecular biological indexes mainly include the expression changes of proteins and exosomes related to brain aging; the neurobiological indexes mainly include the structural changes of the brain and brain regions related to aging; other indexes mainly include the cognitive manifestations of neurodegenerative diseases related to brain aging.
| Subject | Keyword |
|---|---|
| Research object | Mice, rat, transgenic mice, aged mice, aged rat?adult?pig?adult rat |
| Research disease | Alzheimers disease, parkinsons disease, schizophrenia, dementia, stroke, bipolar disorder, autism, huntingtons disease |
| Research contents | |
| Molecular biological index | Messenger RNA, microRNA, miRNA, exosome, circulating microRNA, circular RNA, lncRNA, neurotrophic factor, BDNF, Aβ, amyloid precursor protein, NF-Kb, dopamine, Tau, nerve growth factor, alpha-synuclein, necrosis factor alpha |
| Neurobiologyical index | Brain, hippocampus, neurogenesis, neuroinflammation, DNA methylation, long term potentiation, hypothalamus, dentate gyrus, cerebral ischemia, brain injury, hippocampal neuron, frontal cortex, blood-brain barrier |
| Other index | Aging/senescence ,inflammation, neuron?central nervous system, microglia, cerebrospinal fluid, neuroprotection, cognitive impairment, cognitive decline, neural stem cell |
Table 5. Keywords category list.
Results
In recent years, there is a year-on-year increase in the studies on the influence of brain aging on exosomes at home and abroad; Although significant contributions in this field have been made in the United States, China and Germany, the cooperation between countries is relatively less; Core authors have gathered in groups, and most of the cooperation between authors is limited to their own countries; These studies in this field are mainly conducted by integrating neuroscience, biochemistry and molecular biology, cell biology, clinical neurology, geriatrics and other disciplines; The research objects mainly include aging rats and mice. Additionally, the influence of domestic studies is relatively weak compared with other countries.
Conclusion
Cite Space software analysis provides a novel perspective for the influence of brain aging on exosomes. There is still a certain gap between domestic studies and foreign ones. In the future, it is required to integrate resources among different institutions, and strengthen cooperation and exchange, which would promote the development of this field.
Summary
The exploration on brain aging and exosomes has been gradually developed after 12 years of effort since its starting in 2009. The United States is the mainstay of research in this field, with a high publication rate and centrality. China also plays a certain role in this field, but with a relatively weak influence. Therefore, it is necessary to conduct more transnational and cross-regional cooperative research. Although the prolific authors have made significant contributions to the research and development in this field, the majority of studies in this field are conducted independently. In addition, there is little cooperation between different teams and institutions, and there is an extensive lack of cross-regional cooperation, which limits the exchange and development of research in this field to a certain extent.
The exploration on brain aging and exosomes has been integrated with 70 disciplines, among which neuroscience, biochemistry and molecular biology, cell biology, clinical neurology, geriatrics, and psychiatry are the main disciplines related to this field. In recent five years, the popular disciplines integrated into this research field mainly include hearing and language pathology, rehabilitation, virology, mathematics and computational biology, social sciences and so forth. The research object for the influence of brain aging on the expression of exosomes has developed from animal models to human bodies, and the research symptoms cover most neurodegenerative diseases. With the advancement of the research in this field, there is a significant enrichment in the research contents, mainly including molecular biological indexes, neurobiological indexes and other indexes. In this field, the molecular biological indexes, neurobiological indexes and other indexes are explored and analyzed by integrating multidisciplinary research methods. Besides, different technologies are adopted to perform scientific analysis on the influence of brain aging and exosome expression from different aspects. Moreover, the Cite space research method provides technical support for the research in this field, which conduces to understanding the research hotspots and research trends in this field. However, there are some limitations in this research method. For example, the articles are only selected from Web of science, and the retrieval is not performed in PubMed, CNKI or other databases. In addition, only articles in English are included, and the articles in other languages are not retrieved. Further, Citespace analysis is biased towards quantitative analysis, whose defects would be eliminated by adopting other research methods in subsequent research, which would make the research in this field more comprehensive and objective.
In a nutshell, the research through this method provides a new perspective for the influence of brain aging on exosomes, and also provides valuable information for scholars in this field, such as the potential collaborators, cooperative institutions, hot topics and research trends. In the subsequent research, it is necessary to integrate the resources among different institutions to strengthen cooperation and exchange, which would promote the research development of this field.
Acknowledgements
The authors would like to thank all the subjects who par- Ticipated in the present study. Particularly, we would also like to thank Associate Professor Wang and Doctor Li who assisted us throughout the study. This work received partial financial help from University of Chengdu University.
References
- Mattson MP, Arumugam TV. Hallmarks of brainaging: adaptive and pathological modification by metabolicstates. Cell Metab. 2018;27(6):1176-99.
- Yuan X. Negative global population growth could be brought forward to 2065. Population and health, 2020, No. 279(11):15-18.
- Cheng XJ, Hu GQ. Research progress on health effects of population aging. Chinese Journal of Epidemiology. 2021;1915-20.
- Yankner BA, Lu T, Loerch P. The aging brain. Annu Rev Pathol. 2008;3:41-66.
- Driscoll I, Hamilton DA, Petropoulos H, et al. The aging hippocampus: cognitive, biochemical and structural findings. Cereb Cortex. 13,1344-151.
- Chételat G, Landeau B, Salmon E, et al. Relationships between brain metabolism decrease in normal aging and changes in structural and functional connectivity. Neuroimage.2013;1:167-77.
- Mohammed CPD, Park JS, Nam HG. MicroRNAs in brain aging. Mechanisms of ageing and development. 2017;168:3-9.
- Coppedè F, Migliore. DNA repair in premature aging disorders and neurodegeneration. Curr Aging Sci. 2012;3(1):3-19.
- Morawe T, Hiebel C, Kern A, et al. Protein homeostasis, aging and Alzheimer’s disease. Mol. Neurobiol.2012; 46:41-54.
- Kourtis N, Tavernarakis N. Cellular stress response pathways and ageing: intricate molecular relationships. EMBO J. 2011;30(13):2520-31.
- Eacker SM, Dawson TM, Dawson VL. Understanding microRNAs in neurodegeneration. Nat Rev Neurosci. 2009;10(12):837-41.
- Abe M, Bonini NM. MicroRNAs and neurodegeneration: role and impact. Trends Cell Biol. 2013;23(1):30-6.
- Suzanne M, Steller H. Shaping organisms withapoptosis. Cell Death Differ. 2013;20(5):669-75.
- Akers JC, Ramakrishnan V, Kim R, et al. miRNA contents of cerebrospinal fluid extracellular vesicles in glioblastoma patients. J Neurooncol. 2015;123(2):205-16.
- Zhao D, Sui Y, Zheng X. MiR-331-3p inhibits proliferation and promotes apoptosis by targeting HER2 through the PI3K/Akt and ERK1/2 pathways in colorectal cancer. Oncol Rep. 2016;35(2):1075-82.
- Eacker SM, Keuss MJ, Berezikov E, et al. Neuronal activity regulates hippocampal miRNA expression. PLoS One 6. 2011;6(10):e25068.
- Liu N, Landreh M, Cao K, et al. The microRNA miR-34 modulates ageing and neurodegeneration in Drosophila. Nature. 2012;482(7386):519-23.
- Yang J, Liu XX, Fan H, et al. Extracellular vesicles derived from bone marrow mesenchymal stem cells protect against experimental colitis via attenuating colon inflammation, oxidative stress and apoptosis. PLoS One. 2015;10(10):e140551.
- Cheng Y, Cheng CM, Hu ZG, et al. The methodological function of CiteSpace Knowledge graph. Scientific research. 2015;23(2):242-53.
