Research Article - Biomedical Research (2017) Volume 28, Issue 19
The pathological alternation of hepatic tissues in HBV infected patients with negative peripheral antigen and the expression profile of tissues HBV DNA
Silu Peng, Zhiying Pan, Junjie Ren, Hong Sun and Bing Liu*
The Department of Infectious Diseases, Sichuan Mianyang 404 Hospital, Mianyang City, Sichuan Province, PR China
- *Corresponding Author:
- Bing Liu
The Department of Infectious Diseases
Sichuan Mianyang 404 Hospital, PR China
Accepted on September 24, 2017
Abstract
Objective: During viral hepatitis B, serum HBsAg and HBeAg were important indexes reflecting viral replication and liver inflammation. However, patients with negative serum HBeAg still developed viremia or inflammatory necrosis. Negative HBsAg patients also had serum HBV DNA. This study thus analysed patients who were negative for HABsAg or HBeAg, to provide evidences for diagnosing chronic hepatitis with unknown reasons.
Patients and Methods: A total of 22 patients were collected to detect HBV marker using HBV specific test kit. Biopsy was performed by ultrasonic guidance, followed by hematoxylin-eosin staining. Fluorescent quantitative PCR measured the copy number of HBV DNA from hepatic tissues and peripheral blood samples.
Results: 3 out of 22 cases (13.6%) showed positive HBV DNA in peripheral blood, while the positive rate of hepatic HBV DNA was 86.4%. In serum model positive for anti-HBs or anti-HBs+anti-HBc, pathological examination revealed no significant abnormality. In serum models of anti-HBs+anti-HBc +anti-HBe, anti-HBc or anti-HBc+anti-HBe, partial infiltration of inflammatory cells could be observed. The activity of hepatic tissues was the highest in hepatic tissues positive for anti-HBc, accompanied with higher fibrosis. A further correlation analysis revealed positive relationship between replication number of hepatic HBV DNA copy number and activity/fibrosis of hepatic tissues (r=0.56 or 0.34, p<0.05 in both cases).
Conclusion: In serum models with anti-HBc, anti-HBc plus anti-HBe, the activity and fibrosis degree of hepatic tissues were relatively higher, and were correlated with HBV DNA copy numbers.
Keywords
Antigen negative, HBV infection, HBV DNA.
Introduction
Hepatitis B Virus (HBV) is one of the most common viral infection diseases, as it affects more than 3.5 million people, and causes nearly 600 thousand deaths in one year [1,2]. During the progression of HBV infection, serum HBV surface antigen (HBsAg) and e-antigen (HBeAg) levels are important indexes reflecting viral replication and hepatic inflammation activity [3-5]. Under normal circumstance, the negative expression of HBsAg and HBeAg indicates disease improvement and suppressed viral replication. However, as further observation of clinical cases, some patients who were negative for HBeAg still presented as viremia or hepatic necrosis [6,7]. Therefore the diagnosis/treatment of HBeAg negative patients could improve the prognosis and life quality of hepatitis B patients.
Insidious hepatitis B refers to those patients who were negative for serum HBsAg, and consists of 10%~25% of all patients [6]. Previous study has revealed the potency for insidious hepatitis B to develop into chronic hepatitis, liver cirrhosis or even liver cancer [8]. Therefore HBsAg-negative infection should draw attention in patients with chronic liver disease having unclear reason [9]. The identification rate of serum HBV DNA is significant among all HBsAg negative patients. Due to relatively lower level of serum virus, minimal HBsAg might not be detected by traditional assays such as radioimmunology. The utilization of fluorescent quantitative PCR with higher sensitivity is thus critical for such patients.
In this study, fluorescent quantitative PCR was performed to detect peripheral and liver tissue levels of HBeAg and HBsAg, in an attempt to provide further evidences for diagnosis of chronic hepatitis patients with unknown reasons, and to provide more knowledge for early diagnosis of hepatitis.
Materials and Methods
Clinical samples
A total of 22 cases were recruited from both out-patient and inpatient clinics of Sichuan Mianyang 404 Hospital. There were 13 males (25~75 y old, average age=51.15 ± 23.12 y) and 9 females (47~78 y old, average age=58.22 ± 22.14 y).
Inclusive criteria: (1) Not received HBV vaccine; (2) No history of chronic hepatitis B; (3) Not received anti-viral treatment; (4) Negative for HBsAg and HBeAg but positive for serum core antibody for HBV (HBcAb) and/or HBeAb and/or HBsAb; (5) Serum enzymatic index for liver less or equalled to 2 ULN (80 U/L), and Total Bilirubin (TBIL) less or equal to 85.5 μmol/L.
Exclusive criteria: (1) Accompanied with other viral infection; (2) Alcohol abuse patients; (3) With auto-immune or metabolic liver disorders; (4) Abnormal liver development; (5) Complicated with major disorder (tumor, chronic kidney disease or diabetes); (6) Long term usage of liver-toxic drugs; (7) Using immune suppressant drugs; (8) Pregnant women.
The experimental protocol has been pre-approved by the ethical committee of Sichuan Mianyang 404 Hospital and written consents have been obtained from all patients and healthy volunteers.
Reagents
HBV marker test kit (Xinbo Bio, China); ZTLYB nucleic acid extraction kit (Tianlong Bio, China); Hematoxylin and eosin dyes (Beisuo Bio, China); Automatic biochemical analyzer (Beckman, US).
Serum HBV marker assay
The detection of HBV markers in peripheral blood was performed using HBV marker test kit following the manual instruction.
Liver tissue biopsy
Liver biopsy was performed under the guidance of colored ultrasound (indicator: serum TBIL<85.5 mmol/L, PTA>60%, excluding congestive liver lesion, hepatic echinococcosis, hepatic vascular disease, severe jaundice/ascites, infection on right pleura, worse general condition or severe anemia patients). Tissues collected were fixed in 4% formaldehyde, embedded in paraffin, and stained by hematoxylin-eosin. The morphology of tissues was examined by standards. The judgement of liver inflammation and fibrosis was deduced based on the standard stipulated by Chinese Medical Association (2000).
HBV DNA extraction and assay
Paraffin tissues sections were de-waxed, rinsed in ethanol to remove xylene, weighted and lysed. DNA was precipitated, filtered in column for binding. The column was rinsed to wash out, centrifuged and eluted to keep DNA at -20°C. HBV reaction mixture from the test kit was mixed for centrifugation. The reaction system consisted for 19.4 μl HBV reaction mixture and 0.6 μl Taq polymerase. Fluorescent quantitative PCR was performed under the following conditions: 95°C for 3 min, followed by 45 cycles each containing 94°C for 15 s and 60°C for 30 s.
Statistical method
SPSS16.0 software was used to analyse all collected data. Spearman was used for correlation analysis. The significant level was defined as 0.05.
Results
Serum levels of HBV markers
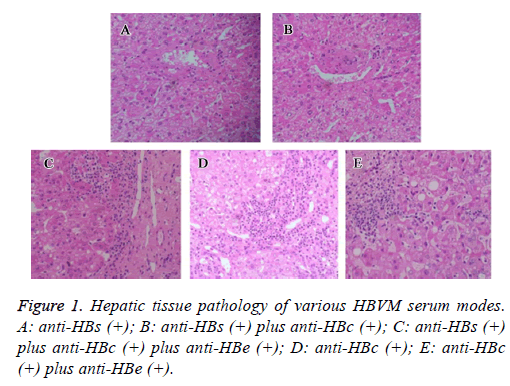
HBV surface markers were divided into 5 groups based on serum test results. As shown in Figure 1, in all HBV patients negative for HBsAg and HBeAg, there were 3 cases (13.6%) with positive peripheral HBV DNA, suggesting the existence of HBV replication even in some patients who were negative for HBeAg or HBsAg. In all 6 patients negative for HBsAg, no one showed HBV DNA existence (Table 1).
| HBVM serum mode | N | HBV DNA (+) | HBV DNA (+) rate |
|---|---|---|---|
| Anti-HBs | 6 | 0 | 0% |
| Anti-HBs+Anti-HBc | 7 | 2 | 28.60% |
| Anti-HBs+Anti-HBc + Anti-HBe | 2 | 0 | 0% |
| Anti-HBc | 5 | 1 | 20.00% |
| Anti-HBc+Anti-HBe | 2 | 0 | 0% |
Table 1. Peripheral HBV DNA expression in various serum modes of HBV patients.
From HBV DNA test results on hepatic tissues from biopsy, there were a total of 19 cases which were positive for HBV DNA replication (86.4%). Therefore there were still lots of HBV infected patients even with negative expression for HBsAg and HBeAg, which should draw attention in clinical diagnosis and treatment (Table 2).
| HBV serum mode | N | HBV DNA (+) | HBV DNA (+) rate |
|---|---|---|---|
| Anti-HBs | 6 | 5 | 83.30% |
| Anti-HBs+Anti-HBc | 7 | 6 | 85.70% |
| Anti-HBs+Anti-HBc+Anti-HBe | 2 | 2 | 100% |
| Anti-HBc | 5 | 4 | 80% |
| Anti-HBc+Anti-HBe | 2 | 2 | 100% |
Table 2. Analysis of hepatic tissue expression of HBV DNA across HBV infection modes.
Hepatic tissue pathology and HBVM serum modes
In hepatic tissues samples collected from patients with different HBV serum markers, hematoxylin-eosin staining was performed (Figure 1). In anti-HBs (+) or anti-HBs (+) plus anti-HBc (+) patients, no significant abnormality was observed. In tissues with anti-HBs (+) plus anti-HBc (+) plus anti-HBe (+), or anti-HBc (+), or anti-HBc (+) plus anti-HBe (+), infiltration of inflammatory cells were observed.
HBV serum modes and hepatic tissue fibrosis
In different HBV serum modes, we analysed the activity of hepatic tissues and fibrosis. Results showed the highest activity in anti-HBc (+) patients, who also had highest fibrosis grade (Table 3). A further correlation analysis revealed the positive relationship between HBV DNA replication and activity/ fibrosis of hepatic tissues (r=0.56 and 0.34, p<0.05 in both).
| HBV serum modes | Hepatic tissue activity | Fibrosis |
|---|---|---|
| Anti-HBs | 1.2 ± 0.3 | 0.2 ± 0.1 |
| Anti-HBs+Anti-HBc | 1.3 ± 0.2 | 0.4 ± 0.2 |
| Anti-HBs+Anti-HBc+Anti-HBe | 1.8 ± 0.4 | 2.4 ± 0.6 |
| Anti-HBc | 2.6 ± 0.2 | 2.6 ± 0.2 |
| Anti-HBc+Anti-HBe | 1.5 ± 0.2 | 1.5 ± 0.3 |
Table 3. HBV serum modes and fibrosis.
Discussion
Most studies so far have confirmed the chronic hepatitis B with HBeAg negative expression as one status of continuous HBV infection, as shown by re-activation of hepatitis and elevated viral replication in clinics [10]. Such phenomena might be caused by the mutation of anterior C domain and basic core start codon. Lots of studies showed that the mutant strain caused by mutation usually escape from host immune response, facilitating continuous replication of HBV. Moreover, the level of hepatic tissue inflammation is even severer in HBeAg-negative patients compared to HBeAgpositive individuals [11].
Results from this study showed the existence of positive results by HBV DNA replication quantification even in those patients with negative serum HBsAg or HBeAg expression. In a total of 22 patients, there were 3 of them (13.6%) with positive HBV DNA in peripheral blood, suggesting the existence of HBV infection and replication even in patients who were negative for HBsAg or HBeAg. Due to the limited sample size (N=22), no large-scale population study has been performed, making the current conclusion largely uncertain. Further assay for HBV DNA in hepatic tissues found that, although most HBV patients had no positive expression of HBsAg or HBeAg, in hepatic tissues, abundant HBV replication still existed in hepatic tissues. This sub-group of patients thus should draw more attention, making the routine assay for serum HBV surface markers necessary. However, due to the limited availability of hepatic tissues in clinics, serum HBV DNA assay can work as an assistant method for HBV diagnosis. The contradictory results that HBV infection but negative for HBsAg is mainly caused by lower serum HBsAg contents, which had higher lower limit in current assays. Most studies have found relatively lower HBV DNA in serum and lower viral copy number in hepatocytes in those patients who were positive for HBV DNA but negative for HBsAg [12-14]. Moreover, some studies found the negative correlation between HBsAg and age, indicating the possible suppression of HBsAg antibody level with aging [15].
Tissue pathological examination for patients with different HBV-surface markers found no significant abnormality in anti- HBs (+) or anti-HBs (+) plus anti-HBc (+) patients by hematoxylin-eosin staining, and partial inflammatory cell infiltration in tissues with anti-HBs (+) plus anti-HBc (+) plus anti-HBe (+), or anti-HBc (+), or anti-HBc (+) plus anti-HBe (+). Severer inflammatory response existed in the existence of anti-HBc. Some scholars calculated the sensitivity of anti-HBc and found its sensitivity as 22% in all 2505 research objects, as four-fold of the sensitivity of HBsAg [16]. Therefore it is speculated that during HBV infection diagnosis, HBsAg may not be the sole sensitive marker due to individualized difference.
We further analysed hepatic tissue activity and fibrosis across different HBV serum modes. Results showed the highest activity in hepatic tissues with anti-HBc, which also had higher fibrosis level. Further correlation analysis revealed the positive relationship between HBV DNA replication number or hepatic tissue activity with fibrosis level, suggesting that anti-HBc might work as one index for HBV replication. Other study revealed no existence of such correlation between HBV DNA and hepatic tissues activity no matter in HBsAg negative or positive patients [17]. Such inconsistency might be attributed to: (1) This study selected HBV patients with HBsAg and HBeAg; (2) This study selected Chinese population while the abovementioned study recruited Bangladesh people. Hepatic HBV DNA level is one important parameter measuring the treatment efficacy for HBsAg positive patients [5]. For HBsAg negative patients, however, no reliable evidence has been found so far. Other study also revealed the potency of HBV DNA in predicting hepatic fibrosis in HBV patients who were negative for HBeAg.
Another study revealed the existence of the correlation between HBV DNA and hepatic injury only in those patients with anterior C domain mutation [18]. Normally e-antigen may develop with the progression of chronic viral hepatitis. Therefore it is unreliable for differentiating between active hepatitis and non-active carriers solely based on the single value of HBV DNA [19,20]. The combination between clinical symptom, serum study and HBV DNA replication number should benefit effective diagnosis of viral hepatitis B.
Acknowledgments
This work was supported by the scientific research subject of Sichuan province health department in 2011 (NO.110560).
References
- Goldstein ST. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol 2005; 34: 1329-1339.
- Perz JF. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006; 45: 529-538.
- Su TH. Longitudinal change of HBsAg in HBeAg-negative patients with genotype B or C infection. PLoS One 2013; 8: 55916.
- Dow BC. Significance of tests for HBeAg and anti-HBe in HBsAg positive blood donors. J Clin Pathol 1980; 33: 1106-1109.
- Lu HY. Intrahepatic HBV DNA as a predictor of antivirus treatment efficacy in HBeAg-positive chronic hepatitis B patients. World J Gastroenterol 2007; 13: 2878-2882.
- Chan HL. Serum HBsAg quantification to predict response to peginterferon therapy of e antigen positive chronic hepatitis B. Aliment Pharmacol Ther 2010; 32: 1323-1331.
- Montazeri G. Serum hyaluronate as a non-invasive marker of hepatic fibrosis and inflammation in HBeAg-negative chronic hepatitis B. BMC Gastroenterol 2005; 5: 32.
- Kaviani MJ. Occult hepatitis B virus infection and cryptogenic chronic hepatitis in an area with intermediate prevalence of HBV infection. World J Gastroenterol 2006; 12: 5048-5050.
- Volz T. Impaired intrahepatic hepatitis B virus productivity contributes to low viremia in most HBeAg-negative patients. Gastroenterology 2007; 133: 843-852.
- Ganji A. Correlation between HBsAg quantitative assay results and HBV DNA levels in chronic HBV. Hepat Mon 2011; 11: 342-345.
- Chu CJ, Hussain M, Lok AS. Quantitative serum HBV DNA levels during different stages of chronic hepatitis B infection. Hepatology 2002; 36: 1408-1415.
- Conjeevaram HS, Lok AS. Occult hepatitis B virus infection: a hidden menace? Hepatology 2001; 34: 204-206.
- Marusawa H. Latent hepatitis B virus infection in healthy individuals with antibodies to hepatitis B core antigen. Hepatology 2000; 31: 488-495.
- Mason AL. Molecular basis for persistent hepatitis B virus infection in the liver after clearance of serum hepatitis B surface antigen. Hepatology 1998; 27: 1736-1742.
- Kim YJ. The change of the quantitative HBsAg level during the natural course of chronic hepatitis B. Liver Int 2011; 31: 817-823.
- Ramia S. Frequency and significance of antibodies against hepatitis B core (anti-HBc) antigen as the only serological marker for hepatitis B infection in Lebanese blood donors. Epidemiol Infect 2005; 133: 695-699.
- Alam S. Characteristics of treatment naive chronic hepatitis B in Bangladesh: younger populations are more affected; HBeAg-negatives are more advanced. Saudi J Gastroenterol 2008; 14: 15-19.
- Lindh M. Hepatitis B virus DNA levels, precore mutations, genotypes and histological activity in chronic hepatitis B. J Viral Hepat 2000; 7: 258-267.
- Manesis EK, Papatheodoridis GV, Hadziyannis SJ. Serum HBV-DNA levels in inactive hepatitis B virus carriers. Gastroenterology 2002; 122: 2092-2093.
- Lee CZ. Correlation of HBV DNA levels in serum and liver of chronic hepatitis B patients with cirrhosis. Liver 2002; 22: 130-135.