Review Article - Journal of Plant Biotechnology and Microbiology (2022) Volume 5, Issue 1
The effect of plant growth-promoting Bacillus species on growth, nutrient, and hormone content of cabbage (Brassica oleracea).
Abdissa Deberssa Gutama*
Department of Biology, Dilla University College of Natural and Computational Science, P.O.Box 419 Dilla, Ethiopia
- *Corresponding Author:
- Abdissa Deberssa Gutama
Department of Biology
Dilla University College of Natural and Computational Science
Dilla
Ethiopia
Phone: 251920442291
E-mail: abdisad@du.edu.et
Received: Dec 30, 2020, Manuscript No. AAPBM-22-23993; Editor assigned: 04-Jan-2022, PreQC No. AAPBM-22-23993(PQ); Reviewed: 15-Jan-2022, QC No. AAPBM-22- 23993;Revised: 24-Jan-2022, Manuscript No. AAPBM-22-23993(R); Published: 10-Feb-2022, DOI:10.35841/aapbm-5.1.101
Citation: Gutama AD. The effect of plant growth-promoting Bacillus species on growth, nutrient, and hormone content of cabbage (Brassica oleracea). J Plant Bio Technol. 2022;5(1):101
Abstract
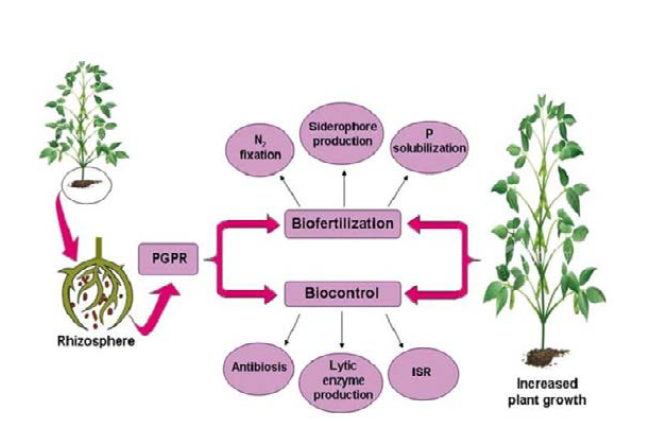
Like any other living things plants also need food for their development, growth and productivity. So there are 16 essential elements which are required by plants for healthy development and productive growth. Agricultural chemical application has been used for a long time. This is the rout terrestrial and aquatic pollution; and ecological destruction. So it is must to replace pollutant source with eco- friendly approaches. Rhizobacteria, Strains of Bacillus species, including B. subtilis, B. cereus, B. amyloliquefaciens, B. pumilus, B. pasteurii, B. mycoides, B. sphaericus, P. polymyxa, P. azotofixans, and B. endophyticus influence the growth, development, and yield of crops under controlled and varied natural conditions either directly or indirectly through various mechanisms. Bacillus species like any other PGPR has capacity to induce plant growth promotion by either direct or indirect modes of action. Bacillus is the most abundant genus in the rhizosphore which has the ability to secrete and release a number of metabolites that are released by these strains, which strongly influence the nutrient availability of the plants. This is through direct mechanisms; by producing bacterial volatiles phyto-hormones which has stimulatory role and, reduction of the ethylene level in plant, upgrading plant nutrient status (liberation of phosphates and micronutrients from insoluble sources; non-symbiotic nitrogen fixation) and prompting disease-resistance mechanisms (induced systemic resistance).
Introduction
Cabbage is an important source of vitamin A, B and C and contains minerals like P, K, Ca, Na, and Fe. It is highly consumed vegetables all over the world. They belong to the genus Brassica of the family Cruciferae. Vegetables, like all other living things, need food for their survival, development, growth and productivity. There are 16 essential elements which are required vegetables like any other plants for the achievement of healthy development and productive growth. These essential elements are divided in to macro and microelements [1]. The elements are Carbon, hydrogen, and oxygen; are derived from the atmosphere, soil and water. The other 13 essential elements are (nitrogen, phosphorus, potassium, calcium, magnesium, sulfur, iron, zinc, manganese, copper, boron, molybdenum, and chlorine). They are supplied from the sources either from soil minerals and soil organic matter or by organic or inorganic fertilizers [2].
In developing nations all over the world, and especially among resource-poor farmers, soil infertility is the most constraint limiting vegetable yield. There are three well known mechanisms used for soil fertility restoration; adopting the concept of integrated soil fertility management encompassing a strategy for nutrient management-based on natural resource conservation, biological nitrogen fixation, and increased efficiency of the inputs [3].
Important components of integrated nutrients managements are called Biofertilizers. It is cost effective, eco-friendly and renewable source of plant nutrients to supplement chemical fertilizers in sustainable agricultural system [4]. Biofertilizers are products of different types of living cells (microorganisms). They promotes plant growth by converting nutritionally essential elements (nitrogen, phosphorus) from unavailable to available form through biological process such as nitrogen fixation and solubilization of rock phosphate [5]. There for these beneficial microorganisms in biofertilizers accelerate and improve plant growth and protect plants from pests and diseases [6].
The conversion of Rhizobacteria cells from vegetative bacteria into nitrogen-fixing bacteroids involves a modification of cell structure and function, most probably with an underlying developmental pathway. The rhizosphere is the area of soil that is instantly near to the root surface and that is affected by root exudates. There are different types of substances such as carbohydrates (sugars and oligosaccharides), organic acids, vitamins, nucleotides, flavonoids, enzymes, hormones, and volatile compounds that diffuse from the roots and stimulate the microbial activity. This activation makes a dense and active microbial population that interacts with the roots and within it [7].
Bacillus species
Bacillus is the most abundant genus in the rhizosphere, and the PGPR activity of some of these strains has been known for many years, resulting in a broad knowledge of the mechanisms involved. There are a number of metabolites that are released by these strains, which strongly influence the environment by rising nutrient availability of the plants. Bacillus species are naturally present in the closely contact with plant roots. Bacillus subtilis is able to continuously manage stable contact with higher plants and promote their growth [8]. Bacterial inoculation in plant micro propagation system, at the beginning of the acclimatization phase can be observed from the perspective of the establishment of the soil microbiota rhizosphore [9]. Rhizobacteria, Strains of Bacillus species, including B. subtilis, B. cereus, B. amyloliquefaciens, B. pumilus, B. pasteurii, B. mycoides, B. sphaericus, P. polymyxa, P. azotofixans, and B. endophyticus influence the growth, development, and yield of crops under controlled and varied natural conditions either directly or indirectly through various mechanisms [10].
Bacillus species used as biofertilizers which is directily influence plant growth through the synthesis of plant growth hormones, Phosphate solubilizing, improved P solubilizzation and availability, raising N, P, K and iron (Fe) nutrition availability and uptake . Disease control roles of Bacillus are complex interrelated processes performed by direct and indirect mechanisms that embrace synthesis of some metabolites (auxin, cytokinin and gibberellins), induction of 1- aminocyclopropane 1 carbocylate (ACC) deaminase, production of siderophore, antibiotics, hydrogen cyanide (HCN), and volatile compounds [11].
Advantages and application of bacillus species in vegetable production
Implementation of biotechnology especially concerning Plant growth-promoting rhizobacteria has been hindered by the lack of consistency and variation in responses, that are obtained in field trials from site to site, year to year, or for different crops [12,13]. Today PGPR are widely used in developing countries, and inoculants are used on millions of hectares of land. The plant growth promoting effects of PGPR mainly on vegetable seedling is mainly depends on morphological and physiological changes of the inoculated plant roots and their functions, and the enhancement of water and mineral uptake [14].
Role of bacillus species on yield and productivity of vegetable seedlings
Balanced quantities and sufficient availability of nutrients is compulsory for optimum plant growth and productivity [15]. Worldwide, soil infertility is the most important constraint limiting crop yield. Adopting the concept of integrated soil fertility management encompassing a strategy for nutrient management-based on natural resource conservation, biological nitrogen fixation, and increased efficiency of the inputs are well known mechanisms used for soil fertility restoration [16]. Important components of integrated nutrients managements are called Biofertilizers. Biofertilizers has multidirectional advantage for the users; for example cost effective, eco-friendly and renewable source of plant nutrients to supplement chemical fertilizers in sustainable agricultural system [17].
Biofertilizers are products of different types of living cells (microorganisms) which inoculated to seed, plant surface or soil, colonize the rhizosphere or the interior of the plant and promotes growth by converting nutritionally essential elements (nitrogen, phosphorus) from unavailable to available form through biological process such as nitrogen fixation and solubilization of rock phosphate [18]. Therefore, these beneficial microorganisms in biofertilizers accelerate and improve plant growth and protect plants from pests and diseases [19].
For example the major influencing factors for vegetable plant growth regulators are nutrient availability and up taking like any other plants. This is highly determined by Rhizobacteria specifically Bacillus species in cabbage plant and seedling. According to nutrient content of cabbage seedlings was highly influenced by PGPR treatments [20]. PGPR applications increased the plant nutrient element content, with the exception of Na and Cu. The highest concentrations for N and P were recorded in B. megaterium TV-91C, while in B. subtilis TV- 17C for Ca, Na, and Fe and in P. agglomerans RK-92 for K, Mg, and Mn (Figure 1).
Mechanisms used by bacillus species for plant growth promoting
Bacillus species like any other PGPR has the capacity to induce plant growth either direct or indirect modes of action. Direct mechanisms includes producing bacterial volatiles phyto-hormones which has stimulatory role and, reduction of the ethylene level in plant, upgrading plant nutrient status (liberation of phosphates and micronutrients from insoluble sources; non-symbiotic nitrogen fixation) and prompting disease-resistance mechanisms (induced systemic resistance) [21-23].
Direct plant growth promotion role of bacillus species
Biofertilization role of bacillus species: Bacteria (Bacillus species) generally influence physical and physiology of plant in various ways. This physiological change mostly is the cause for increasing plant growth and yield via increasing level of plant hormone within their tissue; this enhances growth of plants in a various directions. Biofertilizing role of PGPR, particular by Bacillus species has capability of initiating plant growth by increasing nutrient uptake of plants and regulating amount of hormone production in plants tissue [24].
Rhizobacteria determines plant growth specifically vegetable plant growth, health and productivity by different types of mechanisms such as enhancing solubilization of mineral nutrients, stimulation of root growth, and suppression of root diseases [25]. This types plant growth promoting ways, make easy nutrient up take, increase nutrient availability and stimulate plant root growth and are commonly referred as biofertilizers. Biofertilizers are considered as the major and incomparable role to increase the production of vegetable crops in low input agricultural systems [26].
There are many reports which show from different perspectives concerning PGPR activities of vegetable plant growth enhancement. The most cited mechanisms used by PGPR to promote plant growth are as follows; as soon as colonization occurs, PGPR interact with the plant host and enhance the nutrient uptake of host plants by (1) biological N2 fixation, (2) increasing the availability of nutrients in the rhizosphere, (3) inducing increases in root surface area, (4) enhancing other beneficial symbiosis of the host, and (5) combination of modes of action (Vessey, 2003). Some studies also indicate that the effective PGPR increase plant growth basically by changing the whole microbial community structure in rhizosphore. PGPR are soil microbes which has the capacity of fixing N2, solubilize mineral nutrients and mineralize organic compounds [27, 28].
Role of bacillus species in phosphate solubilization
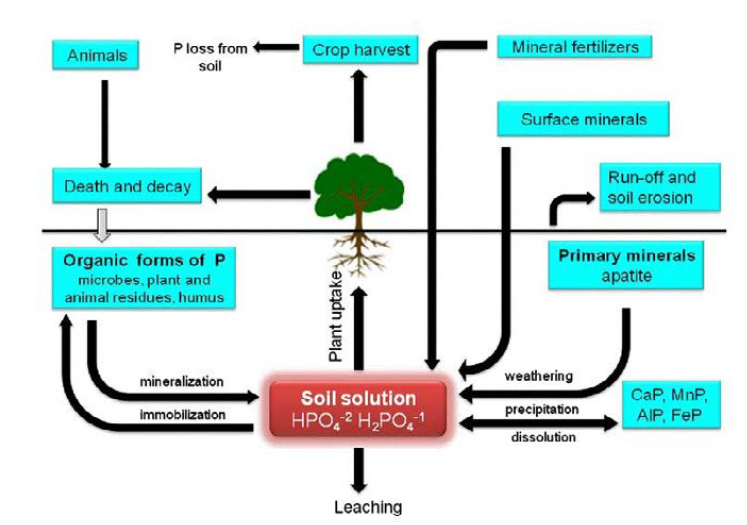
Phosphorus (P) is very important for plant growth and is found in every living plant cell. It is involved in various physiological activities of plant functions, including energy transfer, photosynthesis, transformation of sugars and starches, nutrient movement within the plant and transfer of genetic characteristics from one generation to the next. On the other side deficiency of P can severely limit plant growth and productivity, particular by vegetables’ [29].
In agricultural systems and crop production, abiotic stress which is, phosphorus deficiency results into significant contribution in diminution of crop productivity and yields of vegetables [30]. So in order to avoid or mitigate diminution of vegetable yield and environmental disaster, microorganisms especially Bacillus sp. (Bacillus megaterium TV-91C, Pantoea agglomerans RK-92, and Bacillus subtilis TV-17C) play significant role in influencing the availability of soil P to plant roots; and increasing P mobilization in soil [31].
For example 50% to 80% of the total soil P is represented by Organic P represents, and most plants are unable to utilize these sources of P [32]. So participation of Several bacterial species specifically Bacillus sp [33]. has a great advantage for phosphate-solubilizing. The idea of P solubilization is justified by the study by bacterial strains were confirmed with B. megaterium TV-91C, P. agglomerans RK-92, and B. subtilis TV-17C strains showed capacity to solubilize phosphate after inoculation (Figure 2).
Role of bacillus species in ammonia production
Biological nitrogen fixation performed yearly, up to 100 million tons of N2 for terrestrial ecosystems and from 30 to 300 million tons for marine ecosystems. Additionally, 20 million tons result from chemical fixation due to atmospheric phenomena. On the beginning of 19th century the first industrial production of Rhizobium inoculants began (Mosier, 2002). According to an FAO report, manufacture of nitrogen fertilizer for 2007 was 130 million tons of N2, and this should further rise in the coming years [34].
On the other side extensive use of chemical fertilizer has a great impact on environment. A amount of added fertilizer is lost as a result of denitrification and leaching of soil by rainfall and irrigation [35]. The major source of water pollution caused by eutrophication is this leaching. As a consequence, extending application of biological nitrogen fixation by any means is an important issue. So application of PGPR has the capacity to solve the problems of environmental pollution, waste management and nutrient availability and up taking role. While biological nitrogen fixation (BNF) is performed, nitrogen is reduced in electron-transfer reactions. This does synthesis of ammonia and release of hydrogen. After that ammonium which is produced used for the next synthesis of bio-molecules (Table 1).
| Bacterial strains | Isolated from | Nitrogen fixation | Phosphate solubilization |
|---|---|---|---|
| Bacillus megaterium TV-91C | Sugar beet | + | wt |
| Pa11toea agglomerans RK-92 Bacillus subti/is TV- I7C |
Pear Rye |
+ t |
st Wt |
Table 1. Nitrogen fixation and phosphate-solubilizing activity of the tested bacterial strains after inoculation of Bacillus sp.to Brassica oleracea.
Role of bacillus species in nitrogen availability and uptake by vegetables
In the soil nitrogen occurs in the forms of both organic and inorganic and within different seasonal changes characterized by a heterogeneous distribution in the soil. Nitrogen inputs by the processes of fixation reactions and transformations of N2 between different pools have significant implications for plant growth and for the loss of N2 from soil systems.
Mineralization of organic forms of N2 to ammonium (NH4+) is mediated by Microbial and its succeeding nitrification to nitrate (NO3 -) is a significant role for N2 availability and has pressure on root activity and rhizosphere dynamics. According to evidences from researchers the soluble organic forms of N2 (e.g. low molecular weight compounds such as amino acids) may also play a significant role. The rhizosphore of particularly Bacillus species has significantly affects the uptake of different N2 forms. It is directly influenced by soil pH in the immediate vicinity of the root and subsequent influence on nutrient acquisition, especially in relation to the availability of P and various micronutrients (e.g. Zn, Mn and Fe) [36].
Protons uptake occur by influence of NO3- influx changes the rhizosphore pH, this net release protons for NH4+ uptake, this can also bring about changes in the nature of substrates exuded from roots or the quantities of exudates released, and consequently may have major impact on the structure of microbial communities around the root . Mostly mass flow and diffusion are the mechanism through which NO3− and NH4+ reach the root surface [37].
The concentration of nitrate in soil solution is typically present in the form of mM and, and its mobility is relative to orthophosphate, is more mobile. Thus indicate that it has the potential able to move in the soil up to several mm per day. On the other side ammonium has less motion or mobile since it eagerly adsorbs to the cation exchange sites in soil. But it has lower rates for both mass flow and diffusion. Diffusion and mass flow is the major pathway for inorganic N2 uptake and additionally it is difficult to differentiate diffusion from root interception. It is generally accepted that interception of N2 in soil solution following root extension accounts for a minute amount of N2 taken up by plants.
Siderophore production of rhizobacteria (Bacillus species)
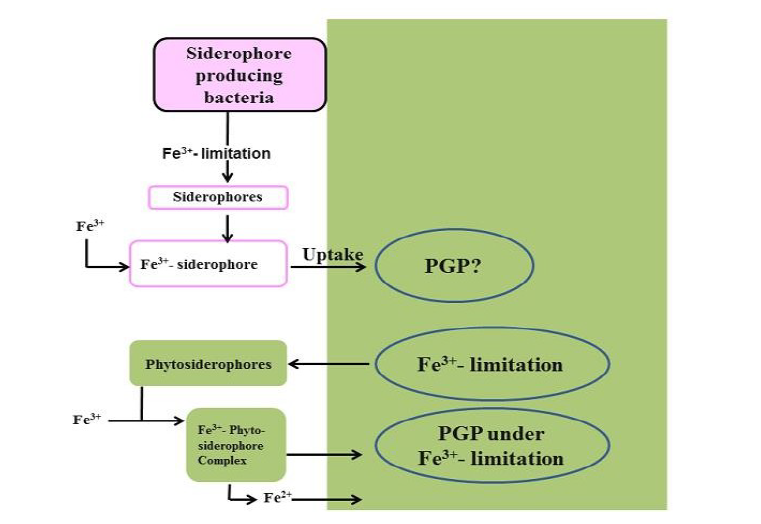
Siderophores play a vital role in the bio-control of numerous soil-borne plant diseases and in plant iron nutrition. Extracellular metabolites released by PGPR for the action of microbial Fe- chelating low molecular weight compounds. The formation of siderophore occurs due to the presence of PGPR in rhizosphere amplifies the rate of Fe3+ supply to plants and due to this plant growth and productivity enhanced. Additionally, this compound after chelating Fe3+ makes the soil Fe3+ deficient for other soil microbes and consequently inhibits the activity of competitive microbes.
Siderophores has the capability to enhance affinity of iron (III) chelators, low molecular weight that transport iron into bacterial cells. The composition of the systems is ferricspecific ligands (siderophores) and their cognate membrane receptors as chelating agents in bacteria. Consequently, siderophores have been shown to be involved in the suppression of Fusarium oxysporum. Because siderophores causes the limited supply of iron (III) in the rhizosphere, and causes its shortage to pathogens and this hinder the growth of pathogens (Figure 3).
Iron is one of an essential element for all vegetables. Even if it the abundant element on the earth crust but it is hardly soluble and therefore not suitable for uptake by plants. The concentration of Fe3+, the form of iron ions available for plants, is only 10-18 M. the combination of microbes Bacillus sp. and vegetable (Brassica oleracea) Plants produce and excrete chelators and/or phytosiderophores which bind Fe 3+ and transport it to the root surface where it is either reduced to Fe2+, that is subsequently taken up by the plant, or it is absorbed as a Fe 3+ phytosiderophore complex by the plant [38].
Indirect plant growth promotion role of bacillus species
Phytostimulation effects of bacillus species: Plants hormones are chemical messengers that determine plant growth and development; and also have the ability to affect a plant's response to its environment. These chemical messengers are organic compounds that are effective at very low concentration. They are typically synthesized in one part of the plant and are taken to another location to the acting site. There are five major classes of plant hormones have been identified. These classes of hormones are; auxins, cytokinins, gibberellins, abscisic acid and ethylene. Directly or indirectly these hormones determine plant growth and developments including cabbage in their cell division, cell elongation, and plant cell differentiation. Hormones are varies based on their site of action, developmental stage of their action and need concentration. They are stimulating and act together with specific target tissues to cause physiological responses, such as growth or fruit ripening. Generally the final physiological response of plants including vegetables is the result of two or more hormones acting together.
Role of Bacillus species in production of IAA for growth improvement
Regulation of plant development including organogenesis, tropic responses, cellular responses such as cell expansion, division, and differentiation, and gene regulation is the major signaling function or role of hormones.
According to BS Saharan of 2011 diversified bacterial species possess different capability of producing phytohormones, auxin or IAA. Different biosynthesis pathways have been identified frequently. Particularly IAA biosynthesis is widespread among plant-associated bacteria. Interactions between IAA-producing bacteria and plants lead to diverse outcomes on the plant side, varying from pathogenesis to phyto-stimulation. Bacteria use this phytohormone to interact with plants as part of their colonization strategies. IAA can also be a signaling molecule in bacteria and therefore, can have a direct effect on bacterial physiology.
Studies additionally mentioned that PGPR influences plant growth and development by the production of phytohormones such as auxins, gibberellins, and cytokinins. The effects of auxins on plant seedlings are determined by their concentration. The bacterial strains which produce highest amount of auxins such as indole acetic acid (IAA) and indole acetamide (IAM) causes maximum growth and yield of wheat crop in non-sterilized soil. Mostly this enhance plants to have a greater amounts of fine roots which have the effect of increasing the absorptive surface of plant roots for uptake of water and nutrients.
Rhizobacteria particularly Bacillus sp. are the first group of bacteria, which determine the ability of vegetable plants to release IAA that can help to promote the growth and pathogenesis in plants. The IAA production is studied in Rhizobium strains associated only with a few legume hosts.
Bacillus megaterium from the rhizosphere is able to produce IAA and thus it helps in the plant growth promotion specifically cabbage (Brassica oleracea). For example inoculation of cabbage (Brassica oleracea) plant with different Bacillus sp. has develop capability of IAA production which highly increases the growth by the N, P, K, Ca and Mg uptake of the vegetable plant. Fundamental variation of growth on rooting and root dry matter of cuttings of cabbage (Brassica oleracea) grown on Bacillus sp. inoculated substrate was observed. The same result were also recorded on cucumber, tomato and pepper are inoculated with different strains of PGPR which produce IAA, there is a significant increase in the growth of the vegetables (Table 2).
| Control | TV-91C | RK-92 | TV-17C | |
|---|---|---|---|---|
| Gibberellic acid (ng L-1) | 190 t b* | 215 a | 216 a | 214 a |
| Salicylic acid (ng L-1) | 44.7 b** | 50.6 b | 76.4 a | 53.8 a |
| Abscisic acid (ng L-1) | 0.23 a* | 0.17 b | 0.21 a | 0.22 a |
| Indole acetic acid (ng L-1) | 6.31 c** | 7.67 b | 8.74 a | 7.82 b |
Table 2. Hormone content of cabbage seedlings in response to PGPR treatments.
Cytokinin role of Bacillus species for plant growth
Plant hormones generally are assumed to interact with specific receptors that reside either on the cell surface or within the cytoplasm. Cytokinin is one of the plant hormones which has important role of regulators of development and environmental responses of plants that execute their action via the molecular machinery of signal perception and transduction. Two identified cytokinin receptor have important role in plant growth promotion. There are two groups of cytokinin such as; steroid hormone receptor model while the other fits the membrane receptor model [39].
According to (Marschner, 2010) there is the links between temperature and Cytokinin signaling, and an involvement of calcium ions in Ck signaling. Tryptophan also enhances the production of IAA in Bacillus amyloliquefaciens FZB42. Plants inoculated with the bacillus sp. together with Ag+ ion and L-tryptophan (Trp), give the highest root dry weight, and significantly increase the uptake of N, P and K compared to non-inoculated control plants.
Ethylene hormone regulating role of rhizobacteria for plant growth
Ethylene is also one of the hormone determine growth and development of plants, however it has different effects on plant growth depending on its concentration in root tissues. When its concentration is increased, it causes reduction of crop yield and growth by inducing defoliation and cellular processes that lead to prevent or retard stem and root growth as well as premature senescence. Based on environmental variation for example, under different types of stress, such as cold, draught, flooding, infections with pathogens, presence of heavy metals, among others, plants respond by synthesizing 1-aminocyclopropane-1- carboxylate (ACC), which is the precursor for ethylene. When 1-aminocyclopropane-1- carboxylate (ACC) is secreted into the rhizosphere and is re- adsorbed by the roots, it is converted into ethylene. These types of accumulation of ethylene leads to a descending spiral effect, causes poor root growth. This hinders and reduces capability to acquire water and nutrients this results additional stress. PGPR in nature has the capacity to degrade ACC in the rhizosphere can help to restore a healthy root system that is needed to cope with environmental stress. Rhizobacteria degrade ethylene via the enzyme ACC deaminase. This enzyme can reduce or avoid some of the dangerous effects of the high ethylene levels [40].
Bacillus species role on growth rate of cabbage (brassica oleracea) seedling
The seedling determines high yield production of vegetable crops. Because the final yields of most vegetable crops are depends on their Seedling qualities, establishment and uniform plant growth and development. Therefore in order to increase yield of vegetable crops and maintain plant health growth, application or inoculation of Plant growth promoting rhizobacteria specifically Bacillus sp. has significant potential in nitrogen fixation, mineral solubilization and disease suppression.
Nitrogen is one of the essential substances in plant growth and the production of food and feed. Because it is the key elements in cellular synthesis like; enzymes, proteins, chlorophyll, DNA and RNA. Nitrogenase in rhizobial bacteroids are essential enzymes for symbiotic N2 fixation action of vegetable crop with the combination of Bacillus sp. This process of biological nitrogen fixation (BNF) and recycling nitrogen is currently utilized in agriculture, and it will be also provide promising yields for important in crop and vegetable crop productivity for the future, especially in sustainable systems. Studies show that PGPR treatments improve the growth parameters of cabbage seedlings significantly.
Conclusion
Bacillus megaterium strain TV-91C, Pantoea agglomerans strain RK-92, and B. subtilis strain TV- 17C bacillus species inoculation has a great effect on the growth, nutrient, and hormone content of cabbage seedlings. So application of Rhizobium genus specifically bacillus species has not only the advantage of healthy growth of seedling, role of enhancing yields, plant diseases suppression and cost minimizing role but also provide a significant advantage in waste management (phythoremidation) and environmental pollution mechanism. Plant growth-promoting rhizobacteria (PGPR) particularly bacillus species treatments increased fresh and dry shoot and root weight, stem diameter, seedling height, chlorophyll reading values, and leaf area of cabbage seedlings compared with the control.
References
- Alikhani N, Saleh-Rastin H, Antoun. Phosphate solubilization activity of rhizobia native to Iranian soils. Plant Soil. 2006,287:35-41.
- Maheshwari DK. Bacteria in agrobiology: Crop ecosystems. Springer Science Business Media. 2011;19.
- Saharan BS, Nehra V. Plant growth promoting rhizobacteria: A critical review. Life Sci Med Res. 2011;(1):30.
- Ajilogba CF, Babalola OO, Ahmad F. Antagonistic effects of Bacillus species in biocontrol of tomato Fusarium wilt. Stud Ethno-Med. 2013;7(3):205-16.
- Cassán F, Perrig D, Sgroy V, et al. Azospirillum brasilense Az39 and Bradyrhizobium japonicum E109, inoculated singly or in combination, promote seed germination and early seedling growth in corn (Zea mays L.) and soybean (Glycine max L.). Eur J Soil Biol. 2009;45(1):28-35.
- Chakraborty U, Chakraborty BN, Chowdhury PR, et al. Investigation on plant growth promoting rhizobacteria of tea rhizosphere. In6th International workshop on PGPR, IISR, Calicut, Kerala 2006 (pp. 78-82).
- Chen JH. The combined use of chemical and organic fertilizers and/or biofertilizer for crop growth and soil fertility. Soil Environ Sci. 25:232-45.
- Chen YP, Rekha PD, Arun AB, et al. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl Soil Ecol. 2006;34(1):33-41.
- Choudhary DK, Johri BN. Interactions of Bacillus spp. and plants–with special reference to induced systemic resistance (ISR). Microbiol Res. 2009;164(5):493-513.
- Dakora FD, Keya SO. Contribution of legume nitrogen fixation to sustainable agriculture in Sub-Saharan Africa. Soil Biol Biochem. 1997;29(5-6):809-17.
- Matiru VN, Dakora FD. Potential use of rhizobial bacteria as promoters of plant growth for increased yield in landraces of African cereal crops. Afr j biotechnol. 2004;3(1):1-7.
- Dobbelaere S, Vanderleyden J, Okon Y. Plant growth-promoting effects of diazotrophs in the rhizosphere. Crit Rev Plant Sci. 2003;22(2):107-49.
- Abou El-Yazeid A, Abou-Aly HE, Mady MA, et al. Enhancing growth, productivity and quality of squash plants using phosphate dissolving microorganisms (Biophos-phor®) combined with boron foliar spray. Res J Agric Biol Sci 2007;3(4):274-86.
- Miyamoto T, Ochiai K, Takeshita S, et al. Current World Fertilizer Trends and Outlook to 2011/12, 2008. Soil Sci. Plant Nutr. 2012;58(6):728-36.
- George TS, Gregory PJ, Hocking P, et al. Variation in root-associated phosphatase activities in wheat contributes to the utilization of organic P substrates in vitro, but does not explain differences in the P-nutrition of plants when grown in soils. Environ Exp Bot. 2008;64(3):239-49.
- Richardson AE. Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Funct Plant Biol. 2001;28(9):897-906.
- Rodri´guez H, Fraga R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv. 1999;17(4-5):319-39.
- Somasegaran P, Hoben HJ. Methods in legume-Rhizobium technology. University of Hawaii 4th ed:320-325.
- Issazadeh K, Rad SK, Zarrabi S, et al. Antagonism of Bacillus species against Xanthomonas campestris pv. campestris and Pectobacterium carotovorum subsp. carotovorum. Afr J Microbiol Res. 2012;6(7):1615-20.
- Ahemad M, Kibret M. Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Univ Sci. 2014;26(1):1-20.
- Kidoglu F, Gul A, Ozaktan H, et al. Effect of rhizobacteria on plant growth of different vegetables. ISHS Acta Horticulturae: International. 801:342-354.
- Kokalis–Burelle N, Vavrina CS, Rosskopf EN, et al. Field evaluation of plant growth-promoting rhizobacteria amended transplant mixes and soil solarization for tomato and pepper production in Florida. Plant and Soil. 2002;238(2):257-66.
- Malfanova NV. Endophytic bacteria with plant growth promoting and biocontrol abilities (Doctoral dissertation, Leiden University).
- Marius Stefan, Neculai Munteanu, Simona Dunca. Plant-microbial interactions in the rhizosphere -strategies for plant growth-promotion. Genetica si Biologie Moleculara. 2012;46:432-452.
- Clarkson DT, Hanson JB. The mineral nutrition of higher plants. Annu Rev Plant Physiol. 1980;31(1):239-98.
- Martínez-Viveros O, Jorquera MA, Crowley DE, et al. Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J Soil Sci Plant Nutr 2010;10(3):293-319.
- Turan M, Ekinci M, Yildirim E, et al. Plant growth-promoting rhizobacteria improved growth, nutrient, and hormone content of cabbage (Brassica oleracea) seedlings. Turk J Agric For. 2014;38(3):327-33.
- Mosier AR. Environmental challenges associated with needed increases in global nitrogen fixation. Nutr Cycl Agro ecosys. 2002;63(2):101-16.
- Sajid M, Rab A, Wahid F, et al. Influence of rhizobium inoculation on growth and yield of groundnut cultivars. Sarhad J Agric. 2010;27(4):573-6.
- Saharan BS, Nehra V. Plant growth promoting rhizobacteria: A critical review. Life Sci Med Res. 2011;21(1):30.
- Pedraza RO. Recent advances in nitrogen-fixing acetic acid bacteria. Int J Food Microbiol. 2008;125(1):25-35.
- Berendsen RL, Pieterse CM, Bakker PA. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012;17(8):478-86.
- Sivasakthi S, Usharani G, Saranraj P. Biocontrol potentiality of plant growth promoting bacteria (PGPR)-Pseudomonas fluorescens and Bacillus subtilis: A review. Afr J Agric Res. 2014;9(16):1265-77.
- Sudharani M, Shivaprakash MK, Prabhavathi MK. Role of consortia of biocontrol agents and PGPRs in the production of cabbage under nursery condition. Int J Curr Microbiol Appl Sci. 2014;3(6):1055-64.
- Tien TM, Gaskins MH, Hubbell D. Plant growth substances produced by Azospirillum brasilense and their effect on the growth of pearl millet (Pennisetum americanum L.). Appl Environ Microbiol. 1979;37(5):1016-24.
- Osorio Vega NW. A review on beneficial effects of rhizosphere bacteria on soil nutrient availability and plant nutrient uptake. J National Faculty Agronomy Medellin. 2007;60(1):3621-43.
- Vessey JK. Plant growth promoting rhizobacteria as biofertilizers. Plant and soil. 2003;255(2):571-86.
- Vlek PL, Vielhauer K. Nutrient management strategies in stressed environments. Stressed ecosystems and sustainable agriculture. 1993.
- Willigen PD. Supply of soil nitrogen to the plant during the growing season. In Fundamental, ecological and agricultural aspects of nitrogen metabolism in higher plants 1986 (pp. 417-432). Springer, Dordrecht.
- Aziz ZF, Saud HM, Rahim KA, et al. Variable responses on early development of shallot (Allium ascalonicum) and mustard (Brassica juncea) plants to Bacillus cereus inoculation. Malays J Microbiol. 2012;8(1):47-50.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref