Case Report - Journal of Cardiovascular Medicine and Therapeutics (2018) Volume 2, Issue 3
Trans-catheter closure of RPA to LA fistula by duct occluder device
- *Corresponding Author:
- Mahua Roy
Departments of Pediatrics and Surgery North Bengal Medical College and Hospital Darjeeling, West Bengal 700074, India
Tel: 919830769550
E-mail: drmahuaroy@gmail.com
Accepted date: Aug 17, 2018
Citation: Roy M, Chattopadhyay A, Bandhyopadhyay B, et al. Trans-catheter closture of RPA to LA fistula by duct occluder device. J Cardiovasc Med Ther. 2018;2(2):6-8.
Abstract
Right pulmonary artery (RPA) to left atrium (LA) fistula is a rare variant of pulmonary arteriovenous fistula (PAVF) which can cause central cyanosis. We are reporting a case with central cyanosis with structurally normal heart on 2-dimensional (2D) echo which subsequently was diagnosed as a case of RPA to LA fistula. He underwent successful trans- catheter closure of the fistula by duct occlude device.
Keywords
Central cyanosis, Pulmonary artery-LA fistula, Duct occluder device.
Introduction
RPA to LA fistula is a rare variant of pulmonary arteriovenous fistula (PAVF) which can present with central cyanosis, dyspnea and if left untreated can be complicated with hyperviscosity syndrome. Investigative modalities include Transthoracic echocardiography (TTE)/Transesophageal echocardiography (TEE) with contrast echocardiography, Multi Slice Computerized Tomographic (MSCT) angiography or by cardiac catheterization. It can be treated surgically by ligation of fistulous tract, but now a day’s trans-catheter closure has become an alternative option.
Case Report
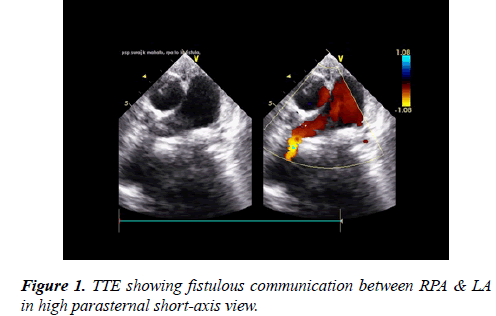
A 7-year-old boy weighing 18 kg presented to outpatient department with central cyanosis, grade III clubbing and shortness of breath (NYHA class III). On examination he had oxygen saturation of 65% in room air without any significant cardiovascular findings. Except high hematocrit (60%) level routine blood investigations were within normal limit including coagulation profile. Chest-X ray showed homogenous opacity in right hilar region.12 lead ECG showed features of LV volume overload. 2D echo showed dilated left atrium (LA) and ventricle (LV), a fistulous communication from RPA was suspected (Figures 1 and 2).
A contrast echocardiography with agitated normal saline was done to rule out any pulmonary arterio-venous fistula. Contrast echocardiography study revealed appearance of microbubbles in LA within 3 cardiac cycles. So, a provisional diagnosis of pulmonary AV malformation (PAVM) was made.
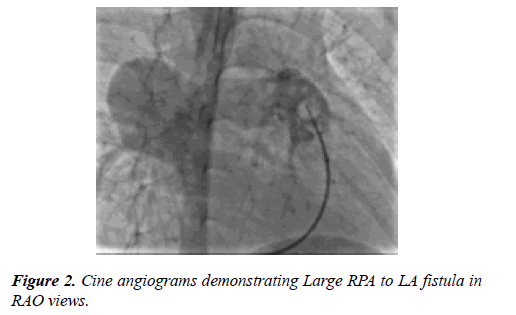
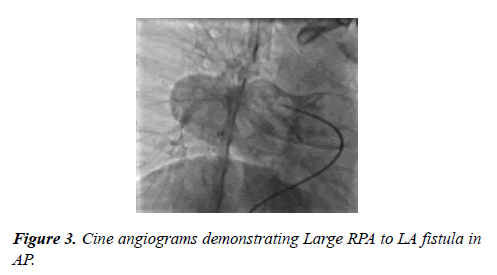
A Multi Slice CT angiogram revealed a large tortuous fistulous communication between RPA and LA. Trans-catheter device closure was planned. An informed consent was obtained from the parents. Patient was electively intubated and ventilated. Two venous accesses by 6(F) short sheaths (Terumo Medical Corporation, Somerset, New Jersey) were taken in both the groins. One arterial access (RFA, 4F) was taken for continuous hemodynamic monitoring. The child was fully heparinized (100 IU/kg). Pulmonary artery (PA) angiogram was done by the Berman catheter (Arrow International Inc, Pennsylvania). Fistulous communication between RPA and LA was best profiled in RAO view (Figure 3). The narrowest segment in angiogram measured 16 mm. We could cross to LA via PFO with a 5 F Multipurpose catheter (Cook Inc, Bloomington, IN).
The fistula was crossed from LA side with an angulated 0.032” × 260 cm glide wire (Terumo Medical corp, Somerset, NJ). The wire was kept in RPA. A veno-venous loop was made by snaring the wire from RPA by a gooseneck snare (ev3 Endovascular Inc., Plymouth, Minnesota, USA).
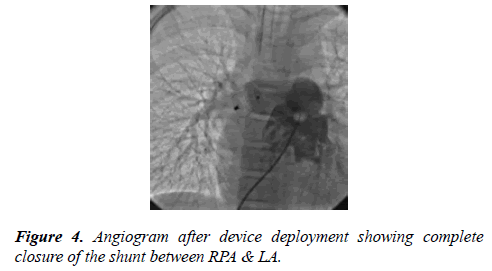
A 18/20 mm duct occlude device (Heart R, Life tech Scientific, Shenzhen) was selected to close the fistulous tract. We didn’t do balloon sizing for selection of device. We had selected the device based upon angiographic and CT images. A 9 (Fr) Lifetech ASD delivery sheath was advanced over the glide wire from the left femoral vein across the PFO and it was parked in RPA. The sheath was completely de-aired. The aortic retention disc of the device was deployed under fluoroscopy in the RPA and the waist of the device into the fistulous tract. Post procedure selective RPA angiogram showed complete closure of the fistula without any pulmonary arterial or venous obstructions (Figure 4).
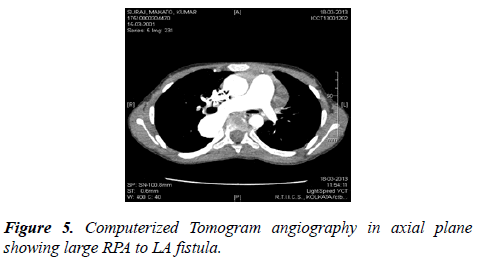
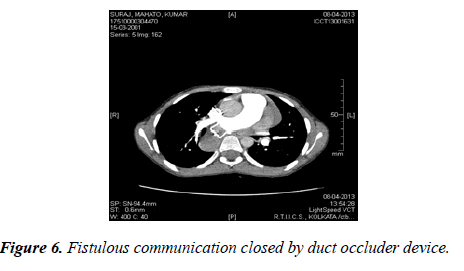
After device deployment the systemic saturation immediately jumped to 98%. The total fluoroscopic time was 38 min and total contrast used was 3 ml/Kg. The patient was extubated on table and discharged after 48 hours of observation on aspirin 5 mg/kg/day for 6 months (Figures 5 and 6).
On follow up after 3 months CT angiogram was done which showed device was perfectly in place without any obstruction or erosion of the nearby structures.
Discussion
Pulmonary artery (PA) to LA fistula is a direct communication between these two structures. The 1st description of such anomaly was given by Friedlich and co-authors in 1950 [1]. Some authors consider this anomaly as a variant of a PAVF however; unlike classical PAVF, PA to LA fistulas do not involve direct communication between an artery and a vein. PA to LA fistula is an uncommon variant of PAVF, but it shares the same clinical features like central cyanosis, normal cardiovascular examination, and a shadow parallel to the left heart border on chest x-ray.
Except few reported cases of post traumatic RPA to LA fistula, most of the cases are congenital in origin [1-3]. In about 95% cases, this communication identifies between RPA and LA [4]. Only few cases of left pulmonary artery (LPA) to LA fistula have been reported in the literature so far [3,5,6].
Although definite embryological explanation is not available till now, hypothesis is that this anomaly result from a communication between the RPA and a primitive pulmonary vein and later the pulmonary vein would become incorporated into the wall of the LA, resulting in a fistulous connection between the RPA and LA [7,8].
Regarding associated cardiac malformation, atrial septal defects (ASD) are the most common. Other concomitant cardiac malformations are rare, but anomalies of the right lung, such as absence of the lower or middle lobe, right lung sequestration, and diverticulum of the right main bronchus have been reported [5]. Chauhan et al., reported a transcatheter closure of large RPA to LA fistula in a child after surgical closure of a large OS-ASD. That patient’s desaturation was obvious only after surgical closure of ASD [9].
If left untreated it can be complicated with systemic embolism or cerebral abscess. First surgical repair of such fistula was done by Blalock; after that nearly 70 cases of RPA to LA fistula have been described in literature [4-6,8,10].
Previously surgical repair was the only available treatment for such lesion, however now-a day’s trans-catheter closure of such fistulous tract has become a suitable alternative option. Presence of a small PFO in our patient facilitated the retrograde approach. In fact, the technique of trans-septal retrograde approach to close the RPA to LA fistula using duct occluder devices has already been reported in literature. Like our case Abe T et al., also utilized retrograde approach to close the fistulous tract after doing septal puncture [7]. We used the large duct occluder device in a small child without any complications.
Another alternative route for RPA to LA fistula closure is antegrade approach through the right ventricle to the main pulmonary artery to fistulous tract. In such situation the aortic disc of the device would be in LA side and PA disc would be in RPA side.
As like our case Karunakar Vadlamudi et al., had used PDA device to close fistulous tract [11]. On the other hand, Chauhan el al reported transcatheter closure by 12 mm muscular device. Other suitable option through this approach would be a vascular plug.
Conclusion
Transcatheter closure of RPA to LA fistula has almost replaced the surgical closure. So, we conclude that even the larger defects can be closed safely by trans-septal approach using a duct occluder device.
References
- Friedlich A, Bing RJ, Blount SG Jr. Physiological studies in congenital heart disease. IX Circulatory dynamics in the anomalies of venous return to the heart including pulmonary arteriovenous fistula. Bull Johns Hopkins Hosp. 1950;86:20-57.
- Zeebregts CJ, Nijveld A, Lam J, et al. Surgical treatment of a fistula between the right pulmonary artery and the left atrium: Presentation of two cases and review of literature. Eur J Cardio thorac Surg. 1997;11:1056-61.
- Saatvedt K, Stake G, Lindberg H. Fistula between the right pulmonary artery and the left atrium-an unusual cause of cyanotic heart disease. Cardiol Young. 1995;5:85-7.
- Mohanty SR, Yadav R, Kothari SS, et al. Right pulmonary artery left atrium communication. Ann ThoracSurg. 2000;69:279-71.
- Lucas RV Jr, Lund GW, Edwards JE. Direct communication of a pulmonary artery with the left atrium. An unusual variant of pulmonary artery with the left atrium. An unusual variant of pulmonary arteriovenous fistula. Circulation. 1961;24:1409-14.
- Tuncali T, Aytac Aj. Direct communication between the right pulmonary artery and the left atrium: A case report and review of the literature. Pediatrics. 1967;71:384-89.
- Abe T, Kuribayashi R, Sato M, et al. Direct communication of the right pulmonary artery with left atrium: A case report and review of the literature. J Thorac Cardiovasc Surg. 1972;64:38-44.
- Ohara H, Ito K, Kohguchi N, et al. Direct communication between the right pulmonary artery and the left atrium. J Thorac Cardiovasc Surg. 1979;77:742-47.
- Chauhan A, Agarwal S, Gupta U, et al. Pulmonary artery-to-left atrial fistula discovered after the closure of atrial septal defect: A rare clinical scenario. Ann Pediatr card. 2018;11:211-13.
- Sheikhzadeh A, Hakim H, Ghabusi P, et al. Right pulmonary artery-to-left atrial communication: Recognition and surgical correction. Am Heart J. 1984;107:396?98.
- Vadlamudi K, Verma S, Christopher J, et al. Transcatheter closure of large right pulmonary artery-to-left atrial fistula. Annals of pediatric cardiology. 2013;6(2):188.