- Biomedical Research (2008) Volume 19, Issue 3
The possible mechanism of action of palm oil ?-tocotrienol and ?-tocopherol on the cervical carcinoma CaSki cell apoptosis
1Narimah AH Hasani, 2Permeen A Yusoff, 3Khalid BAK, 4A Ghapor MT, 2*Wan Ngah WZ.
1Faculty of Medicine, Universiti Teknologi MARA, 40450 Shah Alam, Selangor, Malaysia.
2Department of Biochemistry, Faculty of Medicine, Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, 50300 Kuala Lumpur, Malaysia.
3School of Medicine and Health Sciences, Monash University Malaysia Campus, 46150 Petaling Jaya, Selangor, Malaysia.
4Division of Chemistry and Technology, Palm Oil Research Institute of Malaysia, Selangor, Malaysia.
- *Corresponding Author:
- Wan Zurinah Wan Ngah
Department of Biochemistry
Faculty of Medicine
Universiti Kebangsaan Malaysia
Jalan Raja Muda Abdul Aziz
50300 Kuala Lumpur
Malaysia
Phone: +603-92897222/92897292
Fax: +603-26938037
E-mail: zurina@medic.ukm.my
Accepted date: December 17 2007
Abstract
Alpha-, γ-, δ-tocotrienols and γ-tocopherol have been reported to exhibit anti-proliferation ef-fects in several human cancer cells i.e., breast (estrogen-responsive, MDA-MB-435 and estrogen non-responsive, MCF7) and prostate (androgen-sensitive, LNCaP and androgen-resistant, PC-3) via controlling the signal transduction pathways that resulted in an increase in apoptosis. In this study, we tested the effects of γ-tocotrienol, α-tocopherol and α-tocopherol acetate on the prolif-eration and apoptosis in human cervical carcinoma CaSki cells. The cells were treated with dif-ferent doses (0 to 300μM) of γ-tocotrienol, α-tocopherol and α-tocopherol acetate and then the proliferation activity were determined using 5-Bromo-2'-deoxy-uridine (BrdU) detection method. Data obtained show that γ-tocotrienol efficiently inhibited the proliferation activity of CaSki cells by 93.5% to 97.8% (p<0.01, n=4) beginning with a dose of 100μM and above with IC50 value of 75μM, while α-tocopherol inhibited the proliferation activity of CaSki cells at lesser magnitude of 19.7% to 39.4% (p<0.01, n=4) beginning with a dose of 50μM and IC20 value of 300μM. On the contrary, α-tocopherol acetate showed no effect on the cell proliferation. The cells apoptotic activity after treatment with different doses of γ-tocotrienol and α-tocopherol (0 to 500μM) was measured using cellular DNA fragmentation ELISA method. In this assay, treatment with 150μM of γ-tocotrienol and 300μM of α-tocopherol had shown to enhance the maximum apoptotic activity of CaSki cells by 6.8 fold (p<0.01, n=4) and 2.7 fold (p<0.01, n=4), respectively as compared to untreated cultures. At the same doses as above, both compounds in-duced a 50% (p<0.05, n=4) and 40% (p<0.05, n=4) of nuclear apoptotic morphological changes in CaSki cells, respectively as detected using propidium iodide staining. The mechanism of ac-tion involved in γ-tocotrienol and α-tocopherol-induced apoptosis was investigated through Western blot analysis. The exposure of both compounds at 150μM and 300μM, respectively for 0, 1, 2, 3, 4, 5, 6, 12, 18, and 24 hours enhanced the expressions of p53, Bax and Caspase-3, and the activity of Caspase-3. These data suggest that p53, Bax and Caspase-3 are involved in the apoptotic signaling cascade induced by γ-tocotrienol and α-tocopherol.
Keywords
γ-tocotrienol, α-tocopherol, CaSki cell apoptosis
Introduction
Palm oil vitamin E consists of two groups of structurally-related fat soluble compounds, α-, γ-, δ- tocopherols (78% to 82%) and (18% to 22%) α-, γ-, δ- tocotrienols [1]. The unique differences in the unsaturated isoprenoid tail is be-lieved to enable tocotrienols to display efficient anti-cancer activity compared to that of the saturated tocopherols [2].