- Chen C. Science mapping: A systematic review of the literature. Data Inf Sci. 2017;2:1-40.
- Zhang JH, Hu ZG. Review and prospect of CiteSpace software application research. J Mod Inf Ser. 2013;33:99-103.
- Su HY. An Analysis of the research hotspots of Library and Information Science in China in recent ten years Based on the method of scientific knowledge Graph. Books intelligence, 2019;000(007):1-3.
- Zhang XM, Zhang X, Luo X, et al. Knowledge mapping visualization analysis of the military health and medicine papers published in the web of science over the past 10 years. Mil Med Res. 2017;4:23.
- Liang YD, Li Y, Zhao J, et al. Study of acupuncture for low back pain in recent 20 years: a bibliometric analysis via Cite Space. J Pain Res. 2017;10:951-64.
- Cosentino M, Colombo C, Mauri M, et al. Expression of apoptosis-related proteins and of mRNA for dopaminergic receptors in peripheral blood mononuclear cells from patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 2019;23(1);88-90.
- Wang DH, WU FF, Wang SM, et al. Progress in human brain science research programs. Medical Innovation in China, 2019;16(007):168-72.
- Neurology TL.The Human Brain Project?Mutiny on the flagship. Lancet Neurol.2014;13(1):855.
- Zhao Y, Jaber V, Alexandrov PN, et al. (2020). microRNA-based biomarkers in Alzheimer’s disease (AD). Front. Neurosci. 1028.
- Jaber VR, Zhao Y, Sharfman NM, et al. Addressing Alzheimer's Disease (AD) Neuropathology Using Anti-microRNA (AM) Strategies. Mol Neurobiol. 2019;56(3):8101-08.
- Van Rheenen TE, Cropley V, Fagerlund B, et al. Cognitive reserve attenuates age-related cognitive decline in the context of putatively accelerated brain ageing in schizophrenia-spectrum disorders. J Psychol Med. 2020;50(9):1475-89.
- Kindler J, Lim CK, Weickert CS, et al. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol Psychiatry. 2019: 25(11):2860-72.
- Zhai HS. Knowledge graph analysis of table tennis technique and tactics analysis in China [D]. Shanxi university.
- Ding XD. Bibliometrics fundamentals. Med Libr Assoc. 2015;103(4): 217-18.
- Somel M, Guo S, Fu N, et al. MicroRNA, mRNA, and protein expression link development and aging in human and macaque brain. Genome Res. 2010;20(9):1207.
- Kosaka N, Iguchi H, Ochiya T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer science. 2010;101(10):2087-92.
- C López-Otín, Blasco MA, Partridge L, et al. The Hallmarks of Aging. J Cell. 2013;153(6):1194-217.
- Lau P, Bossers K, Janky RS, et al. Alteration of the micro RNA network during the progression of Alzheimer's disease. EMBO Mol Med.2013;5(10):1613-34.
- Chen B, Shin S. Bibliometric analysis on research trend of accidental falls in older adults by using Citespace Focused on Web of Science Core Collection (2010–2020). Int J Environ Res.2021;18(4):1663.
- Huang WQ, Lin Q, Chen S, et al. Integrated analysis of microRNA and mRNA expression profiling identifies BAIAP3 as a novel target of dysregulated hsa-miR-1972 in age-related white matter lesions. J Aging. 2021;13(3):4674-95.
- Xi YB, Wang Yc, Zhao Y, et al. Sodium channel voltage-gated beta 2 plays a vital role in brain aging associated with synaptic plasticity and expression of cOX5A and FGF-2. Mol Neurobiol. 2016;53(2):955-67.
- Tan YX, Hong Y, Jiang S, et al. MicroRNA?449a regulates the progression of brain aging by targeting SCN2B in SAMP8 mice. Int J Mol Med. 45(4):1091-102.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref