Research Article - Current Pediatric Research (2022) Volume 26, Issue 5
Vigabatrin is associated with lower risk of infantile spasms relapse compared to ACTH in tuberous sclerosis patients.
Naim Zeka1,2, Ramush Bejiqi1,3, Abdurrahim Gerguri1, Ragip Retkoceri1, Armend Vuciterna1, Arlinda Maloku1,2, Leonore Zogaj1, Eris Zeka2 and Haki Jashari1,4*
1Department of Neurology, University Clinical Center of Kosovo, Pediatric Clinic, Pristina, Kosovo
2Departments of Medicine, University of Pristina, Pristina, Kosovo
3Departments of Medicine, University of Gjakova, Pristina, Kosovo
4Department of Pediatric, University Children’s Hospital, Pediatric Clinic, Skopje, Republic of Macedonia
- Corresponding Author:
- Haki Jashari, Department of Neurology, University Clinical Center of Kosovo Pediatric Clinic, Pristina, Kosovo, E-mail: hjashari.md@gmail.com
Received: 18 April, 2022, Manuscript No. AAJCP-22-57647; Editor assigned: 21 April, 2022, PreQC No. AAJCP-22-57647(PQ); Reviewed: 05 May, 2022, QC No. AAJCP-22-57647; Revised:12 May, 2022, Manuscript No. AAJCP-22-57647(R); Published: 26 May, 2022, DOI:10.35841/0971-9032.26.5. 1351-1356.
Abstract
Background and Aim: Vigabatrin (VGB) and Adrenocorticotropic Hormone (ACTH) are the two first line of treatment of Infantile Spasm (IS) in Tuberous Sclerosis Complex (TSC) patients. The aim of this study was to assess the efficacy and safety of these two drugs in TSC patients and to evaluate the EEG changes during treatment. Methods: We prospectively studied 22 consecutive infants with TSC and infantile spasm. Based on the initial therapy we divided the patients into two groups: Group-I: TSC who received VGB (n=15) and Group-II: TSC who received ACTH (n=7). The children who showed a partial or no response on treatment after 8 weeks, they were switched on another drug (VGB to ACTH and vice versa). The primary outcome was relapse of IS and secondary outcomes were peripheral visual field constriction, drowsiness, hypotonia, irritability, ear infection, hypertension, weight gain, irritability and stomach irritation. Results: Twenty-two TSC patients with IS were included. The median age of patients was 3 months (1-8 months) and 8 of them (36.8%) were females. At 12 months follow-up on initial therapy the frequency of complete responders (40% vs. 28.6%; p=0.02) and partial responders (33.3% vs. 14.3%; p=0.01) were higher and non-responders less prevalent (27.7% vs. 57.1%; p<0.001) in VGB arm compared to ACTH arm. Similarly, after switched on another drug, the non-responders to treatment were more prevalent in group switched on ACTH compared to group switched on VGB (50% vs. 25%; p=0.001). During follow up at 3, 6, 9 and 12 months the frequency of hypsarrhythmia was lower and multifocal activity was not significant compared to first recorded EEG in those two groups of treatment. In addition, at 12 months follow-up the hypsarrhythmia and multifocal activity were more prevalent in the group switched to VGB compared to the group switched on ACTH. Conclusions: Although refractory epilepsy is common, many patients achieve seizure control. This study suggests that the risk of infantile spasms relapse in TSC may be more reduced by vigabatrin treatment compared to ACTH.
Keywords
Tuberous sclerosis complex, Infantile spasm, Vigabatrin, Adrenocorticotropic hormone.
Introduction
Tuberous Sclerosis Complex (TSC) is a genetic disorder caused by a mutation in either the TSC1 or TSC2 gene leading to formation of hamartomas in multiple organ systems [1]. Classically, TSC was identified by the triad of facial angiofibromas, intellectual disability, and epilepsy. Discovering the responsible genes which encode hamartin and tuberin, improved the diagnosis of TSC, particularly in patients who do not have seizures or intellectual disabilities [2]. Despite such evidence, seizure are the most common presenting features of
TSC affecting 73% to 90% of patients, commonly including Infantile Spasm (IS) [3,4].
IS presents in a distinctive fashion with clusters of brief seizures called epileptic spasms and a spectrum of severe electroencephalographic abnormalities that include hypsarrhythmia [5]. First line of treatment for IS including natural and synthetic Adrenocorticotropic Hormone (ACTH) such as prednisolone and Vigabatrin (VGB) [6]. Despite the established efficacy of these drugs, especially during short-term duration, the cumulative long-term risk of IS relapse is still
high [7]. In another hand, although the VGB among children with TSC was shown to be very effective [8-10], the adverse event of retinal toxicity remains a clinically relevant challenge [11]. The aim of this study was to assess the efficacity and safety of these two drugs in TSC patients and to evaluate the EEG changes during treatment.
Methods
Study population
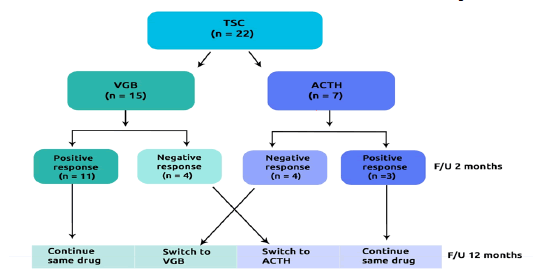
We prospectively studied 22 consecutive infants with TSC and infantile spasm [12]. Patients were referred to the pediatric clinic, university clinical centre of Kosovo between 2015 and 2020. Based on the initial therapy we divided the patients into two groups: Group-I: TSC who received VGB (n=15) and Group-II: TSC who received ACTH (n=7) (Figure 1).
The starting dose of VGB varied from 50 mg/kg/day to 150 mg/kg/day in two daily doses. For ACTH arm the starting dose was 40 UI/day, however, in the case where the initial response was considered inappropriate the dose was increased up to 80 UI/day.
The children who showed a partial or no response on treatment after 8 weeks, they were switched on another drug (VGB to ACTH and vice versa). Reason for exclusion was not recorded.
This study was approved by the institutional review boards. All patients’ guardians provided written informed consent prior to any study procedures.
Data collection
Demographic and clinical indices were collected from medical records in all children, in whom routine biochemical testes were assessed in Table 1. EEG, MRI and ophthalmologic examination were also performed.
| Variable | Patients |
|---|---|
| (n=22) | |
| Demographic data | |
| Age (months) | 3 (1-8) |
| Sex (female, n, %) | 8 (36.3) |
| Concomitant abnormalities | |
| Hypochromic macules (n, %) | 17 (77.2) |
| Facial angiofibroma (n, %) | 16 (72.7) |
| Gingival fibromas (n, %) | 9 (40.9) |
| Gingival angiomyolipoma (n, %) | 14 (63.6) |
| Rhabdomyoma cordis (n, %) | 14 (63.6) |
| Pulmonary LAM (n, %) | 1 (4.5) |
| Cortical tubes (n, %) | 18 (81.8) |
| Hepatic hamartoma (n, %) | 6 (27.3) |
| Retinal hamartoma (n, %) | 9 (40.9) |
| Subependymal nodules (n, %) | 2 (9.1) |
| Subependymal giant cell astrocytoma (n, %) | 1 (4.4) |
Table 1. Demographic and clinical data among TSC patients. TSC: Tuberculosis Sclerosis Complex; LAM: Lymphangioleiomyomatosis.
EEG examination
All the patients underwent an initial EEG. The serial outpatient video-EEGs (1 hour duration, awake and sleep) were performed at each visit and interpreted in a blinded fashion by a team of electroencephalographers. The EEG was performed using a minimum of 19 electrodes according to the 10–20 system and a reduced array with nine electrodes was allowed in infants under 3 months of corrected by age (Fp1, Fp2, C3, C4, T4, T3, O1, O2, Cz).
The EEG was repeated every 3 months in each patient. The first EEG was done before the start of therapy while the other EEG was done after 3, 6 and 9 and 12 months after starting therapy.
Adverse clinical events
Adverse Clinical Events (ACEs) were collected and information on participants’ clinical outcome was obtained from electronic medical records, clinical visits and personal communication with general physicians.
The primary outcome was relapse of IS and secondary outcomes were peripheral visual field constriction, drowsiness, hypotonia, irritability, ear infection, hypertension, infection, weight gain, irritability and stomach irritation.
Definition of response and relapse of IS
The response to treatment was defined as the cessation of IS, according to clinical and EEG criteria (not demonstrating either infantile spasms or hypsarrhythmia for at least one month after treatment).
In partial responders infantile spasms were considerably less frequently, but not stopped completely. The IS relapse was defined as the presence of either epileptic spasm (according seizure diary or hypsarrhythmia recorded on EEG) at least 1 month after initial response.