In vitro studies have shown that α-, γ-, δ-tocotrienols and γ-tocopherol effectively inhibit the proliferation of several human cancer cell lines including cervical carcinoma (HeLa), breast cancer (non-estrogen responsive, MDA-
MB-435 and estrogen responsive, MCF-7), prostate cancer (androgen-resistant, PC-3 and androgen-sensitive, LNCaP) and lung cancer (A549) cells without affecting the normal prostate epithelial (PrEC) cells [3-5].
The mechanism of the chemotherapeutic properties of vi-tamin E may be mediated via the repression of cell prolif-eration, blockage of cell cycle at G1-S transition phase, reduction in DNA synthesis or induction of apoptosis [5-8]. Of these postulated mechanisms, apoptosis has been studied most extensively [9].
Apoptosis-associated activation of transforming growth factor-β (TGF-β) and Fas/CD95 signaling pathways up-stream the JNK activation. Once activated, JNK will then phosphorylate the transcription factor, c-Jun and mediated the translocation of Bax to mitochondria resulting in the released of cytochrome c, Caspases activation and apop-tosis [10-13]. Studies have shown that δ-tocotrienol in-duces apoptosis in human breast cancer cells, MDA-MB-435 via stimulation of the TGF-β, Fas- and JNK-signaling pathways [13].?VES has also been shown to induce apop-tosis in human breast cancer cells via translocation of Bax from the cytosol to the mitochondria and releasing cyto-chrome c from the mitochondria to the cytosol [12]. An-other study has demonstrated that VES also induces apop-tosis in prostate cancer cells through inducing Bcl-xL/Bcl-2 function [8]. Gamma-tocopherol alone or in combination with δ-tocopherol, induces apoptosis in androgen-sensitive (LNCaP) but not in the androgen-resistant (PC-3) human prostate cancer cells through the induction of cytochrome c release, activation of caspase-9 and -3, cleavage of poly-ADP-ribose polymerase and by an involvement of caspase-independent pathways [5].
Recent evidence suggests that p53, a tumor suppressor protein, induces apoptosis primarily through the intrinsic mitochondrial pathway [14,15]. Western blot analysis showed that estradiol markedly increases the p53 protein level in apoptotic estrogen receptor-positive breast cancer cells, MCF-7:5C. Estradiol treatment also increases the p53 mRNA expression in MCF-7:5C cells compared with control. Ethanol extract of Chinese licorice root G. uralensis inhibits cell proliferation by upregulating p53 and p2lwaf1/cipl and downregulating of cyclin E and cdk2, and induces apoptosis through overexpression of Bax in MCF-7 human breast cancer cells [16].
The present study therefore, aims to investigate the possi-ble mechanism of action of palm oil γ-tocotrienol and α-tocopherol on the human cervical carcinoma CaSki cell apoptosis.
Materials and methods
Cell culture
CaSki cells were purchased from American Type Cell Col-lection (Manassas, VA, USA) and cultured in RPMI con-taining 10% fetal bovine serum, 20mM Hepes, 20mM so-dium bicarbonate, 2mM L-glutamine and 1% penicillin and streptomycin. The cells were grown in culture flask (Falcon, Becton Dickinson, NJ, USA) as monolayer to 80% to 90% of confluence in 5% CO2, at 37°C. Culture media and the above chemicals were purchased from FLOWLAB, Sydney, Australia.
Vitamin E treatment
Palm oil γ-tocotrienol and α-tocopherol were obtained as 80% concentrate (single peak by HPLC) from the Palm Oil Research Institute of Malaysia, Kuala Lumpur. Stock solu-tions of both compounds were dissolved in absolute alco-hol at 500μM and then diluted so that the final concentra-tion of alcohol in the culture media was <0.1%. Alpha-tocopherol acetate was purchased from Sigma Chemical Co, St. Louis, MO, USA.
Cell proliferation assay
The effect of γ-tocotrienol, α-tocopherol and α-tocopherol acetate in the cell proliferation was determined using a 5-Bromo-2'-deoxy-uridme (BrdU) labelling and detection method (Bohrienger Mannheim, Mannheim, Germany).
Apoptosis assay
The cellular DNA fragmentation was measured using a Cellular DNA Fragmentation-ELISA method (Bohrienger Mannheim, Mannheim, Germany) and the morphological evaluation of propidium iodide-stained cells was determi-ned using a Leitz Dialux 20 EB fluorescent microscope at 630 magnification with a minimum of 4 counts involving >200 to 300 cells/field from different five locations per slide.
