Research Article - International Journal of Pure and Applied Zoology (2022) Volume 10, Issue 8
THE FUNCTION OF PECTEN OCULI. CONUS PAPILLARIS IN REPTILES AND ITS ANALOGUE PECTEN OCULI IN BIRDS EVOLVED IN TANDEM WITH INCREASING URIC ACID IN SERUM
Amund Ringvold*
Eye Department, Oslo University Hospital, Oslo, Norway
- Corresponding Author:
- Amund Ringvold
Eye Department, Oslo University Hospital
Oslo, Norway
E-mail: a.d.ringvold@medisin.uio.no
Received: 07-July-2022, Manuscript No. IJPAZ-22-68793; Editor assigned: 08-July-2022, PreQC No. IJPAZ-22-68793(PQ); Reviewed: 22-July-2022, QC No. IJPAZ-22-68793; Revised: 26-July-2022, Manuscript No. IJPAZ-22-68793(R); Published: 02-August-2022, DOI: 10.35841/2320-9585-10.8.136
Abstract
The aim of this work is to identify the function of conus papillaris (CP) in reptiles and its analogue pecten oculi (PO) in birds. The study is based exclusively on information available in literature.
Currently the two apparently incompatible hypotheses at the forefront are: PO provides nutrition to the retina, or, alternatively, PO regulates intraocular pH to counter anaerobic retinal metabolism.
The present survey demonstrates that the development of CP occurred while reptiles evolved from aquatic to terrestrial life, and nitrogen waste excretion in urine changed from being predominantly ammoniotelic to mainly uricotelic during the same period of evolution. Accordingly, a correlation in time exists between the development of CP and the gradual alteration in protein catabolism. Furthermore, both the serum uric acid (UA) and the vascular permeability of PO increased during this period. Assuming that the retina is nourished through trans-vitrial diffusion from PO, a consequence could be that the heavily soluble UA leaks into the vitreous body compromising light transparency of the eye media.
Conclusion/new hypothesis: The ocular impact of increasing serum UA is minimized through three documented mechanisms: the well-known removal of UA via the trabecular meshwork, along with the here established selective blood-ocular barrier to UA, and the improved UA solubility through intraocular alkalization by PO's carbonic anhydrase.
Keywords
Birds, Pecten Oculi, Avian Eye, Reptiles, Conus Papillaris, Evolution, Uric Acid.
Introduction
According to Wingstrand and Munk, the first description of PO in the bird’s fundus was, in all likelihood, presented by the Danish scientist Olaus Borrichius in 1674, although he may have been preceded by Peiresc in 1641 [1]. Ever since these reports some 350 years ago, this unique structure has been one of the enigmas in comparative ophthalmology. The term pecten means comb, and so PO is a comb-like, pigmented tissue projecting
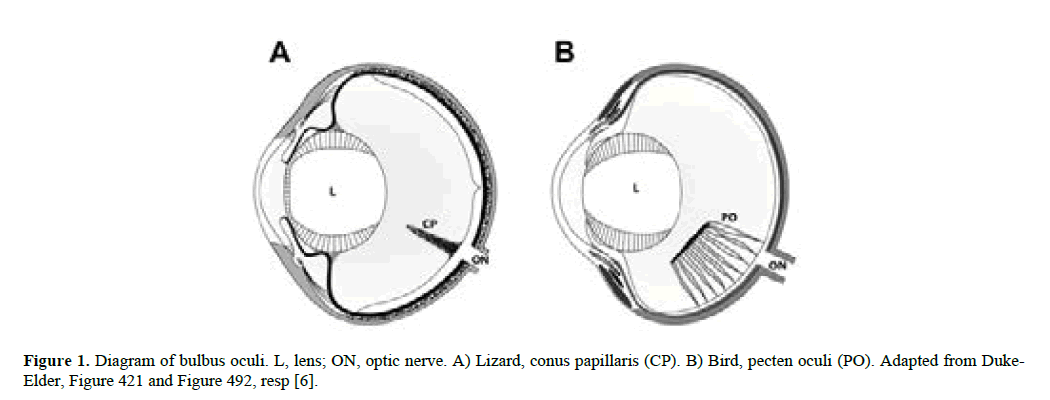
Figure 1: Diagram of bulbus oculi. L, lens; ON, optic nerve. A) Lizard, conus papillaris (CP). B) Bird, pecten oculi (PO). Adapted from Duke- Elder, Figure 421 and Figure 492, resp [6].
While Petit pointed out that its localization is such to interfere as little as possible with retinal function, more than thirty theories have been advanced to explain its function, many of them agree that the main role of PO is to assist in the nutrition of retina and the inner eye in general [1-10]. As PO is the only vascular tissue in the posterior avian eye, it could compensate for lacking an intra-retinal blood circulation [11]. In addition, ablation experiments (applying retro bulbar surgical cautery of arteries supplying PO) are said to support this interpretation. It has also been argued that the observed correlation between PO’s functional morphology and lifestyle of the bird lends support to the nutritive role of PO [12].
However, the nutritional hypothesis is controversial, and it has been predicted that PO may have specialized functions in addition to nutrition, for instance neutralizing any sudden changes in the intraocular pressure, or that PO has both an optical as well as a trophic function [13,14]. A striking feature of PO is not only the dominating location at the posterior part of the bulbus, but also its high content of alkaline phosphatase and carbonic anhydrase [15-18]. The latter indicates that the function of PO could be to regulate the carbon dioxide pressure in the avian eye.
An interesting objection to the view that PO is needed for retinal nutrition has been presented by Brach [19]. He argued that, due to methodological shortcomings, Wingstrand and Monk's results may have been caused by compromise to the circulation of the optic nerve head itself by use of retrobulbar cautery. Therefore, he performed intrabulbar ablation of pecten in chicken with modified bipolar electrocautery forceps. It transpired that removal of PO changed pH in the vitreous cavity from 7.56 to 7.35 [19]. Given this refers to a logarithmic scale (10 logarithm), the difference is significant. Thus, he “suggested that the function of pecten is not primarily nutritive and may instead be related to intraocular pH regulation". More specifically, he speculated that PO is needed to prevent intraocular acidification due to an anaerobic glycolytic metabolism in the avascular bird retina, an interpretation which could explain why PO is extraordinary rich in carbonic anhydrase [20].
The bird’s PO varies considerably in form (conical, vaned, pleated) and size [21]. In species with small PO, like the owl (Bubo virginianus), it projects out into the vitreous cavity 5-6 mm, whereas in the dove (Leucosarcia picata) it reaches almost as far anteriorly as the equatorial lens [21,22]. It has been advocated that there is a correlation between a bird’s behaviour towards light and activity levels and pectin's size and number of folds [11,23,24].
In short, several interesting hypotheses on the function of PO have been presented over the previous centuries, and at present, the following two, evidence-based options, seem to be at the forefront: Firstly, that PO is the primary source of nutrition and oxygen to the inner retina, and secondly, that it may acts as a pH-regulator in the vitreous body preventing intraocular acidification from anaerobic retinal glycolysis [1,19]. Both of these hypotheses address important qualities of PO, but neither explain all observations. This poses the first question in this study: Are these two functions exclusive or can they be unified?
In addition, it has been reported that CP, an anatomical structure analogue to PO in birds, is also found in some reptiles [25]. In case CP and PO are equivalent structures functionally, CP may reveal the initial steps in the cascade of events leading towards the development of PO in birds. It should be underlined that, according to our present knowledge, birds are not the direct successors of reptiles in the process of evolution. However, the two classes of animals are somehow linked to each other, and so the various steps in the development of reptiles’ CP might have some bearing on the more elaborate structure of PO in birds. Thus, the second question in the present study is how CP and PO relate to each other. The evaluation of these two issues has been based exclusively on information available in the literature.
Material and Method
A literature review was performed, collecting information on CP and PO from various digital databases (PubMed, Medline, Ovid, Scopus, Web of Science), in some cases also from old library collections still not digitalized. The focus has been on reptiles, but relevant information on amphibians, birds, and mammals has also been included.
From the large number of studies on this subject over the centuries, it is noteworthy that no single bird species, diurnal or nocturnal, has been identified that doesn’t have PO. Thus, the present survey is based on the assumption that PO is present in all bird species, whereas fish, amphibians, and mammals are devoid of it. The fact that CP, a tissue element similar to PO, is found on the optic disc in some reptile species, indicates that this “comb-like structure” is not unique to the bird eye. Based on macroscopic as well as light microscopic observations many authors have considered CP in reptiles analogous to the avian PO, and this idea is also supported by the fact that CP and PO are similar at the electron microscopic level [14,25-28]. Accordingly, both morphological and biochemical aspects as well as functionality of CP and PO could be examined together.
Morphology: The reason why CP is present in some, but not all reptile species, is unknown [11]. However, the behaviour of reptiles is clearly influenced by the environment in which they live, and so it is interesting to evaluate the relationship between habitat and anatomical development of CP in the various species.
Biochemistry: During evolution, when animals were migrating from water to land, specific challenges had to be overcome to ensure adequate oxygen supply, temperature regulation, and osmotic control in the body. These were “instant” demands for survival out of water, and so adequate adaptations had to be present at the amphibian stage. Accordingly, evolution-induced changes in any of these vital functions do not represent a timely good match with the occurrence of CP in reptiles. Intuitively, however, the development of CP should be synchronized with the gradual changes in protein and purine catabolism. Accordingly, the present study is designed to examine how these two phenomena correlate to each other in time.
Functionality: The interplay between morphological and biochemical observations will be highlighted searching for a unifying hypothesis on CP/PO’s significance. The validity of this hypothesis will be evaluated both separately, and versus the two prevailing hypotheses (retinal nutrition and intraocular pH regulation).
Results
The class: Reptilia, has been evaluated according to the classification in Halliday and Adler (2002), indicating that there are four living groups [29].
Testudines (turtles and tortoises).
Squamata (lizards: geckos, chameleons, etc., snakes).
Rhynchocephalia (tuatara).
Crocodylia (crocodilians: crocodile, alligator, caiman, gharial).
These animals have been extensively studied for decades, and so a significant amount of information is available in the literature on 1) the morphology of the optic disc area, and 2) on the biochemistry of nitrogen metabolism [29]. However, some detail is lacking when evaluating for a unifying functional hypothesis:
Morphology: According to Johnson, “No trace of a pecten can be found in any Amphibian eye” [30]. He added that this class of animals exhibit an exceptionally well-developed vascular system (membrana vasculosa retinae) in the hyaloid membrane in front of the retina. This differs from reptiles both according to ophthalmoscopical examinations, and comprehensive histological surveys [4,6,11,30].
Regarding CP in aquatic reptiles, it has been shown that in crocodilians “the disc is circular, quite white, and covered with brownish pigment, almost black in C. frontatus [30]. This pigment covering the disc resembles an abortive attempt at a pecten and is entirely functionless”, whereas in chelonian it looks more as “an irregular brownish patch in the centre of the disc". According to Walls, there is no CP in diurnal turtles [11]. It should be added though that so far very few studies have been conducted among the roughly 350 turtle species. Unfortunately, the distinction between aquatic (turtle) and terrestrial (tortoise) chelonians concerning presence or not of CP is incomplete in the literature. However, the following statement indicates that differences may exist in this group: “If provided with a pecten, it is either a vestigial relic, or of the simplest possible type, i.e., a simple unconvoluted cone or thalamus” [30]. In short, despite a paucity of specific information, it seems that faint signs of a CP are present in aquatic reptiles, at least in some species.
On the other hand, terrestrial reptiles (lizards, geckos, chameleons, iguanids, varanoids) reveal a distinct CP, though varying considerably in size between species [4,6,11,30]. Even the shape of CP varies from one species to the next, from a small, vascularised tissue covering the nerve head in geckos, to a fane-like body in some iguanids imitating a bird’s PO.
According to Duke-Elder, it is likely that the CP came into being when the preretinal vascular system present in amphibians was collected into a solitary vascularised tissue protruding into the vitreous body from the optic disc area [6]. This view made him conclude that reptiles in general have an avascular retina. However, this is not entirely true, because there are two groups of reptiles with mainly terrestrial behaviour, though without CP (snakes and tuatara).
Snakes (ophidians): Several authors have noticed the remarkable evolution of snakes’ eye. In one early report it is concluded that “in these species the retina is supplied from the superficial hyaloid vessels or directly by vessels entering the retina” [30]. However, he added (without separating between aquatic and terrestrial snakes) that a minority of species showed “an abortive pecten-like growth occupying the central third of the disc". Similarly, Franz stated that a small CP may be seen in some snakes [4]. This view was supplemented by the observation that in snakes the CP has been exchanged for a vitreo-vessel system in immediate contact with the retina, as seen in amphibians [11]. Furthermore, Halliday and Adler pointed out that, “snake’s evolutionary history, which included a long spell as burrowing animals, has been the driving force behind some of the unique ways they have of sensing the world around them [29]. Sight became largely superfluous when they lived underground, and their eyes therefore became redundant. More advanced species, which made the move back to the surface, needed to reinvent the eye or a satisfactory substitute, leading to fundamental differences between their eyes and other animals’ visual organs”. And finally, in a more specified version by Schwab, “most snakes rely on the choroid for retinal nutrition with diffusion, but some snakes have a network of vessels spreading across the retinal surface much like that seen in fish” [26]. In short, retinal nutrition in snakes is apparently ensured through vessels on or inside the retinal layer, may be even from the choroid.
Tuatara (sphenodon punctatus): Tuatara is another terrestrial reptile without CP. This is a unique cryophilic animal endemic to New Zealand. It is active at body temperature as low as 5.2°C [29,31]. As Russell and Zoo put it: “The tuatara brain and mode of locomotion resemble that of amphibians and the heart is more primitive than all other reptiles [32]. The eye is specialized with a “duplex retina” that contains two types of visual cells for vision by both day and night”. Tuataras are also unusual in having a pronounced third eye (parietal eye), along with special features in the skeleton, evolutionarily retained from fish. These reptiles are considered the most unspecialized living amniote, older than squamates, and often called a “living fossil” [33]. As judged ophthalmoscopically, “there is no trace of a pecten, or blood vessels either retinal or choroidal, but the commencement of a vascular system in the small network of capillary vessels which are restricted to and cover the disc more or less completely, which usually takes the form of a capillary network” [30]. However, choroidal vessels are seen in light microscopic sections (Schwab, Figure 15.8) [26]. In short, in tuatara retina seems to be nourished mainly through diffusion from the choroidal circulation.
Biochemistry: As the reptiles moved from aquatic to terrestrial life, profound biochemical adaptations occurred, one of them in protein metabolism. In general, nitrogenous waste products are ammonia, urea, and UA, and animals excreting mainly one of these substances are termed ammoniotelic, ureotelic, or uricotelic, respectively. Which of these wastes an animal excretes is determined by the environment in which it lives, a statement made by Delaunay, and amply confirmed and extended by other authors [34]. As Cragg put it: “It should be noted that ammoniotelism is restricted to completely aquatic Amphibia [35]. Species such as Rana esculenta and Triturus cristatus, which may spend much of their time in water even outside the breeding season, produce mainly urea. In other words, in Amphibia which live partly in and partly out of water, nitrogen excretion is fitted for the terrestrial mode of life”. Interestingly, this shift takes also place during amphibian development, as nitrogen waste being mainly ammonia in the aquatic larva, changing to more urea in the terrestrial adult [36].
Significant variation has been reported, and diverging conclusions have been drawn concerning nitrogen waste excretion in reptiles, partly because urine precipitates in the bladder sometimes have been neglected. Furthermore, the type of nitrogen waste excreted is to some extent influenced by the state of hydration/dehydration of the single species [37-39]. Aquatic reptiles like crocodylia excrete mainly ammonia, or they are ammonio-uricotelic, compared with terrestrial species like lacertilians (geckos, chameleons, lizards) being solely uricotelic [35,40-44]. A similar difference is seen in chelonian reptiles, with aquatic species (turtles) excreting roughly equal amounts of ammonia and urea, with terrestrial species (tortoises) being more uricotelic, or ureo-uricotelic [38,45-47]. Moyle, analyzing urines of various species of chelonian reptiles living in different habitats, showed that those living in drier environments tended to secrete more UA [46]. There seemed to be a grading of nitrogen excretion from ureotelic aquatic forms to entirely uricotelic land-dwellers, some species regulate the percentage of UA and ammonia in the urine in response to the degree of dehydration [39,48]. In general, it should be kept in mind that many of the nitrogen waste excretion values presented in the literature refer to urine analysis only, without any correlation to serum values.
As to the “aberrant” species (snakes and tuatara), i.e. terrestrial reptiles without CP, both the oviparous (Zamensis diadema) and the viviparous snakes (Eryx thebaicus) excrete nitrogen waste mainly as UA (60-70% of total) with a small portion of ammonia [49,50]. The tuatara (sphenodon punctatus) excretes a semi-solid deposit that is almost pure UA. In addition, and similarly to snakes, it eliminates a liquid portion containing urea (80% of total nitrogen), and ammonia (10%) [51].
Summary of Main Results
Morphological observations: The bulk of information indicates that the development of CP occurred simultaneously with the reptiles migrating from aquatic to terrestrial life, i.e. aquatic species may show pre-stages of CP against the distinct forms of CP observed in terrestrial species (Table 1). However, it should be noted that tuatara and most snakes are terrestrial species without CP and thus don’t follow this pattern (see comment below). Biochemical observations: As reptiles migrated from aquatic to terrestrial habitat, there was a change in nitrogen waste excretion from mainly ammoniotelic to mainly urico-(urea-) telic modes (Table 1), in line with Delaunay’s conclusions [34].
Discussion
Based on this survey, the following interpretations are valid:
Without specifying which, Virchow showed that CP is found in only some reptile species [25]. The present survey takes these observations one step further by identifying reptiles with CP are terrestrial species (Table 1), i.e. the evolution of CP is linked to the terrestrial way of life. This development took place during the Mesozoic Era (i.e. “the Age of Reptiles”, 251-66 million years ago).
The development of CP and the change in nitrogen metabolism occurred in tandem as the reptiles came ashore: a) Preretinal vessels in amphibians were slowly collected into CP in the optic disc area (Table 1). b) Nitrogen waste excretion changed from ammoniotelism towards mainly uricotelism (Table 1). The gradual change in nitrogen waste excretion during evolution included a parallel increase in serum UA content as well, though with significant intra and interspecies variations (Table 2). The serum UA also vary considerably both according to food quantity/quality, and lag time between feeding and sampling [52,53].
Functional aspects of CP/PO: The overall trend seems to be that aquatic animals (fish and amphibians) are ammoniotelic, reptiles represent transitional forms (ammoniotelic – uricotelic – ureotelic), whereas birds and mammals are mainly uricotelic and ureotelic, respectively. With increasing serum UA content as evolution continued, this heavily soluble compound could have leaked into the intraocular compartments and eventually threatened light transparency of the ocular media. To test the validity of this postulate, a key question is whether previous observations on morphology, biochemistry, and functional aspects fit in a unifying hypothesis to explain why CP/PO is needed. Thus, the consequences of increasing UA serum level will be evaluated by indicating how the progression of life from water to land called for significant adaptations both in a) the body, and b) the eye.
Systemic affection: When analyzing the impact of exchanging ammonia in amphibia for UA/(urea) in reptile/bird serum, it is important to keep in mind the difference in chemical properties of the three compounds: Ammonia is a very toxic substance to tissues, though extremely soluble in water [54]. Urea is less toxic compared to ammonia, with good water solubility, i.e. moderate amounts of water is needed for its excretion. Finally, the low solubility and toxicity of UA make nitrogen waste excretion possible with limited water loss. This is an advantage for flying birds to reduce weight, and additionally, they thus don’t require a urinary bladder [55].
Fluctuating UA serum values (uricemia, hyperuricemia) is known from various pathophysiological conditions, and it is noteworthy that the systemic impact of these variations is still an enigma, illustrated through the body’s unpredictable reaction to it. On the one hand, increased serum UA content may cause acute gouty arthritis both in reptiles, birds, and mammals [56-58]. It is known from human pathophysiology that when the serum UA level exceeds 6.05 mg/dL (360 μmol/L), defined as upper normal limit, monosodium urate crystals may be formed in synovial tissues [59,60]. However, as a protective measure minimizing the risk for intravascular crystallization, some 30% of serum UA is transported through a weak urate-albumin complex promoting solubilisation [61]. UA does not reach a much higher blood level in reptiles than in mammals, and 6.05 mg/dL is therefore likely to represent the upper limit for UA solubility in serum for all three animal classes [62]. Paradoxically, however, hyperuricemia does not always promote goat, a phenomenon shown in Table 2 where some species, in good health, have serum UA values exceeding 6.05 mg/dl [61]. Which factors predispose some hyperuricemic individuals and not others to develop goat is still unknown. This makes us realize that hyperuricemia occurring intermittently as part of normal fluctuations, do not always induce damaging consequences systemically. Nevertheless, hyperuricemia could pose challenges to the eye.
Ocular effects: Before evaluating the impact of increasing serum UA concentration on the eye during evolution, it is imperative to clarify ocular blood flow and blood-ocular barriers in general. In all vertebrates, blood enters the eye through the choroidal circulation. These fenestrated vessels are separated from the sensory retina by the retinal pigment epithelium, a monolayer of cells firmly connected through intercellular junctions (zonulae occludentes). In addition, species with intraretinal circulation (most mammals), show a similar barrier in the non-fenestrated endothelial vessel wall, whereas in species with avascular retinas, like birds, the blood-vitreous barrier is localized in PO’s vascular system in the posterior segment of the eye. Finally, there is also a blood-aqueous barrier in the anterior eye segment, i.e. the epithelium covering the ciliary body. Hence, large serum components like proteins are largely excluded from the intraocular compartments (vitreous body and aqueous humour) through cell barriers at four levels: retinal pigment epithelium, retinal capillary endothelium, capillary walls in PO, and the ciliary epithelium. Even though this combination has not been verified in every species, it is likely to apply to vertebrates in general, preventing blood cells and serum proteins from interfering with the light pathway in the eye.
On the other hand, the quality and function of blood-ocular barriers to small serum components are still not well understood [63-65]. Serum components smaller than proteins have obviously easier access to the intraocular compartments than the larger once, demonstrated by the observed oxygen gradient between PO and retina, the fact that fluorescein (mol w 374) readily leaks from PO into the vitreous cavity, and from the ciliary body into the aqueous humor, with systemic application [1,66-68]. (Observations on the permeability of CP are not available). As UA is an even smaller molecule (168 mol w), it is suggested that significant amounts of serum UA could leak into the eye. Bearing in mind the low solubility of UA, and the lack of albumin binding capacity in the vitreous body, this could imply intraocular UA precipitation, like hyperuricemia inducing mono urate crystals in acute gouty arthritis. One could hypothesize that at least three different mechanisms meet this challenge: Blood-ocular barriers, intraocular pH-regulation, and drainage of intraocular UA.
Blood-ocular barriers: Even though small serum components like nutrients, oxygen, and fluorescein have access to the vitreous body, this does not necessarily apply to small molecules in general. This is illustrated in the chicken, one of the most examined species. Pooled values from chicken serum (Table 2) showed UA content of 4.85 mg/dL (289μmol/L), 6.12 mg/dL (364μmol/L), and 4.92 mg/dL (293μmol/L), against 0.47 mg/dL (28μmol/L) in the vitreous body, and 2.19 mg/dL (130μmol/L) in the aqueous humour [69-72]. These concentration gradients indicate that there is indeed a selective blood-ocular barrier for UA in chicken, with a serum: aqueous: vitreous ratio of 1:0.4:0.1. The blood-vitreous UA barrier may be even higher than indicated by these figures because some UA from the aqueous humour may diffuse into the vitreous body. Unfortunately, the level of UA concentration in the vitreous body of other bird species was not available in literature, but values from the anterior eye segment show that the serum: aqueous UA ratio was above 1 in an additional five bird species [71]. The higher UA content in the anterior chamber compared to the vitreous body make sense because this substance is likely to be one of the UV-B absorbing components in the aqueous humour of birds [73]. In short, the intraocular UA level is minimized through selective blood-ocular barriers.
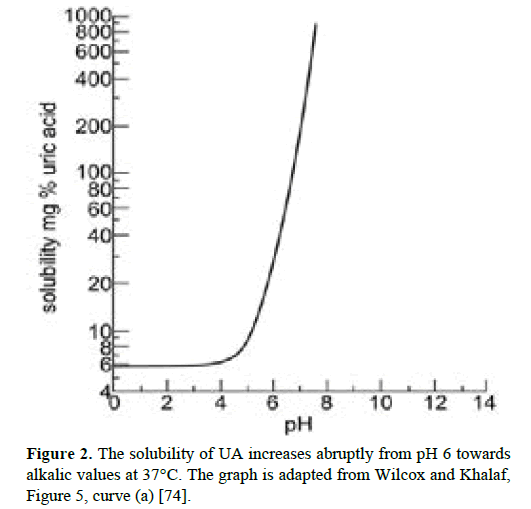
Intraocular pH-regulation: The solubility of UA and monosodium urate depend on pH, sodium ion concentration, and ionic strength. According to Wilcox and Khalaf, focusing on human pathophysiological conditions, there has been some confusion as to the solubility of nitrogen wastes in blood and urine, partly due to the fact that “the terms ‘uric acid’ and ‘urate’ are often used interchangeably without regard to the crystalline phase actually present” [74]. It has been shown that the low solubility of UA at sub-physiological pH is abruptly and strongly increasing upwards from pH 6 [74,75]. Thus, in the alkaline range, UA dissolves to levels far exceeding values obtained at pH 6 and below (Figure 2).
Figure 2: The solubility of UA increases abruptly from pH 6 towards alkalic values at 37°C. The graph is adapted from Wilcox and Khalaf, Figure 5, curve (a) [74].
In this connection it is noteworthy that PO contains significant amounts of carbonic anhydrase ensuring an alkaline intraocular environment [17,76]. This effect has been demonstrated experimentally in chicken where demolition of PO caused a pHdrop in the vitreous body [19]. Accordingly, by alkalizing the vitreous body through carbonic anhydrase, PO increases UA’s solubility and counteracts intraocular precipitation.
Regarding the low UA concentration observed in chicken vitreous (0.47 mg/dL), one may ask why this pH-regulation is needed [72]. To evaluate this, two different aspects should be considered: 1) In serum, UA is partly stabilized through a weak interaction with albumin [61]. However, the vitreous body is a protein-free area, and so UA leaking into this compartment will stay unbound. Drainage of intraocular UA: 2) The UA concentration in chicken vitreous is likely to vary over time in response to fluctuations in blood showing significant variations in response to feeding or fasting. In one report, “plasma uric acid concentrations were increased two to four fold in chicks fed the high protein diets”, and in another, “fasting for an extended period caused marked increases in plasma uric acid concentration; 72 and 240 hours after beginning the fast the plasma uric acid concentration reached approximately 10 and 40 times the initial concentration, respectively” [77,78]. A further illustration of UA serum fluctuations is the annual changes in UA content of the emperor penguin (2.5-24 mg/dL) (Table 2). Similarly, reptiles also show remarkable changes in serum UA content in response to feeding, although it is rather difficult to establish reference intervals for UA in chelonians and other ectotherms because they are capable of profound physiologic adaptation to different habitats, seasons, and environmental changes [53,79]. Thus, due to significant UA fluctuations in blood both in birds and reptiles, one may expect varying amounts of unbound uric acid in the vitreous body despite the selective blood-ocular barriers to UA. This could lead to intraocular UA precipitation unless PO increased its solubility through alkalization of the environment in the area.
Compounds in fluids may be moved through two different processes: diffusion and bulk flow. The vitreous body is composed of a hyaluronic acid gel largely restricting bulk flow. Once inside the vitreous body, UA spreads slowly through diffusion, most likely propelled through the bird’s saccadic bulbar oscillations shown by fluorescein [80]. The drainage of UA from this gel structure is suggested to be a slow process, and the concentration gradient in chicken from aqueous humour (2.19 mg/dL) to vitreous (0.47 mg/dL) indicates that vitreous UA does not leave the eye via the anterior chamber angle, but possibly does so via the choriocapillaris outside the retina.
In contrast, UA in the aqueous humour is drained by bulk flow through the trabecular meshwork. In the human eye, the aqueous humour volume makes up roughly 0,3 mL, i.e. by a steady inflow of some 2 μL/min there would be a total exchange of fluid approximately every 2 hours [81]. These numbers may roughly apply also to bird eyes, (dependent on the eye size of the individual species), and anterior drainage will help maintain a low intraocular UA level.
In short, intraocular UA precipitation is counteracted through selective cell barriers reducing the intraocular UA level, increased UA solubility intraocularly through carbonic anhydrase induced alkalization by PO, and anterior drainage of UA.
Evaluation of the Hypothesis
1) The present review indicates that CP/PO is a crucial structure in the prevention of intraocular UA precipitation. Thus, two aspects should be focused upon when testing the validity of this hypothesis: a) Do we know of any ammoniotelic/ureotelic species with CP/PO? Extensive examination of available literature indicates that the answer is, no. b) Do we know of any mainly uricotelic species without CP/PO? The answer is, yes: Snakes and tuatara.
Most snakes are terrestrial reptiles, and their evolution may have been delayed due to what has been called a “long spell as burrowing animals” [29]. Instead of CP, snakes have intraretinal vessels or vitreous vessels located on the inner retinal surface, resembling some aquatic reptiles [6,11]. Nitrogen waste excretion in these species is mainly as UA (60-70%), with some ammonia [49,50].
Similarly, tuatara is a terrestrial reptile without CP, excreting a mixture of pure, semi-solid UA, urea, and ammonia [51]. However, this is an archaic creature, older than lizards or snakes, without retinal blood vessels, though with a small capillary network on the optic disc and, in addition, choroidal vessels externally.
Conclusion: Tuatara and most snakes behave as terrestrial reptiles. They are lacking CP because the ocular circulation appears to be at an evolutionary premature stage on the pathway towards CP. Their retina does not rely on trans-vitreal nutrition, and measures to prevent vitreal precipitation of UA is apparently not needed. Thus, the aberrant pattern of snakes and tuatara does not seem to contradict the hypothesis.
2). The level of serum UA is different in various animal classes. Terrestrial reptiles and birds have rather high serum concentration of UA (Table 2), excreting it in the urine as a waste product. In contrast, most mammals have low serum UA values, and excrete urea (Tables 1 and 2). Surprisingly, higher primates make up an exception in this pattern, due to a mutation induced loss of uricase in Miocene, 24-5.3 million years ago [82]. Roughly 90% of UA filtered by their kidneys is reabsorbed instead of being excreted [83]. Consequently, higher primates have significant amounts of UA in serum (Table 2) and low UA excretion. It has been suggested that the increased serum UA content in these species may represent an evolutionary advantage, because this “useful waste product” could act as a powerful antioxidant [84,85]. Interestingly, however, it seems that high UA serum concentration may have posed a challenge to the eye in higher primates, because there is indeed a selective blood aqueous barrier to UA in the human ciliary body [86].
| Classes | Habitat | Morphology (blood circulation) |
Biochemistry (nitrogen excretion) |
|---|---|---|---|
| Amphibians | Water/land | Preretinal vessels, no CP [29] | Ammonia (urea) [35] |
| Aquatic reptiles | Water/land | Faint signs of CP on the optic disc in some species [11,29] | Ammonia/UA [35,40-43,45] |
| Terrestrial reptiles | Land | CP of varying size, (2 exceptions, see text) [4,6,11,29] | UA/urea [38,44,46,47] |
| Birds | Land/air | PO in all species (varying size and form) [9,11,55] | UA [91] |
| Mammals (incl. aquatic species, and bats) |
Water/land/air | No PO [26] | Mainly urea [83] (Guanine/allantoin) |
Table 1. The gradual transformation (morphology) of the preretinal vessels in amphibians into CP/PO in reptiles/birds, and the change in nitrogen waste excretion (biochemistry) from ammoniotelism to uricotelism, occurred simultaneously as the amphibians went on shore and subsequently evolved into birds.
| Classes | Species | Serum UA (mg/dL) |
|---|---|---|
| Amphibians | Rana catesbeiana Xenopus laevis Xenopus laevid Rana temporaria Rana argentina |
0.06 [92] 0.2 [93] 0.2 [94] 0.3 [71] 1.34 [95] |
| Aquatic reptiles | Dermochelys coriacea (Saltwater turtle) Crocodylus johnstoni Crocodylus niloticus Aligator mississipiensis Dermatemys mawii (River turtle) Crocodylus siamensis Crocodylus palustris Crocodylus porosus (Saltwater crocodile) |
0.3-0.6 [96] 0.45-1.9 [97] 0.67-5.04 [98] 0.96-4.23 [41] 1.5-6.2 [99] 1.9-5.0 [71] 2.4-9.9 [100] 2.8-16.6 [101] |
| Terrestrial reptiles | Iguana iguana Testudo marginata Gopherus agassizii (Desert tortoise) Gopherus agassizzi Chamaeleo chamaeleon |
1.5 [102] 2.6 [79] 3.5-5.0 [103] 4.8-5.4 [104] 10-10.7 [105] |
| Birds | Emperor penguin Turkey Gallus gallus (Lomann) Galliformes (6 dif. Spec.) Goose Chicken (42 days) African penguins (Spheniscus demersus) Duck Ostrich Hawk |
2.5-24.0 [106] 4.2-4.4 [71] 4.3-5.6 [71] 4.85 [69] 4.9-6.3 [71] 5.5-6.5 [70] 6.6 [107] 7.2-7.7 [71] 7.6-10.0 [71] 14.2 [71] |
| Mammals | Feline Canine White rabbit (New Zeal.) Horse Fin whale (Balaenoptera physalus) Rat (Sprague-Dawley) Cattle Seal (Erignathus barbatus barbatus) Primates: Old world monkeys: Macaca mulatta Macaca mulatta Macaca irus Macaca arctoides Papio cynocephalus New world monkeys: Saguinus Oedipus Cebus albifrons Cebus apella Saimiri sciureus Lagothrix lagotricha Human primates: Chimpanzees, adult (Pan troglodytes) Gorilla (Gorilla gorilla) Orangutan, juvenile (Pongo pygmaeus morio) Human: |
0-0.5 [108] 0.2-0.9 [108] 0.48 [109] 0.10-0.60 [110] 0.50 [111] 0.5-1.4 [112] 0.89-0.93 [113] 1.6 [114] 0.09 [115] 0.3 [116] 0.5 [116] 0.3 [116] 0.4 [116] 2.1 [116] 3.3 [116] 2.8 [116] 0.5 [116] 3.1 [116] f 0.7-3.5 m 1.5-3.3 [117] f 1.8-3.0 m 1.6-3.4 [118] f 3.4-9.3 [119] f 2.0-6.0 m 2.0-7.0 [120] |
Table 2. (serum UA concentrations): In parallel with the gradual change in nitrogen waste excretion, shown in table 1, there was an increasing UA content in serum during evolution from amphibians to birds. Primate values are included for comparison.
How does the new hypothesis relate to the previous observations on CP/PO, and what is the relationship between the new and previous hypotheses?
Previous observations:
• The morphology of CP and PO is similar, i.e. these organs contain a capillary network with endothelial cells showing numerous microvilli on both the luminal and basal side [27,28]. The vessels are surrounded by a marked, basement membrane-like sheath, and pigment cells containing melanosomes are seen in the interstitial tissue particularly in the apex of the organ [87].
• PO readily leaks fluorescein, in contrast to mammal retinal vessels [66,67]. Fenestrated endothelium is found in neither CP/PO nor in mammal retinal vessels. The permeability of intraocular vessels in reptiles is unknown.
• Oxygen does diffuse from the PO to the retina. Thus, PO is a substitute for intraretinal vessels supplying the inner layers of the retina [1].
• Significant amounts of alkaline phosphatase and carbonic anhydrase are present in PO [15-18]. These are both polyfunctional enzymes present ubiquitously in the body, in PO even co-localized in the endothelial microvilli [76,88]. Alkaline phosphatase has optimal activity at alkaline pH environments. Thus, the alkaline environment created in the vitreous body by the carbonic anhydrase in PO is favourable both for the alkaline phosphatase activity and the solubility of UA [19]. In addition, the activity of alkaline phosphatase in PO decreases 25% on dark adaptation [89].
• In birds there is a selective barrier to UA both in PO, and in the ciliary body [71].
While these all observations are compatible with the new unifying hypothesis, there remain some unanswered questions, such as why alkaline phosphatase is needed in PO, and why the size and form of PO vary considerably between birds.
Previous hypotheses versus the new hypothesis:
The validity of the numerous hypotheses on PO’s function has been carefully evaluated by previous authors, showing an overwhelming support of PO as an organ supplying nutrients to the avascular inner retina [1,7,11]. However, Walls’ view p 652-653 seeing PO’s function from a diurnal-nocturnal perspective, i.e. based on the notion that the “highly metabolic cones” are in need of more nutrients compared to the “pure rod retina”, concluded that “most birds do seem to have either large and many folded pectens, or small ones, depending upon their behaviour toward illumination and their general level of activity” [11]. Accordingly, he found it paradoxical that in reptiles, “neither the diurnal turtles nor the nocturnal crocodilians have preserved the ancestral conus papillaris in a useful condition. Its loss in the crocodilians (and in Sphenodon) makes good sense; but the turtles all have many cones some, perhaps, only cones in their retina”. Interestingly, in contrast to Walls’ view, the fact that these species (crocodilians, turtle) are lacking CP is predicted according to the new hypothesis, because both are aquatic reptiles with mainly ammoniotelic/ureotelic excretion.
A different aspect is that the PO could have other “subsidiary functions”, indicated by Wingstrand and Munk [1]. They supported a previous suggestion that the high content of carbonic anhydrase in PO facilitates the removal of carbon dioxide from the vitreous, and they added that “oxygen supply and removal of carbon dioxide are combined in the pecten” [17,18]. This interpretation is compatible with Brach’s statement: “As the anaerobic metabolism of the avian retina liberates an acidic waste product, it is possible that the pecten may be involved in the regulation of intraocular pH [19,20].” It should be kept in mind though, that also some mammals have an avascular retina (guinea pig, rabbit), likely to accumulating acidic waste products, but they function adequately without PO [90].
Furthermore, the main message in Wingstrand and Munk’s reports on PO as a nutritive organ has been contradicted by Brach, who argued that “the function of pecten is not primarily nutritive and may instead be related to intraocular pH regulation” [1,19]. Thus, the main conclusions in the two studies are obviously incompatible. However, this does not necessarily mean that the respective observations are invalid. In fact, these observations are unified in the new hypothesis.
Concluding Remarks
The present study is tracking the evolution of CP from the first sign of capillary network on the optic disc in aquatic reptiles to a distinct CP tissue in terrestrial species. Simultaneously, the nitrogen metabolism changed from ammoniotelic to uricotelic mode, causing high serum UA values. It is noteworthy that the development of PO, though unknown pre-stages, ended up with a distinct PO along with high serum UA values, similar to terrestrial reptiles. This may indicate that CP in reptiles and PO in birds are comparable structures fulfilling the same function.
As to the function specifically, according to previous views CP/ PO is nourishing the retina or stabilizing the intraocular pH level. Both options are included as significant mechanisms in the new hypothesis. In addition, a further aspect is presented indicating that the increasing serum UA level occurring during evolution may have represented a crucial event threatening the transparency of the eye media through intraocular UA precipitation. This damage was apparently counteracted through selective ocular barriers to UA, and carbonic anhydrase induced alkalization of the intraocular compartments. Considering these phenomena, it is shown that the two competing options regarding function are not at all excluding each other, on the contrary they are seemingly completing each other in a unifying, new hypothesis on the function of CP/PO.
Interestingly, the new hypothesis explains not only why CP is found in some reptiles only, but also why PO is present in all birds, and why CP/PO is redundant in amphibians and mammals. The driving force behind the restructuring of the intraocular blood supply when the animals went ashore, and the simultaneous alteration in protein catabolism is unknown. However, the suggested functional link between these two phenomena seems to explain why CP/PO is needed.
Acknowledgements
Thanks to Geir Aksel Qvale for computer generated graphics.
Competing Interests
The author declares no competing interests.
References
- Wingstrand, K.G., and Munk, O., 1965. The pecten oculi of the pigeon with particular regard to its function. Biol. Skr. Dan. Vid. Selsk., 14: 1-64.
- Petit, M., 1735. Anatomical description of the eye of the guinea-cock. Memoirs of the Royal Academy of Sciences, Paris. 123-152.
- Kajikawa, J., 1923. Contributions to the anatomy and physiology of the bird's eye. Graefes Arch. Clin. Exp. Ophthalmol., 112: 260-343.
- Franz, V.I.C.T.O.R., 1934. Higher sense organs. Manual of the comparative anatomy of the vertebrates, 2(Part 2), 989-1292.
- Prince, J.H., 1956. Comparative anatomy of the eye. Springfield, IL: Charles C. Thomas, USA. 267.
- Duke-Elder, S., 1958. The eye in evolution. System of Ophthalmol., 1: 416-417.
- Rohen, J.W., 1964. Manual of human microscopic anatomy. Springer.
- Brach, V., 1977. The functional significance of the avian pecten: A review. The Condor., 79: 321-327.
- Meyer, D.B., 1977. The avian eye and its adaptations. In The visual system in vertebrates., 549-611. Springer, Berlin, Heidelberg.
- Wolburg, H., Liebner, S., Reichenbach, A., and Gerhardt, H., 1999. The pecten oculi of the chicken: a model system for vascular differentiation and barrier maturation. Int. Rev. Cytol., 187: 111-159.
- Walls, G.L., 1942. The vertebrate eye and its adaptive radiation. Cranbrook Institute of Science. 607-662.
- Kiama, S.G., Maina, J.N., Bhattacharjee, J., Weyrauch, K.D., 2001. Functional morphology of the pecten oculi in the nocturnal spotted eagle owl (Bubo Wey Rauch africanus), and the diurnal black kite (Milvus migrans) and domestic fowl (Gallus migrants var. domesticus): a comparative study. J. Zool., 254: 521-528.
- Mann, I.C., 1924. The function of the pecten. Brit. J. Ophthalmol. 209: 209-226.
- Menner, E., 1938. The importance of the pecten in the bird's eye for the perception of movements, together with remarks on its ontogeny and histology. Zool. Yearb. Dept. General Zool. Physiol. Animals., 58: 481-538.
- Friess, A.E., 1969. Zur Topochemie der Vogelretina. Verh. Anat. Ges., 64: 89-91.
- Welsch, U., 1972. Enzyme histochemical and fine structural observations on the pecten oculi of pigeon (Columba livia) and black-headed gull (Larus ridibundus). J. Cell. Sci., 132: 231-244.
- Leiner, M., 1950. About the meaning of the pecten in bird's eye. Verh. dtsch. Zool. Ges, Zool. number of suppl., 15: 117-123.
- Kauth, H., Sommer, H., 1953. The ferment carbonic anhydrase in the animal body. IV.On the function of the pecten in the bird's eye. Biol. Zbl., 72: 196-209.
- Brach, V., 1975. The effect of intraocular ablation of the pecten oculi of the chicken. Invest. Ophthalmol. Vis. Sci., 14: 166-168.
- Krebs, H.A., 1927. About the metabolism of the retina. Biochem. Zeitschr., 189: 57-59.
- Wood, C.A., 1917. The fundus oculi of birds: especially as viewed by the ophthalmoscope; a study in the comparative anatomy and physiology. Lakeside Press. 1-188.
- Braekevelt, C.R., 1993. Fine structure of the pecten oculi in the great horned owl (Bubo virginianus). Histol. Histopathol., 8: 9-15.
- Wagner R., 1837. Contributions to the anatomy of birds. Abh. Math. Phys., 2: 270-305.
- Giersberg, H., 1967. Comparative Anatomy of Vertebrates. 162.
- Virchow, H., Fan, Z., and Bar, P., 1900. Vertebrate vitreous vessels and related issues. Anatomical notebooks. Merkel bonnet. II. Dept. Results.
- Schwab. I.R., 2012. Evolution’s witness. How eyes evolved.Oxford University Press..
- Raviola, E., and Raviola, G., 1967. A light and electron microscopic study of the pecten of the pigeon eye. Am. J. Anat., 120: 427-461.
- Brach, V., 1976. Structure and function of the ocular conus papillaris of Anolis equestris (Sauria: Iguanidae). Copeia., 552-558.
- Halliday, H., and Adler, K., 2002. The new encyclopedia of Reptiles & Amphibians. Oxford University Press.181.
- Johnson, G.L., 1927. VII. Contributions to the comparative anatomy of the reptilian and the amphibian eye, chiefly based on ophthalmological examination. Philos. Trans. R. Soc., 215: 315-353.
- Thompson, M.B., and Daugherty, C.H., 1998. Metabolism of tuatara, Sphenodon punctatus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol., 119: 519-522.
- Russell M., and Zoo, D., 1998. Tuatara, relics of a lost age. Cold Blooded News, 25: 1-5.
- Dawbin, W.H., 1962. Tuatara in its natural habitat. Endeavour., 21: 16.
- Delaunay, H., 1931. Nitrogen excretion of invertebrates. Biol. Rev., 6: 265-301.
- Cragg, M.M., Balinsky, J.B., and Baldwin, E., 1961. A comparative study of nitrogen excretion in some amphibia and reptiles. Comp. Biochem. Physiol., 3: 227-235.
- Munro A.F., 1953. The ammonia and urea excretion of different species of Amphibia during their development and metamorphosis. Biochem. J., 54: 29-36.
- Balinsky, J.B., Cragg M.M., and Baldwin E., 1961. The adaption of amphibian waste nitrogen excretion to dehydration. Comp. Biochem. Physiol., 3: 236-244.
- Dantzler, W.H., and Schmidt-Nielsen, B., 1966. Excretion in fresh-water turtle (Pseudemys scripta) and desert tortoise (Gopherus agassizii). Am. J. Physiol., 210: 198-210.
- Rogers, L.J., 1966. The nitrogen excretion of Chelodina longicollis under conditions of hydration and dehydration. Comp. Biochem. Physiol., 18: 249-260.
- Hopping. A., 1923. Seasonal changes in the gases and sugar of the blood and the nitrogen distribution in the blood and urine of the alligator. Amer. J. Physiol., 66: 145-163.
- Coulson, R.A., Hernandez, T., and Brazda, F.G., 1950. Biochemical studies on the alligator. Proc. Soc. Exp. Biol. Med., 73: 203-206.
- Khalil, F., and Haggag, G., 1958. Nitrogenous excretion in crocodiles. J. Exp. Biol., 35: 552-555.
- Haggag, G., 1964. Ammonotelism and uricotelism in crocodiles. Comp. Biochem. Physiol., 11: 155-159.
- Khalil, F., 1951. Excretion in reptiles – IV. Nitrogenous constituents in excreta of lizards. J. boil. Chem., 189: 443-445.
- Baze, W.B., and Horne, F.R., 1970. Ureogenesis in chelonia. Comp. Biochem. Physiol., 34: 91-100.
- Moyle. V., 1949. Nitrogenous excretion in chelonian reptiles. Biochem. J., 44: 581-584.
- Khalil, F., and Haggag, G., 1955. Ureotelism and uricotelism in tortoises. J. exp. Zool., 130: 423-432.
- Dantzler, W.H., 1967. Stop-flow study of renal function in conscious water snakes (Natrix sipedon). Comp. Biochem. Physiol., 22: 131-140.
- Khalil, F., 1947. Nitrogen constituents of the urinary concretions of the oviparous snake zamenis diadema, schlegel. J. Biol. Chem., 172: 101-103.
- Khalil F., 1948. Nitrogen constituents of the urinary concretions of the viviparous snake eryx thebaicus, reuss. J. Biol. Chem. 172: 105-106.
- Hill. L., and Dawbin, W.H., 1969. Nitrogen excretion in the tuatara, sphenodon punctatus. Comp. Biochem. Physiol., 31: 453-468.
- McNabb, R.A., and McNabb, F.M.A., 1975. Urate excretion by the avian kidney. Comp. Biochem. Physiol., 51: 253-258.
- Maixner J.M., Ramsay, E.C., and Arp L.H., 1987. Effects of feeding on serum uric acid in captive reptiles. J. Zoo. Animal. Med., 18: 62-65.
- Visek, W.J., 1968. Some aspects of ammonia toxicity in animal cells. J. Dairy Sci., 51: 286-295.
- Evans, H.E., Heiser, J.B., Podulka, S., Rohrbaugh. R.W., Bonney, R., 2001. Handbook of bird biology, 2end ed. 159.
- Mader, D.R., 2006. Reptile medicine and surgery. Saunders. 793-800.
- Gilbert, M., Virani, M.Z., Watson, R.T., Oaks, J.L., Benson, P.C., Khan, A.A., and Shah, Q.A., 2002. Breeding and mortality of oriental white-backed vulture Gyps bengalensis in Punjab Province, Pakistan. Bird Conserv. Int., 12: 311-326.
- Kumar, P., and Clark, M., 2009. Clinical medicine, 7th ed. 536-538.
- Janssens, H.J.E.M., Fransen, J., Lisdonk, E.H., Riel, P.L.C.M., Weel, C., and Janssen, M., 2010. A diagnostic rule for acute gouty arthritis in primary care without joint fluid analysis. Arch. Intern. Med., 170: 1120-1126.
- Uhlig, T., Eskild, T., Hammer, H.B. 2016. Gout - new in diagnostics and treatment. Tidsskr. Nor. Legeforen., 136: 1804-1807.
- Bluestone, R., Kippen, I., Campion, D., Klinenberg, J., and Whitehouse, M., 1974. Urate binding: A clue to the pathogenesis of gout. Review. J. Rheumatol., 1: 230-235.
- Dessauer, H.C., 1970. Blood chemistry of reptiles: physiological and evolutionary aspects. Biology of the Reptilia., 3: 1-72.
- Vegge, T., 1972. A blood-aqueous barrier to small molecules in the ciliary processes of the vervet monkey (Cercopithecus aethiops). J. Cell Biol., 135: 483-499.
- Braekevelt, C.R., 1994. Fine structure of the pecten oculi in the American crow (Corvus brachyrhynchos). Anat. Histol. Embryol., 23: 357-366.
- Alan, A., Onuk, B., Alan, E., and Kabak, M., 2020. Light and electron microscopic studies on the pecten oculi showing blood–retina barrier properties in Turkey’s native Gerze chicken. Anat. Histol. Embryol., 49: 478-485.
- Abelsdorff, G., and Wessely, K., 1909. Comparative physiological investigations into the fluid exchange of the eye in the vertebrate series: I. Birds. Arch. Ophthalmology., 64: 65-125.
- Bellhorn, R.W, and Bellhorn, M.S., 1975. The avian pecten. Ophthalmic. Res., 7: 1-7.
- Maurice, D.M., 1967. The use of fluoresce in ophthalmological research. Invest. Ophthalmol. 6: 464-477.
- Balasch, J., Palacios, L., Musquera, S., Palomeque, J., Jimenez, M., and Alemany, M., 1973. Comparative hematological values of several galliformes. Poult. Sci., 52: 1531-1534.
- Bowes, V.A., Julian, R.J., and Stirtzinger, T., 1989. Comparison of serum biochemical profiles of male broilers with female broilers and White Leghorn chickens. Can. J. Vet. Res., 53: 7-11.
- Ringvold, A., Anderssen, E., Jellum, E., Bjerkas, E., Sonerud, G.A., Haaland, P.J., and Kjonniksen, I. 2003. UV-absorbing compounds in the aqueous humour from aquatic mammals and various non-mammalian vertebrates. Ophthalmic. Res. 35: 208-216.
- Gardiner, E.E., Newberry, R.C., and Keng, J.Y., 1990. Avian vitreous humor concentrations of inosine, hypoxanthine, xanthine, uric acid, uracil and uridine as influenced by age and sex: their relevance as indicators of ante-mortem hypoxia. Forensic Sci. Int., 47: 123-127.
- Ringvold, A., Anderssen, E., and Kjonniksen, I., 2000. UV absorption by uric acid in diurnal bird aqueous humour. Invest. Ophthalmol. Vis. Sci., 41: 2067-2069.
- Wilcox, W.R., Khalaf, A., Weinberger, A., Kippen, I., and Klinenberg, J.R., 1972. Solubility of uric acid and monosodium urate. Med. Biol. Eng. Comput., 10: 522-531.
- Fried, F.A., and Vermeulen, C.W., 1964. Artificial uric acid concentrations and observations on uric acid solubility and supersaturation. Invest. Urology., 2: 131-144.
- Eichorn, M., and Flugel, C., 1988. Histochemical demonstration of carbonic anhydrase and Na?/K?-ATPase in the pecten oculi of the fowl. Exp. Eye. Res., 47: 147-153.
- Featherston, W.R., 1969. Nitrogenous metabolites in the plasma of chicks adapted to high protein diets. Poult. Sci., 48: 645-652.
- Okumura J.I., and Tasaki, I., 1969. Effect of fasting, refeeding and dietary protein level on uric acid and ammonia content of blood, liver and kidney in chickens. J. Nutr., 97: 316-320.
- Lopez-Olivera, J.R., Montane, J., Marco, I., Martinez-Silvestre, A., Soler, J., and Lavin, S., 2003. Effect of venipuncture site on hematologic and serum biochemical parameters in marginated tortoise (Testudo marginata). J. Wildl. Dis., 39: 830-836.
- Pettigrew, J.D., Wallman, J., and Wildsoet, C.F., 1990. Saccadic oscillations facilitate ocular perfusion from the avian pecten. Nature., 343: 362-363.
- Moses, R.A., 1975. Adler's Physiology of the Eye. Clin. Appl.
- Kutzing, M.K., and Firestein, B.L., 2008. Altered uric acid levels and disease states. J. Pharmacol. Exp. Ther., 324, 1-7.
- Alvarez-Lario, B., and Macarron-Vicente, J., 2010. Uric acid and evolution. Rheumatology., 49: 2010-2015.
- Wu, X., Muzny, D.M., Chi Lee, C., and Thomas Caskey, C., 1992. Two independent mutational events in the loss of urate oxidase during hominoid evolution. J. Mol. Evol., 34: 78-84.
- Costantini, D., 2011. On the measurement of circulating antioxidant capacity and the nightmare of uric acid. Methods Ecol. Evol., 2: 321–325.
- Lobo, A.M., Mokete, B., Firth, G., Firth, M., Karim, A., Collins, C., and Thompson, G., 2006. A Study of Aqueous Humour Levels of Ascorbate and Uric Acid in Patients Undergoing Cataract Surgery. Invest. Ophthalmol. Vis. Sci., 47: 676-676.
- Kiama, S.G., Bhattacharjee, J., Maina, J.N., and Weyrauch, K.D., 1994. Scanning electron microscope study of the pecten oculi of the black kite (Milvus migrans): possible involvement of the melanosomes in protecting the pecten against damage by ultraviolet light. J. Anat., 185: 637-642.
- Amemiya, T., 1982. Electron histochemical study of alkaline phosphatase activity in the pecten oculi of the Chick. Graefe’s Arch. Clin. Exp. Ophthalmol., 219: 11-14.
- Bawa, S.R., and YashRoy, R.C., 1972. Effect of dark and light adaptation on the retina and pecten of chicken. Exp. Eye. Res., 13: 92-97.
- Yu, D.Y., and Cringle, S.J., 2001. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog. Ret. Eye. Res., 20: 175-208.
- Wright, P.A., 1995. Nitrogen excretion: three end products, many physiological roles. Review. J. Exp. Biol., 198, 273-281.
- Cathers, T., Lewbart, G.A., Correa, M., and Stevens, J.B., 1997. Serum chemistry and hematology values for anesthetized American bullfrogs (Rana catesbeiana). J. Zoo. Wildl. Med., 28: 171-174.
- Wilson, S., Felt, S., Torreilles, S., Howard, A., Behan, C., Moorhead, R., and Green, S., 2011. Serum clinical biochemical and hematologic reference ranges of laboratory-reared and wild-caught Xenopus laevis. J. Am. Assoc. Lab. Anim. Sci., 50: 635-649.
- Chang, A.G., Hu, J., Lake, E., Bouley, D.M., and Johns, J.L., 2015. Biochemical and hematologic reference intervals for aged Xenopus laevis in a research colony. J. Am. Assoc. Lab. Anim. Sci., 54: 465-470.
- Coppo, J.A., Mussart, N.B., and Fioranelli, S.A., 2005. Blood and urine physiological values in farm-cultured Rana catesbeiana (Anura: Ranidae) in Argentina. Rev. Biol. Trop., 53: 545-559.
- Stewart, K., Mitchell, M.A., Norton, T., and Krecek, R.C., 2012. Measuring the level of agreement in hematologic and biochemical values between blood sampling sites in leatherback sea turtles (Dermochelys coriacea). J. Zoo. Wildl. Med., 43: 719-725.
- Scheelings, T.F., Williamson, S.A., and Reina, R.D., 2016. Hematology and serum biochemistry for free-ranging freshwater crocodiles (Crocodylus johnstoni) in Western Australia. J. Wildl. Dis., 52: 959-961.
- Lovely, C.J., Pittman, J.M., and Leslie, A.J., 2007. Normal haematology and blood biochemistry of Wild Nile crocodiles (Crocodylus niloticus) in the Okavango Delta, Botswana. J. S. Afr. Vet. Assoc., 78: 137-144.
- Rangel-Mendoza, J., Weber, M., Zenteno-Ruiz, C. E., Lopez-Luna, M. A., and Barba-Macias, E., 2009. Hematology and serum biochemistry comparison in wild and captive Central American river turtles (Dermatemys mawii) in Tabasco, Mexico. Res. Vet. Sci., 87: 313-318.
- Stacy, B.A., and Whitaker, N., 2000. Hematology and blood biochemistry of captive mugger crocodiles (Crocodylus palustris). J. Zoo. Wildl. Med., 31: 339-347.
- Millan, J.M., Janmaat, A., Richardson, K.C., Chambers, L.K., and Fomiatti, K.R., 1997. Reference ranges for biochemical and haematological values in farmed saltwater crocodile (Crocodylus porosus) yearlings. Aust. Vet. J., 75: 814-817.
- Benson, K. G., Paul-Murphy, J., and MacWilliams, P., 1999. Effects of hemolysis on plasma electrolyte and chemistry values in the common green iguana (Iguana iguana). J. Zoo. Wildl. Med., 30: 413-415.
- Christopher, M.M., Berry, K.H., Wallis, I.R., Nagy, K.A., Henen, B.T., and Peterson, C.C., 1999. Reference intervals and physiological alterations in hematologic and biochemical values of free-ranging desert tortoises in the Mojave Desert. J. Wildl. Dis., 35: 212-238.
- Dickinson, V.M., Jarchow, J.L., and Trueblood, M.H., 2002. Hematology and plasma biochemistry references range values for free-ranging desert tortoises in Arizona. J. Wildl. Dis., 38: 143-153.
- Cuadrado, M., Molina-Prescott, I., and Flores, L., 2003. Comparison between tail and jugular venipuncture techniques for blood collection in common chameleons (Chamaeleo chamaeleon). Vet. J., 166: 93-97.
- Robin, J.P., Frain, M.I.C.H.E.L., Sardet, C.L.A.U.D.E., Groscolas, R.E.N.E., and Le Maho, Y.V.O.N., (1988). Protein and lipid utilization during long-term fasting in emperor penguins. Am. J. Physiol., 254: 61-68.
- Parsons, N.J., Van der Spuy, S.D., and Schaefer, A.M., 2015. Establishment of baseline haematology and biochemistry parameters in wild adult African penguins (Spheniscus demersus). J. S. Afr. Vet. Assoc., 86: 1-8.
- Hines, R., 2012. Normal dog and cat blood chemistry and lab value.
- Ozkan O., and Pekkaya, S., 2019. Normal values of biochemical parameters in serum of New Zealand white rabbits. Turk. Hij. Den. BiyolDerg., 76: 157-162.
- Ju, J.C., Cheng, S.P., Fan, Y.K., Hsu, J.C., Chiang, S.K., Chen, E.V., and Chiou, S.C., 1993. Investigation of equine hematological constituents in central Taiwan. Asian-australas. J. Anim. Sci., 6: 147-153.
- Kjeld, M., 2001. Concentrations of electrolytes, hormones, and other constituents in fresh postmortem blood and urine of fin whales (Balaenoptera physalus). Can. J. Zool., 79: 438-446.
- Nakagawa, T., Mazzali, M., Kang, D.H., Sánchez-Lozada, L.G., Herrera-Acosta, J., and Johnson, R.J., 2006. Uric acid–a uremic toxin?. Blood Purif., 24: 67-70.
- Doornenbal, H., Tong, A.K., and Murray, N.L. 1988. Reference values of blood parameters in beef cattle of different ages and stages of lactation. Can. J. Vet. Res., 52: 99-105.
- Erokhina, I.A., Kavtsevich N.N., 2019. Blood plasma chemistry in White Sea bearded seals across different age groups. Arctic. Environmental. Res., 19: 159-165.
- Chen, Y., Qin, S., Ding, Y., Wei, L., Zhang, J., Li, H., and Cheng, J., 2009. Reference values of clinical chemistry and hematology parameters in rhesus monkeys (Macaca mulatta). Xenotransplantation., 16: 496-501.
- Christen, P., Peacock, W.C., Christen, A.E., and Wacker, W.E.C., 1970. Urate oxidase in primates. Folia. Primat., 13: 35-39.
- Howell, S., Hoffman, K., Bartel, L., Schwandt, M., Morris, J., and Fritz, J., 2003. Normal hematologic and serum clinical chemistry values for captive chimpanzees (Pan troglodytes). Comp. Med. 53: 413-423.
- McClure, H.M., Keeling, M.E., and Guilloud, N.B., 1972. Hematologic and blood chemistry data for the gorilla (Gorilla gorilla). Folia. primat. 18: 300-316.
- Mendonça, R.S., Takeshita, R.S., Kanamori, T., Kuze, N., Hayashi, M., Kinoshita, K., and Matsuzawa, T., 2016. Behavioral and physiological changes in a juvenile Bornean orangutan after a wildlife rescue. Glob. Ecol. Conserv., 8: 116-122.
- Ralston, S.H., Penman, I.D., Strachan, M.W., and Hobson, R., 2018. Davidson's Principles and Practice of Medicine E-Book. Elsevier Health Sciences.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref