Research Article - Current Pediatric Research (2017) Volume 21, Issue 1
The role of pentoxifylline on neuroprotection in neonatal rat model of hypoxic ischemic brain injury.
Hülya Halis1*, Narin Liman2, Osman Baştuğ1, Tamer Güneş11Department of Pediatrics, Division of Neonatology, Faculty of Medicine, Erciyes University, Turkey.
2Department of Histology and Embryology, Faculty of Veterinary Medicine, Erciyes University, Turkey.
- *Corresponding Author:
- Hulya Halis
Department of Pediatrics, Division of Neonatology
Faculty of Medicine, Erciyes University
Talas Street, 38039, Kayseri, Turkey.
Tel : 00-90-3522076666
Fax : 00-90-3524375825
E-mail: drhhalis@yahoo.com
Accepted date: January 05, 2017
Abstract
Despite important progress in obstetric and neonatal care, Hypoxic-Ischemic Encephalopathy (HIE) is one of the common causes of neonatal brain damage. The aim of the present study was to investigate the possible neuroprotective effects of two different dosages of pentoxifylline (60 and 100 mg/kg) (PTX) on a model of perinatal HIE. Seven-day-old Wistar rat pups were subjected to right common carotid artery ligation and hypoxia (92% nitrogen and 8% oxygen) for 2h. They were treated with PTX or saline both immediately after hypoxia-ischemia (HI) and after 2h. In sham group animals, neither ligation, nor hypoxia was performed. Neuronal apoptosis was evaluated by the caspase-3 immunostaining methods. The numbers of caspase-3 immunoreactive cells in the parietal cortex and dentate gyrus were observed to have significantly increased in the HI, PTX60 and PTX100 groups compared to the sham group, and, no differences were detected between the HI, PTX60 and PTX100 groups. The numbers of caspase-3 immunoreactive cells in the cornu ammonis regions of the hippocampus were significantly higher in the HI, PTX60 and PTX100 groups than in the sham group. But, elevated in the PTX60 group was significantly lower than that determined in the HI group. However, the number of necrotic cells in all regions of brain were significantly lower PTX60 than HI and PTX100 groups. These results show that PTX administration after hypoxia-ischemia reduces neuronal apoptosis and necrosis. Therefore, PTX may be an effective drug to use to decrease the severity of neonatal hypoxic-ischemic brain injury.
Keywords
Hypoxia-ischemia; Pentoxifylline; Apoptosis; Necrosis.
Introduction
Despite important progress in obstetric and neonatal care hypoxic-ischemic encephalopathy (HIE) during gestation and perinatal period are common causes of neonatal brain damage which are frequently associated with neurodevelopmental disorders, including cerebral palsy, epilepsy, memory deficits, learning disabilities, and other cognitive impairments [1-3]. Incidence is 1 to 2 per 1000 live births, but is 2 to 3 times more common in developing countries [4]. Although, hypothermia is the only standard neuroprotective treatment used in infants with HIE, it has a number of limitations [5]. Therefore, new pharmacologic and therapeutic approaches continues to be investigated.
Today, it is better known that inflammation plays an important role in brain injury induced by neonatal HIE [6- 9]. Cytokines are important inflammatory mediators, and also plays role both in maintain brain tissue homeostasis and in the response of the brain to diverse forms of injury [7,8]. Recent evidences shown that tumor necrosis factor alpha (TNF-α) and interleukin-1 beta (IL-1β) protein and gene expression increase within hours after a cerebral hypoxic-ischemic insult in rats [8-10]. Furthermore, recent data indicate that TNF-α can potentiates glutamate neurotoxicity by inhibiting glutamate uptake in induce neuronal cell death [11]. In addition to this, Zhao et al. have shown that TNF-α may play an important role as an effector of receptor-mediated apoptotic cell death in the central nervous system and TNF-α induced caspase-3 during apoptotic cell death in hippocampal culture [12]. Systemic administration of either a pharmacologic antagonist of the IL-1 and TNF-α receptor attenuates apoptosis to cause hypoxic-ischemic injury in seven day old rats [13,14]. The results of morphological, biochemical molecular studies denote that both apoptotic and necrotic mechanisms contribute to neurological death after hypoxic-ischemia in different neonatal animal models [15-18]. The evidence so far demonstrates that apoptosis involves the activation of caspases, which are aspartate-specific cysteine proteases and members of the interleukin-1β-converting enzyme family, that serve as the primary mediators of apoptosis [19]. In various ischemia models, caspase-3 is a potent effector of apoptosis triggered via several different pathways in neuronal cells in the brain and caspase inhibitors are neuroprotective in adult ischemia models [16,20-23].
PTX, structurally similar to the methylxanthines is a synthetic theobromine derivative and non-steroidal immunomodulating agent with unique hemorrheologic effects. It has been used in a variety of infectious and inflammatory conditions such as sepsis, necrotizing enterocolitis and chronic lung disease in newborns (24). PTX downregulates pro-inflammatory cytokines such as TNF-α, IL-1β, interleukin -6 (IL-6), platelet activating factor (PAF) and Interferon gamma (IFNγ) [24-26]. Experimental studies have shown that PTX treatment improves brain edema, neurological and neurochemical deficits in rats subjected to transient focal cerebral ischemia and stroke [27,28]. Previous studies reported that the pretreatment with PTX decreased the incidence and severity of hypoxic-ischemic injury in immature rat brain, by attenuating the expression of IL-1β and TNF-α gene [29]. Furthermore, several studies reported that the anti-inflammatory features of PTX include the inhibition of TNF-α production and the TNF-α inhibition and anti-inflammatory effect of PTX may be responsible for the neuroprotection provided by the drug [27,30].
Given the evidences which suggest TNF-α induces caspase-3 during neuronal apoptotic cell death and TNF-α production inhibited by PTX, we hypothesize that the neuroprotective effect of PTX afforded by the inhibition of caspase-3-dependent apoptosis [12,27]. The objective of the present study was to investigate the possible neuroprotective effects of two different dosages of PTX (60 and 100 mg/kg) on a model of perinatal hypoxic-ischemic brain damage, by evaluating the neuronal apoptosis. To this end, we evaluated the regional and temporal profiles of caspase-3 immunoreactivity in the parietal cortex and hippocampus of neonatal rats after unilateral hypoxicischemic brain injury.
Materials and Methods
Experimental animals
The experiments were carried out on seven-day-old Wistar rat pups of male sex in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) regarding the protection of animals used for experimental purposes, and with the guiding principles for the care and use of laboratory animals approved by Erciyes University. The rat pups were randomly divided into four groups as follows: Sham (n=5) group: The animals were anesthetized, right carotid released but no ligation or hypoxia was done.
Hypoxic-ischemia (HI) (n=6) group: Saline (0.5 ml) was intraperitoneally injected both immediately after ligation and hypoxia exposure and after 2h.
PTX60 (n=6) group: 60 mg/kg pentoxifylline was intraperitoneally administered immediately after ligation and hypoxia exposure and after 2h.
PTX100 (n=6) group: 100 mg/kg pentoxifylline was administered immediately after ligation and hypoxia exposure and after 2h.
Pentoxifylline was dissolved in saline.
Surgical procedure
Hypoxic-ischemia was performed according to the Levine-Rice model ( on postnatal day 7 (P7) since the brain of this developmental stage is similar to the brain of a 32-34-week-old human fetus histologically [31,32]. Rat pups were anaesthetized by izoflurane inhalation and duration of surgery was less than 5 minutes. At supine position and under the microscopic magnification, the right common carotid artery ligated with a 6-0 silk suture through a median cervical incision. After the ligation of carotid artery the animals were given to the dam for 1 h to recover and feeding period. After recovery, except of the sham group, the animals were placed in a plastic chamber and exposed to hypoxia duration of 2 h (8% O2- 92% N2). The air temperature of chamber was maintained at 32.5 ± 0.5°C.
Immunohistochemistry
For the determination of caspase-3 immunoreactivity, 24 hours after cerebral hypoxia-ischemia rats at postnatal day 8 (P8) were anesthetized intraperitoneally with urethane (1.5 g/kg) and transcardic perfusion was performed with 0.9% saline, followed by Bouine’s solution (75% saturated picric acid, 25% formaldehyde, 5% glacial acidic acid). After perfusion the brains were removed and post–fixed in the same fixative for 6 hours, dehydrated, cleared, and embedded in Paraplast. Six-micrometer-thick adjacent serial coronal brain sections were cut at 50-100 μm intervals and mounted on glass slides coated with 3-Aminopropyl-ethoxy-silane (APES) (Sigma-Aldrich Chemicals, St. Louis, MO, USA) and dried at 37°C. Four slides were prepared from each sample, and each slide contained a minimum of six sections.
Immunohistochemistry was performed using a streptavidin–biotin–peroxidase detection system (Thermo Fisher Scientific Lab Vision Corporation, Fremont, CA, USA). Briefly, sections were deparaffinized in xylene and rehydrated through a graded series of ethanol, and then rinsed several times with phosphate-buffered saline (PBS; pH 7.4). Endogenous peroxidase activity was blocked by incubating the slides in 3% H2O2 in methanol for 15 min at room temperature and washed with PBS two times. After, antigen retrieval was performed by boiling in 0.01 M of citrate buffer (pH 6.0) for 30 min at 80°C using a water bath and by cooling for 20 min prior to immunostaining, for the interaction between the antigen and antibody [33]. Sections were then washed in PBS and incubated in a blocking serum (Ultra V Block, Thermo Fisher Scientific Lab Vision Corporation, Fremont, CA, USA; TA-125UB) for 5 min at room temperature to block non-specific binding. The blocking solution was removed by tapping the slides, which were then incubated with primary antibody against Caspase-3 (CPP32 Ab-4, 1:100 dilution, Cat No: RB-1197, Thermo Fisher Scientific Lab Vision Corporation, Fremont, CA, USA) overnight at 40 C. This was followed by incubation with biotinylated goat anti-rabbit IgG for 15 min and thereafter with the avidin-biotin-peroxidase complex for 15 min. Between each step, sections were washed four times in PBS. The reaction was visualized using 3, 3’-diaminobenzidine tetra hydrochloride (DAB; Thermo Fisher Scientific Lab Vision Corporation, Fremont, CA, USA). After counterstaining with Gill’s hematoxylin, the slides were dehydrated through an alcohol series, cleared with xylene, mounted with coverslips using a permanent mounting medium (entellan).
The caspase-3 antibody was obtained from Thermo Fisher Scientific Lab Vision Corporation (Fremont, CA, USA), which guarantees that the antibodies are specific for rat tissues. The specificity of immunohistochemical procedures was also checked by using negative and positive control sections. As positive controls, sections of colon carcinoma were incubated with primary antibody. Negative control staining was performed by omitting the primary antibody and by using normal rabbit IgG (Santa cruz sc-2027) instead of caspase-3 antibody. Non-spesific staining was not detected in tissue samples from different experiment groups. Examination was conducted using conventional light microscope (BX51; Olympus, Tokyo, Japan)
Analysis of the distribution of caspase-3 immunoreactive cells and necrotic neurons
Immunohistochemical staining was performed in duplicate to verify results. Caspase-3-positive neurons and necrotic neurons in coronal sections from-2.28 mm until-4.52 mm were counted in areas of defined size: parietal cortex (PC), 10×0.014 mm2; cornu ammonis (CA) regions CA1, 10×0.014 mm2; CA2, 3×0.014 mm2; CA3, 5×0.014 mm2; CA4, 3×0.014 mm2; dentate gyrus (DG), 10×0.014 mm2. An average number of cells were obtained from 24 coronal sections of a single animal; no variation of statistical significance could be found between individuals of the same experimental groups. Quantifications of caspase-3 expressing cells and necrotic cells were done by counting of positive cells in 2-10 randomly chosen depending on the hippocampal area at x1000 magnifications by two independent researchers (H.H, N.L.), and the mean score of the two observers was calculated.
Statistical analysis
The data were analyzed using the statistical package program SPSS (Statistical Package for the Social Sciences) for Windows Release 11.5.1 (SPSS Inc., 1898- 2002). All data of this study were calculated as mean ± standard deviation (SD) and P value of less than 0.05 was considered significant. Microsoft Office Standard Edition for Students and Teachers (Microsoft ® Office Excel 2003-2007) modules were used for Graphic drawings. In right brain regions, within-group differences in caspase-3 positive, necrotic cells and healthy normal neuron were tested with one-way ANOVAs. In each group, the comparison of the numbers of caspase-3 positive, necrotic cells and healthy normal neuron between the right and left brain regions were analyzed with dependent t-test (called the paired-samples t-test).
Results
The mean weight of the offspring of rats was 11.8 ± 1.2 in the study. Five rats in the all group died during the surgery procedure. One of the dead rats was in sham group, two rats were in HI group, and two rats were in PTX60 group.
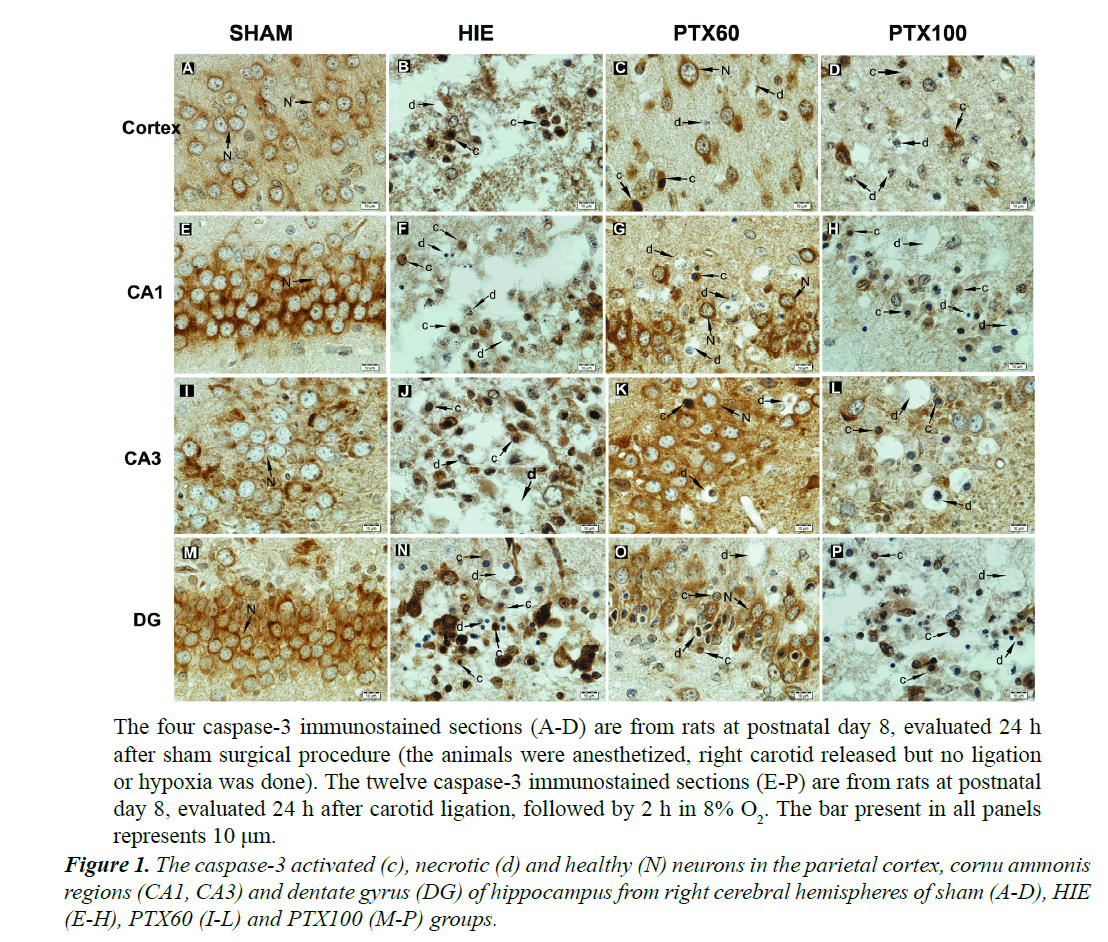
In sham group, caspase-3 immunreactivity was observed in the cytoplasm and nuclear membrane of normal neurons, which had a large round nuclei and prominent nucleoli. However, in treatment groups, the active caspase-3 immunoreactivity was found in neurons with chromatin condensation, nuclear fragmentation and cytoplasmic condensation. Neither necrotic nor apoptotic neurons were observed in sham group. Furthermore, in treatment groups, many large vacuoles were found in cortex, stratum pyramidale of CA regions and granular cell layer of dentate gyrus. These large vacuoles were the residual somata of degenerated or necrotic neurons. The small pieces of chromatin clumps were also observed in some vacuoles. The glial cells were located close to larger vacuoles (Figure 1).
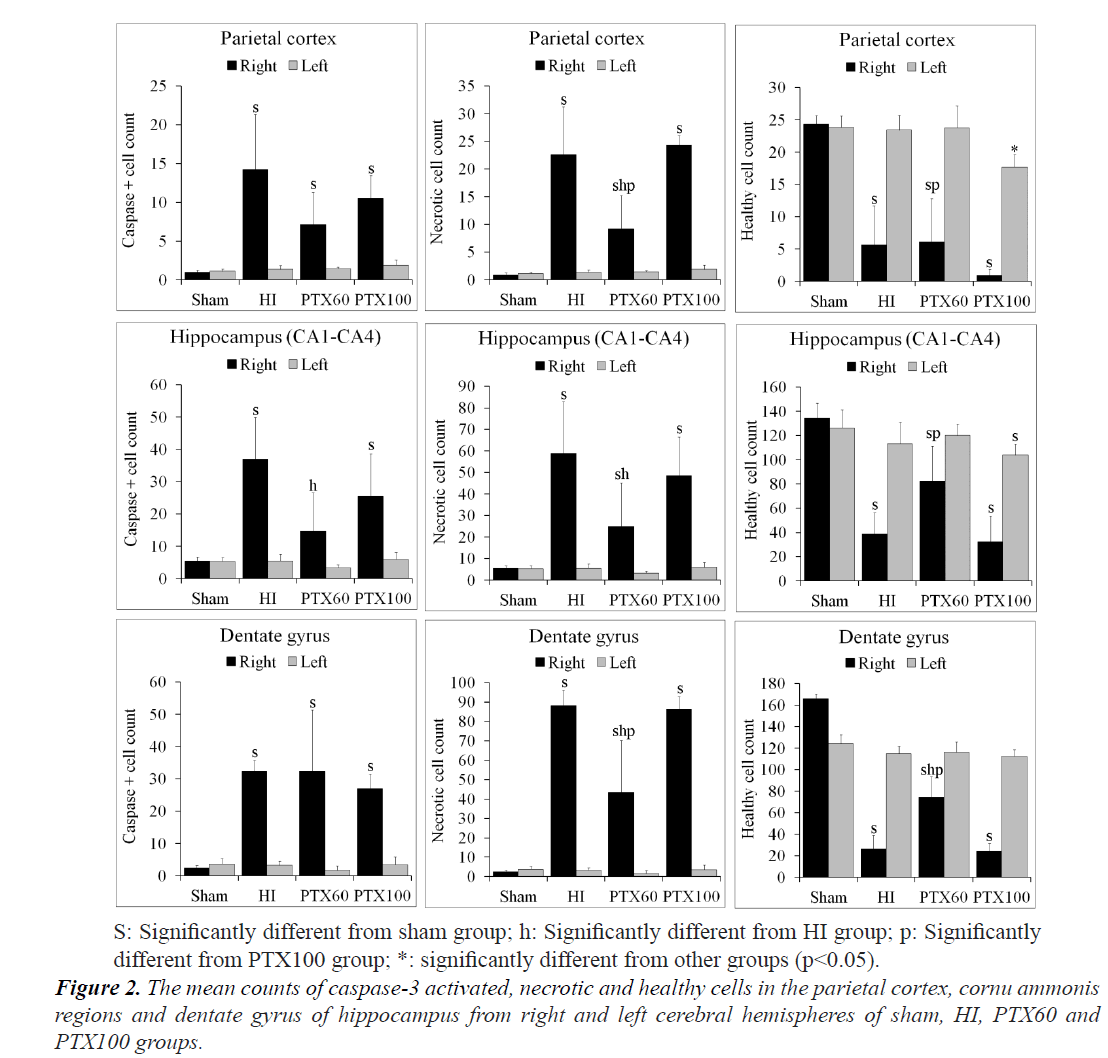
In many brain regions, such as the cerebral cortex, CA regions and dentate gyrus, the numbers of caspase-3 positive apoptotic cells, necrotic cells and healthy cells of each group are shown in Figure 2. Although no difference between the number of healthy normal neuron in the right and left brain region of sham group, their numbers in the HI, PTX60 and PTX100 groups were significantly lower in the right brain regions than in the left regions (P<0.05). However, when the caspase-3 positive and necrotic cell counts were compared between the right and left brain regions, there were increases in the right brain regions of HI, PTX60 and PTX100 groups, which were also found to be statistically significant (P<0.05) (Figure 2). In addition, the number of healthy normal neuron in the left PC of the PTX100 were significantly lower than in the sham, HI and PTX60 groups (P<0.05). As shown in Figure 2, whereas the numbers of healthy normal neuron in the all regions of right brain of PTX60 group were higher than in the PTX100 group (P<0.05), the numbers of healthy normal neuron in the only DG region were significantly higher than in the HI groups (P<0.05).
Evaluation of caspase-3 positive and necrotic cells in right brain regions revealed significantly higher cell count values in the HI, PTX60 and PTX100 groups compared to the sham group (P<0.001). Generally, as shown in Figure 2, the maximum caspase-3 positive and necrotic cells count occurred in the HI group.
The numbers of caspase-3 immunoreactive cells in the PC and DG were observed to have significantly increased in the HI, PTX60 and PTX100 groups compared to the sham group (P<0.05). However, no differences were detected between the HI, PTX60 and PTX100 groups (P>0.05). The numbers of caspase-3 immunoreactive cells in the CA regions of the hippocampus were significantly higher in the HI, PTX60 and PTX100 groups than in the sham group (P<0.05). But, elevated in the PTX60 group was significantly lower than that determined in the HI group (P<0.05) (Figure 2).