Western blot analysis
A total of 10 x 106 cells were cultured and treated sepa-rately with 150μM of γ-tocotrienol and 300μM α-tocopherol (values obtained from apoptosis assay) in 5% CO2 at 37°C at different hours (0, 1, 3, 6, 12, 18, and 24). Cells were lysed with 400μL of ice-cold lysis buffer [1% Nonidet P-40, 0.1% SDS, 0.5% Na deoxycholate, 150mM NaCl, 50mM Tris (pH 8), 10μg/mL aprotinin, 1mM PMSF] for 30 minutes and centrifuged at 14K for another 30 minutes at 4°C. Protein concentration was determined by Bradford protein assay (Sigma Chemical Co, St Louis, Missouri, USA). A total of 100μg of protein was dissolved in 5X loading buffer [0.1% Bromophenol blue, 0.4M Tris-HCl (pH 6.8), 37.5% glycerol, 10% SDS, 7.8% DTT] and heated for 5 minutes at 95°C. The protein was then sepa-rated by 8% to 10% precast SDS/PAGE gel and electro-transferred to a nitrocellulose membrane (Amersham Phar-macia Biotech, Buckinghamshire, England, UK) and pro-bed by antibodies (PharMingen International, San Diego, California, USA). Membranes were exposed to chemilumi-nescent reagent (Boehringer Mannheim, Mannheim, Ger-many) and visualized on a Kodak film (Eastern Kodak Company, Rochester, New York, USA). The above chemi-cals were purchased from Sigma, Chemical Co (St Louis, Missouri, USA).
Caspase-3 activity analysis
Activation of ICE-family proteases (caspases) initiates apoptosis in mammalian cells. Caspase-3 Colorimetric Activity Assay Kit (Chemicon International Inc. Califor-nia, USA) is based on spectophotometric detection of the chromophore p-nitroaniline (pNA) after cleavage from the labeled substrate DEVD-pNA. The free pNA can be quan-tified using a spectrophotometer or a microtiter plate reader at 405nm. Comparison of the absorbance of pNA from an apoptotic sample with an untreated control allows determination of the fold increase in caspase activity.
Statistical analysis
Paired Student's t-test was used to compare between the control and different levels of each treatment on the meas-ured parameters. Significance was set up at p<0.05.
Results
Effect of γ-tocotrienol, α-tocopherol and α-tocopherol acetate in cell proliferation and viability
To determine the effects of γ-tocotrienol, α-tocopherol and α-tocopherol acetate on cell proliferation, we examined the incorporation of BrdU into freshly synthesized cellular DNA.
Results obtained (Figure l) showed that at the lower dose of 50μM, γ-tocotrienol slightly enhanced the proliferation of CaSki cells by 22.0% (p<0.01, n=4). Interestingly, at 100μM and above, γ-tocotrienol effectively suppressed the cells proliferation by 93.5% to 97.8% (p<0.01, n=4) with IC50 value of 75μM. α-tocopherol caused a reduction at lesser magnitude at 50μM by 19.7% to 39.4% (p<0.01, n=4) with IC40 value of 300μM. On the contrary, α-tocopherol acetate had no effect at all concentrations used.
Gamma-tocotrienol (GTT) and α-tocopherol (ATF)-mediated apoptosis
To study the anti-proliferation mechanism induced by γ-tocotrienol and α-tocopherol on CaSki cells, we analyzed the apoptotic properties of both the compounds by mea-suring the cellular DNA fragmentation activity and mor-phological evaluation of propidium iodide-stained cells.
The cellular DNA fragmentation activity of CaSki cells induced by γ-tocotrienol and α-tocopherol was investiga-ted by measuring the BrdU-labeled DNA fragments relea-sed into the cytoplasm during apoptosis.