Statistical analysis
Discrete data are reported as frequencies (percentages) and continuous variables are shown as means and Standard Deviation (SD) if normally distributed, or median and interquartile range (IQR: Q1–Q3) in case of skewed distribution. Continuous data were compared with two?tailed Student t test and discrete data with Chi?square test. A significant difference was defined as p-value <0.05 (2-tailed). Statistical analysis was performed with SPSS Software Package version 26.0 (IBM Corp., Armonk, NY, USA).
Results
Demographic and clinical indices for TSC patients
Twenty-two TSC patients with IS were included. The median age of patients was 3 months (1-8 months) and 8 of them (36.8%) were of female gender. The most concomitant abnormalities were hypochromic macules (77.2%), cortical tubes (81.8%) facial angiofibroma (72.7%), gingival angiomyolipoma (63.6%) and rhabdomyoma cordis (63.6%), while gingival fibromas, retinal hamartoma, hepatic hamartoma, subependymal nodules, pulmonary LAM and subependymal giant cell astrocytoma were less prevalent (40.9, 40.9, 27.3, 9.1, 4.5 and 4.4% respectively).
Treatments response among VGB versus ACTH drugs
At 12 months follow-up on initial therapy the frequency of complete responders (40% vs. 28.6%; p=0.02) and partial responders (33.3% vs. 14.3%; p=0.01) were higher and nonresponders less prevalent (27.7% vs. 57.1%; p<0.001) in VGB arm compared to ACTH arm. Similarly, after switched on another drug, the non-responders to treatment were more prevalent in group switched on ACTH compared to group switched on VGB (50% vs. 25%; p=0.001) (Table 2).
| Treatment response | VGB(n=15) | ACTH (n=7) | P value |
|---|---|---|---|
| Treatment response on initial therapy | |||
| Complete responders (n, %) | 6 (40) | 2 (28.6) | 0.02 |
| Partial responders (n, %) | 5 (33.3) | 1 (14.3) | 0.01 |
| Non-responders (n, %) | 4 (26.7) | 4 (57.1) | <0.001 |
| Treatment response after switching therapy | ACTH to VGB (n = 4) | VGB to ACTH (n = 4) | P value |
| Complete responders (n, %) | 2 (50) | 1 (25) | 0.001 |
| Partial responders (n, %) | 1 (25) | 1 (25) | 0.88 |
| Non-responders (n, %) | 1 (25) | 2 (50) | 0.001 |
Table 2. Comparison of treatment response among VGB versus ACTH drugs.
EEG changes among VGB versus ACTH drugs
During follow up at 3, 6, 9 and 12 months the parameters of EEG changed significantly compared to first recorded EEG in those two groups of treatments.
The VGB therapy was associated with reduced the frequency of hypsarrhythmia during follow-up compared to first recorded EEG (66.7, 46.7, 36.3, 27.3, 18.2%; p<0.05, respectively) while the effect on multifocal activity was not significant (20, 20, 18.2, 18.2, 27.3 respectively). Similarly, the pattern of hypsarrhythmia significantly was decreased (71.4, 42.8, 0, 0, 0%; p<0.05, respectively) on ACTH group but the therapy had no effect on the multifocal activity (14.3, 28.6, 33.3, 33.3, 33.3%; p<0.05, respectively). In contrast, at 12 months followup the hypsarrhythmia and multifocal activity were more prevalent in the group switched to VGB compared to the group switched to ACTH (p<0.05 for both) (Table 3).