When considering the number of necrotic cells, as shown in Figure 2, the maximum necrotic cell count occurred in the HIE group, which was greater than that observed in the control, the PTX60 and PTX100 groups. However, it was also detected that the numbers of necrotic cells in the parietal cortex and dentate gyrus were significantly higher in the HIE and PTX100 groups than in the PTX60 group (P<0.05), and also their numbers in the CA regions of the hippocampus were significantly lower in the PTX60 group than in the HIE group (P<0.05).
Discussion
Perinatal cerebral hypoxia-ischemia is a major cause of neonatal encephalopathy and cerebral palsy. Inflammatory mediators including cytokines (e.g., TNF-α and IL-1β) are implicated in the pathogenesis of hypoxic-ischemic injury in immature brain [7-10]. A large number of diverse interventions such as systemic administration of either a pharmacologic antagonist of the IL-1 receptor or a platelet-activating factor receptor antagonist applied after hypoxia-ischemia has successfully reduced brain damage in neonatal rat hypoxic-ischemic encephalopathy (HIE models) [13,34]. To decrease the severity of neonatal hypoxic-ischemic brain injury, the use of phosphodiesterase inhibitor is one of the effective strategies. PTX is a nonspecific phosphodiesterase inhibitor and a methylxanthine derivative with a variety of anti-inflammatory effects [35]. The mechanism of PTX in reducing inflammation is thought to be due to inhibition of neutrophil activation, down-regulation of pro-inflammatory cytokines such as TNF-α, IL-6 and interferon-γ (IFN-γ), and prevention of endothelial-leukocyte adhesion in postcapillary venules [36-39]. Recent studies reported that PTX has a neuroprotective effect in experimental models of global as well as focal cerebral ischemia and PTX treatment initiated either before or immediately after cerebral hypoxiaischemia attenuated brain damage in immature rats [40,41]. There are several investigations on the effective neuroprotective dose of PTX in the brain ischaemia models [39]. Some of studies showed that PTX had protective effect on the brain trauma on the doses of 30-60 mg kg-1, but others informed that PTX at the doses of 10-20 mg kg-1 had no protective effect on brain trauma [42-45]. In a study conducted by Eun et al. 25, 40, 75 and 150 mg/kg/ doses of PTX were used before and after hypoxia-ischemia and the neuroprotective effect of PTX was evaluated necrotic area measurement. The findings of the study have shown that PTX pretreatment with 40 mg/kg/dose reduced the incidence of liquefactive cerebral infarction, from 75% in controls to 10% with pentoxifylline [40]. Kalay et al. [41] the administration of 60 mg/kg of PTX immediately after hypoxia may reduce brain damage due to hypoxicischemic injury. Bruno et al. have reported that the PTX (50 mg/kg, i.p) treatment of ischemic animals reduced brain injury relatively to controls (10% cell loss) in the CA3 region, but not in the cortex and DG regions. Furthermore they have stated an increase in the number of viable cells in the hippocampus CA1 region, as related to the ischemic animals [29]. However, Honess et al. have determined that at higher doses (200 mg/kg), PTX can induce mild hypothermia (about 2°C core temperature reductions) in mice [46]. Furthermore, LeMay et al. have determined that a high dose of PTX (200 mg/kg) caused hypothermia in control rats and blocked LPS fever, while a low dose (50 mg/kg) did not have these effects. These authors have also determined that injection of the high dose of PTX in control rats caused a rise in plasma IL-6 but not in plasma TNF; however, the peak levels of plasma IL-6 and TNF activities following an injection of LPS are significantly reduced by pretreatment with PTX [47].
Based upon the results of previous studies, in the present study, to determine the effective dose of PTX in neonatal rat model of HIE, two different PTX dosage regimens (60 and 100 mg/kg) were used. PTX has a short elimination half-life (1 hour or less) [24]. High plasma levels of PTX and both major derivatives occur within several minutes after intraperitoneal or subcutaneous injection, but plasma levels are low after oral administration [48]. Therefore, to improve the effectiveness of PTX, we administered the PTX intraperitoneally twice at 2 hours intervals immediately after ligation and hypoxia exposure.
The pathogenesis of perinatal cerebral hypoxic-ischemic injury has been extensively investigated by using rodent models combine unilateral carotid artery ligation with exposure to a period of hypoxia [49]. Several studies have reported that the decrease of cerebral blood flow during the initial phase of HIE causes to rapid depletion of Adenosine-Tri-Phosphate (ATP) leading to failure of Na/K-ATPase pump and this induces depolarization of the cell membrane leading to activation of voltage-gated calcium (Ca+2) channels and an influx of intracellular Ca+2. Increases in the intracellular Ca+2 concentration may produce cellular injury via that activation of Ca+2 – dependent proteases, lipases and endonucleases and by the production of cytokines and other factors can lead to neuronal cell injury and death [50]. Neuronal cell death after hypoxic-ischemic injury takes the form of necrosis or apoptosis. Although necrosis predominates in more severe cases, whereas accumulating data suggests that apoptosis plays an important role in the evolution of hypoxicischemic injury in the neonatal brain [15,23,51-55]. Neonatal HI triggers multiple pathways of apoptosis [56]. Mitochondrial release of cytochrome c and associated stimulation of caspase-3, which this cytochrome c mediated pathway is also referred to as the intrinsic pathway, has been identified as a key mediator of apoptosis in ischemic animal models [15,16,57,58]. Furthermore, Hu et al. have reported that activation of caspase-3 may contribute to HI-induced neuronal death in immature neurons, but would play a minor role in mature neurons [53]. These authors have also reported that caspase-3-mediated and noncaspase-3-mediated neuronal death occur after HI during brain maturation. Zeng et al. have demonstrated that co-existence of necrosis and apoptosis in neonatal rat hippocampus following transient forebrain ischemia [59]. Therefore, to determine neuroprotective effect of PTX in neonatal rat model of HIE, we evaluated the regional and temporal profiles of caspase-3 immunoreactivity and the numbers of necrotic and healthy normal neurons in the PC and hippocampus of neonatal rats after unilateral hypoxic-ischemic brain injury. We determined that hypoxic neuronal apoptosis was characterized by the caspase-3 immunoreactive shrunken cytoplasm and condensed nucleus, but hypoxic neuronal necrosis is characterized by the large vacuoles containing the small pieces of chromatin clumps [59]. These morphological and immunohistochemical results the co-existence of necrosis and apoptosis in the brain region after HI [53,59].
Our results revealed that while no statistically significant differences were detected between healthy normal neuron numbers in the right and left hemispheres of the sham group, the right brain regions healthy normal neuron numbers of the HI, PTX60 and PTX100 groups were significantly lower than those of the left regions (P<0.05), conversely caspase-3 positive and necrotic cell counts were higher in the right brain regions of HI, PTX60 and PTX100 groups (P<0.05). These findings showed that the ligation of carotid artery is done correctly.
In present study we found that in the right brain of PTX60 group, the numbers of healthy normal neuron were higher in the PC and CA regions than in the PTX100 group (P<0.05), whereas in the DG than that observed in the HI and PTX100 groups (P<0.05). These results indicated that treatment with PTX (60 mg/kg) increased the number of healthy neurons in ligature-induced hypoxic-ischemic brain injury in immature rats and are in support of literature reports (27,40,44) indicating that the PTX treatment (40 or 60 mg/kg doses) of ischemic animals reduced brain injury and increased the number of viable cells. Additionally, our findings receive support from the preclinical studies which showed that PTX reduces neuronal damage following ischemia [60,61].
Eun et al. have informed that pretreatment with 150 mg/ kg PTX resulted in 100% mortality; treatment with 75 mg/ kg/dose resulted in approximately 20% mortality [40]. However, these authors have stated that there were no deaths among the rats that received posthypoxic-ischemic PTX treatment, or among their littermate controls. Bruno et al. have administered 100 mg/kg/dose of PTX in adults and they did not detect any toxic effects [27]. Besides, Cunha et al. have reported that PTX (50, 100 and 200 mg/ kg, i.p.) significantly improved learning and memory, in rats with hippocampal lesions induced by glutamate, as evaluated by the T-maze, passive avoidance, and water maze tasks, as compared to the glutamate-lesioned group without PTX treatment [62]. Our findings, which showed that a decrease in the number of healthy normal neurons and an increase in the number of necrotic neurons of the left PC in the PTX100 group, suggest the hypothesis that 100 mg/kg dose of PTX can be toxic on cortical neurons in neonatal rat brain.
Previous studies suggest that caspase-3 is the important factor contributing to delayed caspase-dependent cell death after neonatal HI and caspase-3 inhibitors block caspase-3 activation and cleavage of its substrates, resulting in significant neuroprotection against HI-induced brain injury [23]. Recently, researchers have shown PTX significantly and dose-dependently reduced neuronal cell death via the suppression caspase-dependent apoptostic pathway [63,64]. Kalay et al. [41] demonstrated that caspase-3 activities in the brains of ischemia group were significantly higher than in that of control group and treatment of PTX 60 mg/kg/dose (a single dose) significantly decreased caspase-3 activity in the brain of ischemia group by used spectrophotometric assay. The immunohistochemical evidences obtained from our study indicated that the maximum caspase-3 positive and necrotic cells count occurred in the HI group, which was greater than that observed in the control and the PTX60 and PTX100 groups. Although no differences were detected between the numbers of caspase-3 immunoreactive cells in the PC and DG of the HI, PTX60 and PTX100 groups (P>0.05), the numbers of necrotic cells in the PC and DG were significantly higher in the HI and PTX100 groups than in the PTX60 group (P<0.05). The numbers of caspase-3 immunoreactive and necrotic cells in the CA regions of the hippocampus were significantly lower in the PTX60 group than in the HI group (P<0.05). These findings support information in previous reports and suggest the hypothesis that PTX significantly and dosedependently reduced neuronal cell death caused by HI in neonatal rat model of HI via the inhibition of caspasedependent apoptotic pathway [63,64].
The evidences from this study reveal that the co-existence of necrosis and apoptosis in the brain region after HI and suggest that twice administration of PTX (60 mg /kg dose) after HI reduces the number of apoptotic cells probably via suppressing the activities of caspase-3 and thereby increases the survival rate of healthy neurons.
References
- TrollmanR,GassmanM. The role of hypoxia-inducible transcription factors in the hypoxic neonatal brain. Brain and Dev 2009; 31: 503-509.
- Koelfen W, Freund M, Varnholt V. Neonatal stroke involving the middle cerebral artery in term infants: clinical presentation, EEG and imaging studies, and outcome. Dev Med Child Neurol 1995; 37: 204-212.
- Delsing BJ, Catsman-Berrevoets CE, Appel IM. Early prognostic indicators of outcome in ischemic childhood stroke. Pediatr Neurol 2001; 24: 283-289.
- Levene MI, Vries LS. Fanaroff and Martin?s neonatal-perinatal medicine. In: Martin RJ, Fanaroff AA, Walsh MC, editors. Hypoxic-ischemic encephalopathy (9th ed), United States: Elsevier Publishing Inc 2011; 952-976.
- Perrone S, Stazzoni G, Tataranno ML. New pharmacologic and therapeutic approaches for hypoxic-ischemic encephalopathy in the newborn. J Matern Fetal Neonatal Med 2012; 1: 83-88.
- Volpe JJ. Hypoxic-ischemic encephalopathy. In: Volpe JJ, editor. Neuropathology and pathogenesis. neurology of the newborn. 5th ed. Philadelphia: Elsevier 2008; 259-336.
- Saliba E, Henrot A. Inflammatory mediators and neonatal brain damage. Biol Neonate 2001; 79: 224-227.
- Silverstein FS, Barks JD, Hagan P, et al. Cytokines and perinatal brain injury. Neurochem Int1997; 30: 375-383.
- Liu J, Feng ZC. Increased umbilical cord plasma interleukin-1 beta levels were correlated with adverse outcomes of neonatal hypoxic-ischemic encephalopathy. J Trop Pediatr 2010; 56: 178-182.
- Liu F, McCullough LD. Inflammatory responses in hypoxic ischemic encephalopathy. Acta Pharmacol Sin2013; 34: 1121-1130.
- Zou JY, Crews FT. TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain Res 2005; 1034: 11-24.
- Zhao X, Bausano B, Pike BR, et al. TNF-a Stimulates Caspase-3 Activation and Apoptotic Cell Death in Primary Septo-Hippocampal Cultures. Journal of Neuroscience Research 2001; 64: 121-131.
- Martin D, Chinookoswong N, Miller G. The interleukin-1 receptor antagonist (rhIL-1ra) protects against cerebral infarction in a rat model of hypoxia-ischemia. Exp Neurol 1994; 130: 362-367.
- Büyükakilli B, Atici A, Özkan A, et al: The effect of tumor necrosis factor-a inhibitor soon after hypoxia-ischemia on heart in neonatal rats. Life Sci 2012; 90: 838-845.
- Pulera MR, Adams LM, Liu H, et al. Apoptosis in a neonatal rat model of cerebral hypoxia-ischemia. Stroke 1998; 29: 2622-2630.
- Nakajima W, Ishida A, Lange MS, et al. Apoptosis has a prolonged role in the neurodegeneration after hypoxic ischemia in the newborn rat. J Neurosci 2000; 20: 7994-8004.
- Northington FJ, Ferriero DM, Graham EM, et al. Early neurodegeneration after hypoxia-ischemia in neonatal rat is necrosis while delayed neuronal death is apoptosis. Neurobiol Dis 2001; 8: 207-219.
- Liu CL, Siesjö BK, Hu BR. Pathogenesis of hippocampal neuronal death after hypoxia-ishemia changes during brain development. Neuroscience 2004; 127: 113-123.
- Thornberry NA. Caspases: key mediators of apoptosis. Chemistry and Biology 1998; 5: R97-R103.
- Chen J, Nagayama T, Jin K, et al. Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia. J Neurosci 1998; 18: 4914-4928.
- Namura S, Zhu J, Fink K, et al: Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci 1998; 18: 3659-3668.
- Hara H, Friedlander RM, Gagliardini V, et al. Inhibition of interleukin-1 b-converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc Natl Acad Sci 1997; 94: 2007-2012,.
- Cheng Y, Deshmukh M, D?Costa A, et al. Caspase inhibitor affords neuroprotection with delayed administration in a rat model of neonatal hypoxic-ischemic brain injury. J Clin Invest 1998; 101: 1992-1999.
- Harris E, Schulzke SM, Patole SK. Pentoxifylline in preterm neonates: a systematic review. Paediatr Drugs 2010; 12: 301-311.
- Li SH, Fei X, Chen SF, et al. Pentoxifylline attenuates platelet activating factor-induced permeable edema in isolated perfused guinea pig lungs. Zhongguo Yao Li Wue Bao 1994; 15: 219-222.
- Sei Y, Nishida K, Kustova Y, et al. Pentoxifylline decreases brain levels of platelet activating factor in murine AIDS. Eur J Pharmacol 1997; 325: 81-84.
- Bruno Rde B, Marques TF, Batista TMT, et al. Pentoxifylline treatment improves neurological and neurochemical deficits in rats subjected to transient brain ischemia. Brain Res 2009; 1260: 55-64.
- Vakili A, Mojarrad S, Akhavan MM, Rashidy-Pour A. Pentoxifylline attenuates TNF-a protein levels and brain edema following temporary focal cerebral ischemia in rats. Brain Res 2011; 1377: 119-125.
- Kim KB, Jeon GH, Kim YR, et al. The effect of pentoxifylline on IL-1 beta and TNF-alpha mRNA gene expression in hypoxic-ischemic brain injury of immature rat. J Korean Child Neurol Soc 1999; 7: 181-187.
- Gutierrez M, Tejedor ED, Alonso de Leciñana M, et al. Thrombolysis and neuroprotection in cerebral ischemia. Cerebrovasc Dis 2006; 2: 118-126.
- Rice JE, Vanucci RC, Brierley JB. The influence of immaturity on hypoxic?ischemic brain damage in the rat. Ann Neurol 1981; 9: 131-141.
- Northington FJ. Brief update on animal models of hypoxic?ischemic encephalopathy and neonatal stroke. ILAR J 2006; 47: 32-38.
- Liman N, Alan E, Bayram GK, et al. Expression of survivin, Bcl-2 and Bax proteins in the domestic cat (Felis catus) endometrium during the oestrus cycle. Reprod Domest Anim 2013; 48: 33-45.
- Liu XH, Eun BL, Silverstein FS, Barks JDE. The platelet-activating factor antagonist BN 52021 attenuates hypoxic-ischemic brain injury in the immature rat.Pediatr Res 1996; 40: 797-803.
- Ward A, Clissold SP. Pentoxifylline: a review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacy. Drugs 1987; 34: 50-59.
- Sullivan GW, Carper HT, Novick WJ Jr, Mandell GL. Inhibition of the inflammatory action of interleukin-1 and tumor necrosis factor (alpha) on neutrophil function by pentoxifylline.Infect Immun 1990; 56: 1722-1729.
- Zheng H, Crowley JJ, Chan JC, et al. Attenuation of tumor necrosis factor-induced endothelial cell cytotoxicity and neutrophil chemiluminescence.Am Rev Respir Dis 1990; 142: 1073-1078.
- Mandell GL. Cytokines, phagocytes and pentoxifylline.J Cardiovasc Pharmacol 1995; 25: S20?S22.
- Banfi C, Sironi L, De Simoni G, et al. Pentoxifylline prevents spontaneous brain ischemia in stroke-prone rats. J Pharmacol Exp Ther 2004; 310: 890-895.
- Eun B-L, Liu X-H, Barks JDE. Pentoxifylline attenuates hypoxic-ischemic brain injury in immature rats. Pediatric Research 2000; 47: 73-78.
- Kalay S, Oztekin O, Tezel G, et al. The effects of intraperitoneal pentoxifylline treatment in rat pups with hypoxic-ischemic encephalopathy. Pediatric Neurology 2013; 49: 319-323.
- Sirin BH, Yilik L, Coskun E, et al. Pentoxifylline reduces injury of the brain in transient ischaemia. Acta Cardiol 1998; 53: 89-95.
- Nekoeian AA, Vakili A, Dehghani GA. Pre-ischemic treatment of pentoxifylline reduces cortical and striatal infarcts volume in rat model of focal cerebral ischemia. Iran J Med Sci 2005; 29: 169-173.
- Vakili A, Zahedi Khorasani M. Post-ischemic treatment of pentoxifylline reduces cortical not striatal infarct volume in transient model of focal cerebral ischemia in rat. Brain Res 2007; 1144: 186-191.
- Shohami E, Ginis I, Hallenbeck JM. Dual role of tumor necrosis factor alpha in brain injury. Cytokine Growth Factor Rev 1990; 10: 119-130.
- Honess DJ, Dennis IF, Bleehen NM. Pentoxifylline: its pharmacokinetics and ability to improve tumour perfusion and radiosensitivity in mice. Radiother Oncol 1993; 28: 208-218.
- LeMay LG, Vander AJ, Kluger MJ. The effects of pentoxyfilline on lipopolysaccaride (LPS) fever, plasma interleukin 6 (IL 6), and Tumor necrosis factor (TNF) in the rat. Cytokine 1990; 2: 300-306.
- Raju PI, Tolman KC, Davis PJ, et al. Distribution and metabolism of pentoxifylline in non-tumor-bearing mice. J Med 1993; 24: 353-368.
- Johnston MV, Ferriero DM, Vannucci SJ, Hagberg H. Models of cerebral palsy: which ones are best? J Child Neurol 2005; 20: 984-987.
- Siesjö BK, Bengtsson F. Calcium fluxes, calcium antagonists, and calcium-related pathology in brain ischemia, hypoglycemia, and spreading depression: a unifying hypothesis. J Cereb Blood Flow Metab 1989 9: 127-140.
- Mehmet H, Yue X, Penrice J, et al. Relation of impaired energy metabolism to apoptosis and necrosis following transient cerebral hypoxia-ischemia. Cell Death Differ 1998; 5: 321-329.
- Renolleau S, Aggoun-Zouaoui D, Ben-Ari Y, Charriaut-Marlangue C. A model of transient unilateral focal ischemia with reper fusion in the P7 neonatal rat: morphological changes indicative of apoptosis. Stroke 1998; 29: 1454-1460.
- Hu BR, Liu CL, Ouyang Y, et al. Involvement of caspase-3 in cell death after hypoxia-ischemia declines during brain maturation. Journal of Cerebral Blood Flow and Metabolism 2000; 20: 1294-1300.
- Johnston MV, Trescher WH, Ishida A, Nakajima W. Neurobiology of hypoxic?ischemic injury in the developing brain. Pediatr Res 2001; 49: 735-741.
- Northington FJ, Graham EM, Martin LJ. Apoptosis in perinatal hypoxic?ischemic brain injury: how important is it and should it be inhibited? Brain Res Brain Res Rev 2005; 50: 244-257.
- Fatemi A, Wilson MA, Johnson MV. Hypoxic ischemic encephalopathy in the term infant. Clin Perinatol 2009; 36: 835-858.
- Zhu C, Wang X, Xu F, et al. The influence of age on apoptotic and other mechanisms of cell death after cerebral hypoxia-ischemia. Cell Death Differ 2005; 12: 162-176.
- Wang X, Karlsson JO, Zhu C, et al. Caspase-3 activation after neonatal rat cerebral hypoxia-ischemia. Biol Neonate 2001; 79: 172-179.
- Zhen Y-S, Xu ZC. Co-existence of necrosis and apoptosis in rat hippocampus following transient forebrain ischemia. Neurosci Res 2000; 37: 113-125.
- Labs KH R. Labs R, M. Rossner M. Clinical experience with pentoxifylline. Atherosclerosis 1997; 131: 37-39.
- Teixeira MM, Gristwood RW, Cooper N, et al. Phosphodiesterase (PDE)4 inhibitors: anti-inflammatory drugs of the future? Trends Pharmacol. Sci 1997; 18: 164-171.
- Cunha GMA, Bezerra, PJP, Saldanda MDD, et al. Pentoxifylline improves learning and memory in glutamate-lesioned rats. Pharmacol Biochem Behav 2000; 66: 687-694.
- Zhu FJ, Xia B, Bi Q, et al. Functional protection of pentoxifylline against spinal cord ischemia/reperfusion injury in rabbits: necrosis and apoptosis effects. Chin Med J 2008; 121: 2444-2449.
- Muchhala SK, Benzeroual KE. Pentoxifylline suppressed LPS-induced inflammatory and apoptotic signaling in neuronal cells. Advances in Bioscience and Biotechnology 2012; 3: 731-739.