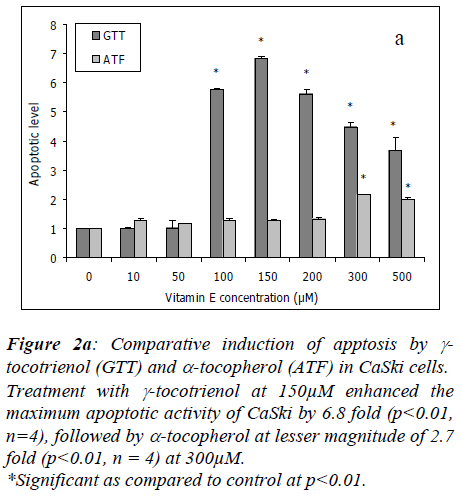
Treatment with γ-tocotrienol at 10μM and 50μM had no effect in CaSki cells, however, at 100μM, it enhanced the apoptotic activity by 5.8 fold (p<0.01, n=4) with the maximum activity of 6.8 fold (p<0.01, n=4) at 150μM. At higher concentrations of 200μM to 500μM, the apoptotic activity induced by γ-tocotrienol was slightly reduced to 5.6 fold to 3.7 fold, but the effect was still significant at p<0.01 as compared to untreated cultures. Alpha-tocopherol enhanced the cell apoptotic activity by 2.7 fold (p<0.01, n=4) beginning at 300μM of treatment (Figure 2a).
Figure 2a: Comparative induction of apptosis by γ-tocotrienol (GTT) and α-tocopherol (ATF) in CaSki cells.
Treatment with γ-tocotrienol at 150μM enhanced the maximum apoptotic activity of CaSki by 6.8 fold (p<0.01, n=4), followed by α-tocopherol at lesser magnitude of 2.7 fold (p<0.01, n = 4) at 300μM.
*Significant as compared to control at p<0.01..
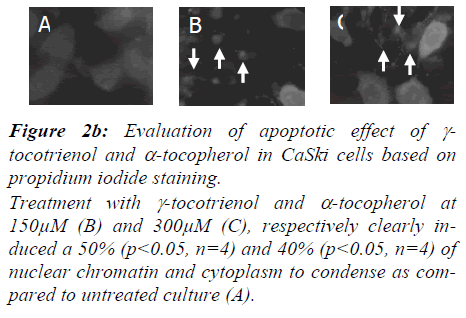
Nuclear changes of CaSki cells induced by γ-tocotrienol and α-tocopherol were confirmed using propidium iodide staining and illustrated in Figure 2b. Apoptotic data are reported as percentage of apoptosis obtained by determin-ing the numbers of apoptotic cells versus the total number of cells, within a cell population by counting >300 cells/field from five different locations per slide.
Figure 2b: Evaluation of apoptotic effect of γ-tocotrienol and α-tocopherol in CaSki cells based on propidium iodide staining.
Treatment with γ-tocotrienol and α-tocopherol at 150μM (B) and 300μM (C), respectively clearly in-duced a 50% (p<0.05, n=4) and 40% (p<0.05, n=4) of nuclear chromatin and cytoplasm to condense as com-pared to untreated culture (A)..
Gamma-tocotrienol (B) and α-tocopherol (C) induced 50% (p<0.05, n=4) and 40% (p<0.05, n=4) of the characteristic nuclear changes, respectively on CaSki cells as visualized by propidium iodode fluorescent DNA dye staining. The chromatin of the treated nuclei was unevenly dispersed and condensed, while the chromatin of the control nuclei was evenly dispersed (A).
Studies on protein expression using Western blot analysis
In order to understand the mechanism of actions of palm oil γ-tocotrienol and α-tocop herol on CaSki cells, we in-vestigated the effect of both compounds in the protein ex-pression of genes that have been implicated in the regula-tion of apoptosis.
Effect of γ-tocotrienol and α-tocopherol at maximum apoptotic activity in the protein expression of p53, Bcl-2, Bax and Caspase-3.
To determine the effect of both compounds on the Cas-pase-3 activity, CaSki cells were cultured with γ-tocotri-enol and α-tocopherol at similar concentration as above up to 24 hours treatment.
Our data showed that γ-tocotrienol at 150μM, efficiently enhanced the Caspase-3 activation in Caski cells by 119.8% to 308.7% (p<0.01, n=4) beginning at 1 hour of treatment. At 300μM, α-tocopherol also exhibited similar effect, however, at lesser magnitude by 123.6% to 305.2% (p<0.01, n=4) of increment. Our finding showed that γ-tocotrienol is more potent as Caspase-3 activator (p<0.01, n=4) at 1 to 6 hours of treatment as compared to α-tocopherol (Figure 4).
Figure 4: Gamma-tocotrienol (GTT) and α-tocopherol (ATF) induced the activity of Caspase-3 in CaSki cells.
Treatment with γ-tocotrienol at 150μM induced the activ-ity of Caspase-3 in Caski cells by 119.8% to 308.7% (p<0.01, n=4, respectively beginning at 1 hour of induc-tion, while α-tocopherol at 300μM, exhibit lesser magni-tude by 123.6% to 305.2% (p<0.01, n=4). Gamma-tocotrienol is more potent as Caspase-3 activator (p<0.01, n=4) at 1 to 6 hours of treatment as compared α-tocopherol.
*Significant as compared to control at each time of treatment : p<0.01.
+Significant as compared between GTT and ATF at each time of treatment : p<0.01.
Discussion
Treatment with γ-tocotrienol at 100μM and above, effec-tively suppressed the proliferation of CaSki cells by 93.5% to 97.8% (p<0.01, n=4) with IC50 value of 75μM, while α-tocopherol caused a reduction at lesser magnitude at 50μM by 19.7% to 39.4% (p<0.01, n=4) with IC40 value of 300μM. On the contrary, α-tocopherol acetate did not show any effect on cell proliferation at all the concentra-tions used (Figure 1).
Apoptosis is an active and physiological process that can be characterized by biochemical and morphological altera-tions. A series of biochemical alterations include cellular fragmentation of nucleus and extensive degradation of chromosomal DNA into non-random fragments of 180 to 200bp by endonucleases. Characteristic of DNA laddering can be seen on agarose gel by electroporesis separation. Morphological characteristic includes shrinkage of cells, membrane blebbing, cytoplasm and chromatin condensa-tion. These changes can be evaluated via propidium iodide staining [17,18].
Our experiments demonstrated that treatment with γ-tocotrienol at 150μM enhanced the maximum DNA frag-mentation activity in CaSki cells by 6.8 fold, followed by α-tocopherol at 300μM with lesser magnitude of 2.0 fold (Figure 2a). Further evidence of apoptosis was obtained by nuclear morphological characteristics as illustrated in Figure 5c. At similar doses as used above, both γ-tocotrienol and α-tocopherol induced a 50% and 40% of nuclear chromatin and cytoplasm condensation, respectively. Pre-vious in vitro studies also reported similar findings, that is, α-, γ- and δ-tocotrienols efficiently inhibit the proliferation of human cervical carcinoma (HeLa), and breast cancer (estrogen responsive, MCF-7 and estrogen non-responsive, MDA-MB-435) cells by inducing apoptosis [3,4,6,7,19] whereas, γ-tocopherol alone or in combination with δ-tocopherol, induced apoptosis in androgen-sensitive (LNCaP) but not in the androgen-resistant (PC-3) human prostate cancer cells [5].
The growth inhibiting effects of vitamins A (retinoic acid), C and E (α-tocopheryl succinate) on cancer cells may be caused by changing the expression of specific genes, level of proteins and translocation of certain proteins from one cellular compartment to another. The alteration in the gene expressions and protein levels are directly related to prolif-eration inhibition and apoptosis [10,20,21].
The tumor suppressor protein p53, is an important regula-tor for the mitochondrion-mediated apoptotic cell death [14,15] by transcriptional activation of genes that encode for proapoptotic proteins such as Bax and by transcrip-tional repression of Bcl-2 [15,22]. The apoptosis-promoting Bax protein led to mitochondrial dysfunction and the release of cytochrome c from the mitochondria. The released cytochrome c then interacts with specific adapter, such as Apaf-1, which in turn converts pro-caspases to active Caspases [23]. Caspases then cleave several substrates including the poly(ADP-ribose) poly-merase, a nuclear enzyme involved in DNA repair and maintenance of genome integrity and post-translational ribosylation of proteins, whereby apoptosis occurs [16,2].
Our finding using Western blot analysis demonstrated that treatment with γ-tocotrienol and α-tocopherol at a maxi-mum apoptotic activity of 150μM and 300μM, respec-tively in CaSki cells, enhanced the protein expression of p53 (53kD) (Figure 3a) and Bax (21kD) (Figure 3b) be-ginning at 3 hours and 6 hours, respectively and continuing through 24 hours after its initiation. At similar concentra-tions, both compounds induced the activation of procas-pase-3 (32kD) in CaSki cells by the formation of active subunit fragments (17 kD) beginning at 1 hour and con-tinuing through 24 hours after its initiation (Figure 3c).
Figure 3b: Treatment with γ-tocotrienol (A) and α-tocophe-rol (B) at maximum apoptotic activity of 150μM and 300μM, respectively in CaSki cells has no effect in the pro-tein expression of Bcl-2 (26kD), however both compounds enhanced the protein expression of Bax (21kD) beginning at 6 hours and continuing through 24 hours after initia-tion..
Figure 3c: Treatment with γ-tocotrienol (A) and α-tocopherol (B) at maximum apoptotic activity of 150μM and 300μM, respectively induced the activation of pro-caspase-3 (32kD) in CaSki cells by the formation of ac-tive subunit fragments (17kD) beginning at 1 hour con-tinuing through 24 hours after initiation.
Both compounds at a similar concentration efficiently en-hanced the Caspase-3 activation in Caski cells by 123.6% to 308.7% (p<0.01, n=4) and 119.8% to 305.2% (p<0.01, n=4), respectively beginning at 1 hour of treatment (Figure 4).
Recent evidence using ethanol extract of Chinese licorice root G. uralensis showed that cell proliferation was inhib-ited via up-regulation of p53, and apoptosis induced through overexpression of Bax in MCF-7 human breast cancer cells [16]. Here, the p53 and Bax protein expres-sions found to be increased at 24 hours and 48 hours, re-spectively after treatment with 100μg/mL licorine root extract which remained elevated up to 72 hours. No change observed in protein expression of Bcl-2 [15]. Treatment with 40μmol/L of RRR-α-tocopheryl succinate in human prostate cancer cells, PC-3 increased the activa-tion of Bcl-2 beginning at 12 hours and continuing through 24 hours after the initiation of the treatment. However, the protein expression was not increased [9]. Another study had shown that treatment of human breast cancer cells, MDA-MB-435 with 20μg/mL of vitamin E succinate re-sulted in the cleavage of Caspase-9 and presented with the appearance of a Mr 37,000 fraction of Caspase-9 appearing by 3 hours after initiation. Activation of ‘exercutioner’ Caspase-3 was detected at 12 and 24 hours after RRR-α-tocopheryl succinate treatment with the appearance of Mr 17,000/20,000 cleaved fragments. Poly-ADP-ribose poly-merase cleavage from the Mr 116,000 intact form to a Mr 84,000 cleavage product served as an additional indicator of caspase activity in MDA-MB-435 cells at 12 and 24 hours after the initiation of RRR-α-tocopheryl succinate treatment. The use of cell-permeable caspase inhibitors indicates that Caspase-9 and Caspase-3 are involved in RRR-α-tocopheryl succinate-induced apoptosis [12].
Conclusion
In summary, we have demonstrated that palm oil vitamin E, especially γ-tocotrienol acts as an effective anti-proliferation agent in human cervical carcinoma cells, CaSki by inducing apoptosis. The increased protein ex-pressions of p53, Bax and Caspase-3, and the activation of Caspase-3 suggest that these genes are critically involved in the γ-tocotrienol and α-tocopherol-induced apoptosis in CaSki cells.
The apoptosis inducing ability of γ-tocotrienol and α-tocopherol makes these compounds as interesting candi-dates for further characterization of their anti-tumor effects in vivo as natural chemoprevention agent in cancer treat-ment. A better understanding of the cellular and molecular events involved in the induction of apoptosis by γ-tocotrienol and α-tocopherol may provide basic rational for cancer therapies in the future.
Acknowledgment
This work was supported by an IRPA grant 06-02-02-0013.
References
- Sambanthamurthi R, Sundram K, Tan YA. Chemistry and biochemistry of palm oil. Progress in Lipid Resea-rch 2000; 39: 507-558
- He L, Mo H, Hadisusilo S, Qureshi AA, Elson CE. Iso-prenoids suppress the growth of murine B16 melanomas in vitro and in vivo. J Nutr 1997; 127: 668-674.
- Komiyama K, Iizuka K, Yamaoka M, Watanabe H, Tsu-chiya N, Umezawa I. Studies on the biological activities of tocotrienols. Chem Phar Bull 1989; 37: 1369-1371.
- Guthrie N, Gapor A, Chambers AF, Carroll KK. Inhibi-tion of proliferation of estrogen receptor negative MDA-MB-435 and -positive MCF-7 human breast cancer cells by palm oil tocotrienols and tamoxifen, alone and in combination. J Nutr 1997; 127: 544S-548S.