| Variable | VGB (n=15) | ACTH (n=7) | P value | VGB to ACTH (n=4) | ACTH to VGB (n=4) | P value |
|---|---|---|---|---|---|---|
| Initial EEG | ||||||
| Normal (n, %) | 2 (13.3) | 1 (14.3) | 0.45 | - | - | - |
| Hypsarrhythmia (n, %) | 10 (66.7) | 5 (71.4) | 0.11 | - | - | - |
| Multifocal (n, %) | 3 (20) | 1 (14.3) | 0.04 | - | - | - |
| EEG after 3 months | ||||||
| Normal (n, %) | 5 (33.3) | 2 (28.6) | 0.34 | - | - | - |
| Hypsarrhythmia (n, %) | 7 (46.7) | 3 (42.8) | 0.27 | - | - | - |
| Multifocal (n, %) | 3 (20) | 2 (28.6) | 0.03 | - | - | - |
| EEG after 6 months | ||||||
| Normal (n, %) | 5 (45.5) | 2 (66.7) | 0.001 | 0 (0) | 0 (0) | 0.91 |
| Hypsarrhythmia (n, %) | 4 (36.3) | 0 (0) | <0.001 | 3 (75) | 2 (50) | 0.01 |
| Multifocal (n, %) | 2 (18.2) | 1 (33.3) | 0.01 | 1 (25) | 2 (50) | 0.01 |
| EEG after 9 months | ||||||
| Normal (n, %) | 6 (54.5) | 2 (66.7) | 0.03 | 1 (25) | 0 (0) | 0.01 |
| Hypsarrhythmia (n, %) | 3 (27.3) | 0 (0) | <0.001 | 2 (50) | 2 (50) | 0.67 |
| Multifocal (n, %) | 2 (18.2) | 1 (33.3) | 0.04 | 1 (25) | 2 (50) | 0.01 |
| EEG after 12 months | ||||||
| Normal (n, %) | 6 (54.5) | 2 (66.7) | 0.03 | 2 (50) | 0 (0) | <0.001 |
| Hypsarrhythmia (n, %) | 2 (18.2) | 0 (0) | 0.001 | 1 (25) | 2 (50) | 0.01 |
| Multifocal (n, %) | 3 (27.3) | 1 (33.3) | 0.12 | 1 (25) | 2 (50) | 0.01 |
Table 3. Comparison of EEG parameters of VGB versus ACTH treatment. ACTH: Adrenocorticotropic hormone; VGB: Vigabatrin; EEG: Electroencephalography.
Adverse Clinical Events (ACEs)
At follow-up on VGB group the most ACEs was peripheral visual field constriction (33.3%), irritability (33.3%), drowsiness (20%), hypotonia (13.4%) and ear infection (13.4%). Likewise, on ACTH group the most ACEs were: irritability (42.8%), hypertension (14.3%), weight gain (28.5%), general infection (28.5%) and stomach irritation (14.3%), (Table 4).
| Variable | VGB (n=15) | ACTH (n=7) | P value |
|---|---|---|---|
| Peripheral visual field constriction (n, %) | 5 (33.3) | 0 (0) | <0.001 |
| Drowsiness (n, %) | 3 (20) | 0 (0) | <0.001 |
| Hypotonia (n, %) | 2 (13.4) | 0 (0) | <0.001 |
| Irritability (n, %) | 3 (33.3) | 3 (42.8) | 0.01 |
| Ear infection | 2 (13.4) | 0 (0) | <0.001 |
| Hypertension | 0 (0) | 1 (14.3) | <0.001 |
| Infection (n, %) | 0 (0) | 2 (28.5) | <0.001 |
| Weight gain (n, %) | 0 (0) | 2 (28.5) | <0.001 |
| Stomach irritation (n, %) | 0 (0) | 1 (14.3) | <0.001 |
Table 4. Adverse clinical events of VGB versus ACTH treatment. ACTH: Adrenocorticotropic hormone; VGB: Vigabatrin.
Discussion
Dr West described infantile spasms 160 years ago in a letter he wrote to Lancet [13]. Since then, a lot of drugs have been investigated for the treatment of infantile spasms, in adults and also in children. However, there is no definitive universal standard treatment up to date.
Our study tried to help understanding the treatment response of infantile spasms in patients with tuberous sclerosis. At 12 month follow-up period, complete and partial responders were more frequent in the VGB arm, either when VGB was used a first line treatment or when switched to VGB from ACTH. Different adverse clinical events were noticed in both groups, except for irritability and infections that were found in both groups (Table 4).
A lot of previous studies compared ACTH to VGB in the treatment of infantile spasms. According to guideline of the American academy of neurology and the child neurology society, both ACTH and VGB may be beneficial for short-term treatment of infantile spasms, with ACTH considered better than VG [14]. Also, a Cochrane review of randomized controlled trials in the treatment of infantile spasms found that treatment with hormones, ACTH or corticosteroids resolves spasms more and earlier than vigabatrin, but it was not yet clear regarding long-term outcomes [13].
However, these studies did not prescribe patients with TS in particular. In this regard, a 1996 study by Aicardi et al. showed that vigabatrin seems to be more effective in patients with TS, where 96% of patients showed complete clinical response after introduction of Vigabatrin [15].
Similar results were also found in some other small prospective studies by Chiron et al. and Vigevano et al. [16,17]. In addition, a retrospective study by Jones et al., found a higher response rate to Vigabatrin in patients with normal development prior to the onset of IS [18].
In contrast to the clinical response, EEG abnormalities were more frequent in VGB arm compared to ACTH arm. At 12 month follow-up evaluation, EEG was normal in 54.5% of patients of the VGB group compared to 66.7% of the ACTH group.
Moreover, no patient showed EEG abnormalities when switched from VGB to ACTH, while 50% of patients still showed EEG abnormalities when switched from ACTH to VGB. Similar findings were also described in other studies [19].
Conclusions
Vigabatrin is associated with lower risk of infantile spasms relapse compared to ACTH in tuberous sclerosis patients. Hence, in accordance with previous studies, our results show that VGB can be considered as a first line therapy, offering a more effective therapy for the management of infantile spasms in patients with tuberous sclerosis. Moreover, VGB is indicated in the treatment of spasms when resistance or intolerance to other anti-epileptic drugs is encountered. Larger clinical trials are needed to further understand the long-term effectivity and the safety of VGB.
References
- Wataya-Kaneda M, Uemura M, Fujita K, et al. Tuberous sclerosis complex: Recent advances in manifestations and therapy. Int J Urol. 2017; 24(9): 681-91.
[Crossref] [Google Scholar] [Indexed]
- Chu-Shore CJ, Major P, Camposano S, et al. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia 2010; 51(7): 1236-41.
- Vignoli A, La Briola F, Turner K, et al. Epilepsy in TSC: Certain etiology does not mean certain prognosis. Epilepsia 2013; 54(12): 2134-42.
- Joinson C, O'Callaghan FJ, Osborne JP, et al. Learning disability and epilepsy in an epidemiological sample of individuals with tuberous sclerosis complex. Psychol Med 2003; 33(2): 335-44.
- Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the international league against epilepsy: Position paper of the ILAE commission for classification and terminology. Epilepsia 2017; 58(4): 522-30.
- Hussain SA, Schmid E, Peters JM, et al. High vigabatrin dosage is associated with lower risk of infantile spasms relapse among children with tuberous sclerosis complex. Epilepsy Res 2018; 148: 1-7.
- Knupp KG, Coryell J, Nickels KC, et al. Response to treatment in a prospective national infantile spasms cohort. Ann Neurol. 2016; 79(3): 475-84.
- Lux AL, Edwards SW, Hancock E, et al. The United Kingdom infantile spasms study comparing vigabatrin with prednisolone or tetracosactide at 14 days: A multicentre, randomised controlled trial. 2004; 364(9447): 1773-8.
- O'Callaghan FJ, Edwards SW, Alber FD, et al. Safety and effectiveness of hormonal treatment versus hormonal treatment with vigabatrin for infantile spasms (ICISS): A randomised, multicentre, open-label trial. Lancet Neurol 2017; 16(1): 33-42.
- O'Callaghan FJ, Lux AL, Darke K, et al. The effect of lead time to treatment and of age of onset on developmental outcome at 4 years in infantile spasms: Evidence from the United Kingdom infantile spasms study. Epilepsia 2011; 52(7): 1359-64.
- Vanhatalo S, Nousiainen I, Eriksson K, et al. Visual field constriction in 91 Finnish children treated with vigabatrin. Epilepsia 2002; 43(7): 748-56.
- Northrup H, Aronow ME, Bebin EM, et al. Updated international tuberous sclerosis complex diagnostic criteria and surveillance and management recommendations. Pediatr Neurol 2021; 123: 50-66.
- Hancock EC, Osborne JP, Edwards SW. Treatment of infantile spasms. Cochrane Database Syst Rev 2013; 6: Cd001770.
- Go CY, Mackay MT, Weiss SK, et al. Evidence-based guideline update: medical treatment of infantile spasms. Report of the guideline development subcommittee of the American academy of neurology and the practice committee of the child neurology society. Neurology 2012; 78(24): 1974-80.
- Aicardi J, Mumford JP, Dumas C, et al. Vigabatrin as initial therapy for infantile spasms: A European retrospective survey. Sabril IS Investigator and Peer Review Groups. Epilepsia 1996; 37(7): 638-42.
- Chiron C, Dumas C, Jambaqué I, et al. Randomized trial comparing vigabatrin and hydrocortisone in infantile spasms due to tuberous sclerosis. Epilepsy Res 1997; 26(2): 389-95.
- Vigevano F, Cilio MR. Vigabatrin versus ACTH as first-line treatment for infantile spasms: A randomized, prospective study. Epilepsia 1997; 38(12): 1270-4.
- Jones K, Go C, Boyd J, et al. Vigabatrin as first-line treatment for infantile spasms not related to tuberous sclerosis complex. Pediatr Neurol 2015; 53(2): 141-5.