- Jiang Q, Wong J, Fyrst H, Saba JD, Ames BN. γ-Tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis. Proc Natl Acad Sci U S A 2004; 101(51): 17825-17830.
- Kline K, Yu W, Sanders BG. Vitamin E and breast can-cer. Journal of Nutrition 2004; 134 (12 Suppl): 3458S-3462S.
- Nesaretnam K, Ambra R, Selvaduray KR, Radhakrish-nan A, Canali R, Virgili F. Tocotrienol-rich fraction from palm oil and gene expression in human breast can-cer cells. Annals of the New York Academy of Sciences 2004; 1031: 143-157.
- Shiau CW, Huang JW, Wang DS, Weng JR, Yang CC, Lin CH, Li CL, Chen CS. α-Tocopheryl succinate in-duces apoptosis in prostate cancer cells in part through inhibition of Bcl-xL/Bcl-2 function. J Biol Chem 2006; 281: 11819-11825.
- Zu K, Hawthorn L, Ip C. Up-regulating of c-Jun-NH2-kinase pathway contributes to the induction of mito-chondria-mediated apoptosis by α-tocopheryl succinate in human prostate cancer cells. Mol Cancer Ther 2005; 4: 43-50.
- Kline K, Yu W, Sanders BG. Vitamin E: mechanisms of action as tumor cell growth inhibitors. J Nutr 2001; 131: 161S-163S.
- Yu W, Sanders BG, Kline K. RRR-α-tocopheryl succi-nate induction of DNA synthesis arrest of human MDA-MB-435 cells involves TGF-β-independent activation of p21 Waf/Cip21. Nutr Cancer 2002; 43: 227-238.
- Yu W, Sanders BG, Kline K. RRR-α-tocopheryl succi-nate-induced apoptosis of human breast cancer cells in-volves Bax translocation to mitochondria. Cancer Res-earch 2003; 63: 2483-2491.
- Shun MC, Yu W, Gapor A, Parsons R, Atkinson J, Sanders, Kline K. Pro-apoptotic mechanisms of action of a novel vitamin E analog (α-TEA) and naturally accurring form of vitamin E (δ-tocotrienol) in MDA-MB-435 human breast cancer cells. Nutr Cancer 2004; 48: 95-105.
- Schuler M, Green DR. Mechanisms of p53-dependent apoptosis. Biochem SocTrans 2001; 29: 684-688.
- Lewis JS, Meeke K, Osipo C, Ross EA, Kidawi N, Li T, Bell E, Chandel NS, Jordan VC. Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resis-tant to estrogen deprivation. J Natl Cancer Inst 2005; 97: 1746-1759.
- Jo EH, Kim SH, Ra JC, Kim SR, Cho SD, Jung JW, Yang SR, Park JS, Hwang JW, Aruoma OI, Kim TY, Lee YS, Kang KS. Chemopreventive properties of the ethanol extract of Chinese licorice (Glycyrrhiza uralen-sis) root: induction of apoptosis and Gl cell cycle arrest in MCF-7 human breast cancer cells. Cancer Letter 2005; 230: 239-247.
- Wyllie AH. Glucocorticoid-induced thymocyte apopto-sis is associated with endogenous endonuclease activa-tion. Nature 1980; 284: 555-556.
- Cotter, TG & Martin, SJ. Technique in apoptosis. A user’s guide. First edition. Portland Press Ltd, London 1996.
- Nesaretnam K, Gutrie N, Chambers AF, Carroll KK. Effects of tocotrienols on the growth of a human breast cancer cell line in culture. Lipids 1995; 30: 1139-1143.
- Neuzil J, Weber T, Gellert N, Weber C. Selective cancer cell killing by alpha-tocopheryl succinate. Br J Cancer 2001; 84: 87-89.
- Prasad KN. Multiple dietary antioxidants enhance the efficacy of standard and experimental cancer therapies and decrease their toxicity. Integrative Cancer Therapies 2004; 3: 310-322.
- Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotheraphy. Cell 2002; 108: 153-164.
- Hu H, Ahn NS, Yang X, Lee YS, Kang KS. Ganoderma lucidum extract induces cell cycle arrest and apoptosis in MCF-7 human breast cancer cell. Int J Cancer 2002; 102: 250-253.
- Yuan JY, Shaham S, Ledoux S, Ellis HM, Horvitz HR. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting en-zyme. Cell 1993; 75: 641-652.