Research Article - Biomedical Research (2017) Volume 28, Issue 7
The effect of nerve block alone or with zinc on wound healing in a rat model of diabetic foot ulcer
Yusom Shin1*, Tae Woo Park1, Joo Won Kim1, Min Jung Jung2 and Hee Bin Park3
1Department of Anaesthesiology and Pain Medicine, Kosin University College of Medicine, Republic of Korea
2Department of Pathology, Kosin University College of Medicine, Republic of Korea
3Department of Anaesthesiology and Pain Medicine, Haeundae Bumin Hospital, Republic of Korea
- *Corresponding Author:
- Yusom Shin
Department of Anaesthesiology and Pain Medicine
Kosin University College of Medicine, Republic of Korea
Accepted date: December 26, 2016
Abstract
Introduction: Patients with Diabetic Foot Ulcer (DFU) have a high risk of limb amputation as well as higher five-year mortality rates than those for several types of cancer. Effective treatment of DFU, therefore, is a pressing issue. Nerve block can have a beneficial wound-healing effect in this regard, due to its sympathectomy-like vasodilation promoting blood circulation. Zinc furthermore, as a cofactor, can facilitate wound healing. In this study, we evaluated the wound-healing effects of nerve block alone and with Intravenous (IV) zinc.
Materials and Methods: Fifteen (15) male Otsuka Long-Evans Tokushima Fatty (OLETF) rats were equally divided into three DFU groups: a non-treated (group 2), a nerve-block (group 3), and a nerveblock and IV zinc group (group 4). Five additional male Long-Evans Tokushima Otsuka (LETO) rats were assigned to a control, non-diabetic group (group 1). A full-thickness wound (5 mm × 5 mm) was made in all of the rats’ left dorsal foot, which, on post-injury day 1, was treated with sciatic nerve block alone (group 3) or additionally with IV zinc (group 4). On day 13, the wound size was measured, and a histological examination was performed with haematoxylin and eosin (H & E) and Masson’s trichrome staining.
Results: The wound-surface-area differences among the three DFU groups were not statistically significant (p=0.40). Neither were there any significant histological differences.
Conclusion: In this study, neither intermittent nerve-block alone or with IV zinc affected DFU healing.
Keywords
Diabetic foot ulcer, Nerve block, Wound healing, Zinc.
Introduction
Diabetes, a worldwide disease, is the seventh cause of death in the United States. Its prevalence increases each year in both developed and developing countries; indeed, it is estimated that by 2035, 590 million people will be diabetics [1,2]. Currently, 20% of diabetics suffer diabetic foot. Among them, 5-10% develops Diabetic Foot Ulcer (DFU), and 3% of those eventually have to undergo foot amputation [3]. Given lowerextremity amputees’ considerably reduced quality of life, effective DFU treatment for prevention of this worst of outcomes is especially important.
One of the highest priorities in the treatment of DFU is improved blood supply [4]. During surgery, increased blood flow can have beneficial effects on vascular-surgical results. Regional anaesthesia’s sympathectomy-like effect can be helpful in this regard [5-7]. Indeed, besides its advantages for postoperative respiratory function improvement and stress response reduction, regional anesthesia (i.e. peripheral nerve block) is known to be in related to lower cardiovascular mortality than is the case with general anesthesia, which is frequently used in non-traumatic major lower-extremity amputations [8,9]. Peripheral nerve block, therefore, is expected to be able to help in the treatment of DFUs by improving blood flow to the wound area. In fact, diabetic patients have shown reduced foot-skin endothelium-dependent vasodilation [10]. Based on this, we can hypothesize that a sciatic nerve block, functioning as a kind of peripheral nerve block that affects the dermatome of the DFU, can improve blood circulation to the DFU, which will help in wound healing.
Additionally, zinc is known to act as a co-factor of enzymes involved in the biochemical process of wound healing, and its requirement increases at the re-epithelialization and granulation stages. It has already been observed that topical zinc hyaluronate promotes ulcer healing in DFU patients [11]. Certainly, diabetic patients might not have enough zinc [12]. This suggests that administration of IV zinc might also, in the manner of topical zinc hyaluronate, facilitate wound healing.
The present animal experimentation was planned and conducted to determine if peripheral nerve block and IV zinc actually promote DFU healing. We aimed to establish the clinical basis of the use of peripheral nerve block and IV zinc for DFU treatment.
Materials and Methods
This study was approved by the Medical Ethical Committee at Kosin University College of Medicine, Busan, Korea. Eighteen (18) OLETF and six LETO rats, all aged four weeks, were housed and cared for until they were 37 weeks old and 575 and 523 g in weight, respectively. Diabetes was evaluated for expression of blood glucose greater than 250 mg/dl in 37- week-old OLETF rats. Rats were assigned two to a cage and maintained under conditions of controlled temperature (20 ± 2°C) and humidity (40-60%) on a 12-h light/dark cycle with sufficient water and feed.
Wounding model
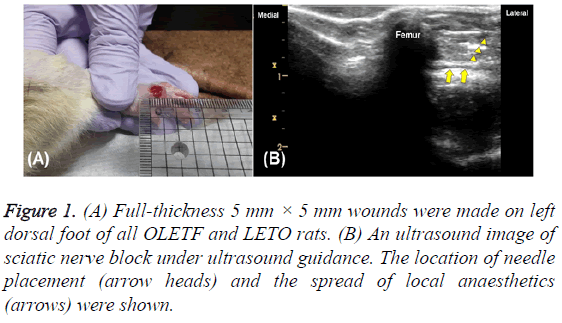
The 24 rats were anesthetized with a single intraperitoneal injection of tiletamine-zolazepam (Zoletil 50, Virbac, France) at a dose of 60 mg/kg body weight. After anesthesia, a fullthickness incisional wound (5 mm × 5 mm; Figure 1A) was formed on the left dorsal foot (day 0). Wounds were maintained without suturing or coverage. During this process, two of the OLETF rats died under the anesthesia, and a third was found to be in poor general condition. The remaining 15 OLETF rats were divided into three DFU groups: a non-treated group (group 2), a nerve-block group (group 3), and a nerveblock and IV zinc group (group 4). One LETO rat in the control group (non-diabetic, group 1) was removed from the study in order to balance the number of rats in each group at five. The optimal number of experimental animals was determined according to the formula E=total number of animals-total number of groups, 10<E<20.
Peripheral nerve block
On post-injury day 1, all of the rats were again anesthetized, this time by injection of 40 mg/kg tiletamine-zolazepam into the peritoneal cavity. Groups 3 and 4 underwent sciatic nerve block under ultrasound guidance without peripheral nerve stimulation. The rats’ breathing was often checked, but no additional oxygen was provided. The region of the left sciatic nerve was observed with the rats in their right lateral position. After shaving the left thigh, the target area near the buttock was contacted with a gel-applied linear probe. Slowly moving the probe up and down anterior-to-posteriorly, the hyperechogenicity of the left femur was observed and the biceps femoris came into view. The expected injection site was wiped with an alcohol-applied pad, and a 26G needle with a 1 ml syringe was placed under the fascia of the biceps femoris using the outside-to-inside in-plane approach (Figure 1B). The standard of successful nerve block confirmation was the rat’s walking, with dragging of the left foot, after fully awakening from the anesthesia. The second nerve block (post-injury day 3) was not performed until two days after the first block, and the third nerve block (day 10) was performed only one week after the second, by the same process. Thus the experimentation entailed a series of three nerve blocks (postinjury days 1, 3 and 10).
Zinc administration
On post-injury day 1, the group 4 rats were anesthetized and underwent the first IV zinc injection. After left sciatic nerve block, the well-vascularized lateral saphenous vein was observed in the ipsilateral leg with the ulcer. In cases where this vein was unsuitable for injection, the ischial vein was injected. If injection on the same side of the wound failed, it was carried out on the opposite side in the same way. After cleaning of the injection site with an alcohol swab, a diluted solution of 0.1 ml zinc sulphate hydrate (Zinc Trace, Huons, Korea) (4.4 mg/ml, 1 mg as Zn) in 0.9 ml normal saline was injected slowly through a 26G needle with a 1 ml syringe, applying light pressure to stop any bleeding. The second IV zinc injection (day 3) was performed two days after the first injection, and the third (day 10) was performed one week after the second, by the same process. Thus, the experimentation entailed a series of three IV zinc injections (post-injury days 1, 3 and 10).
Macroscopic assessment
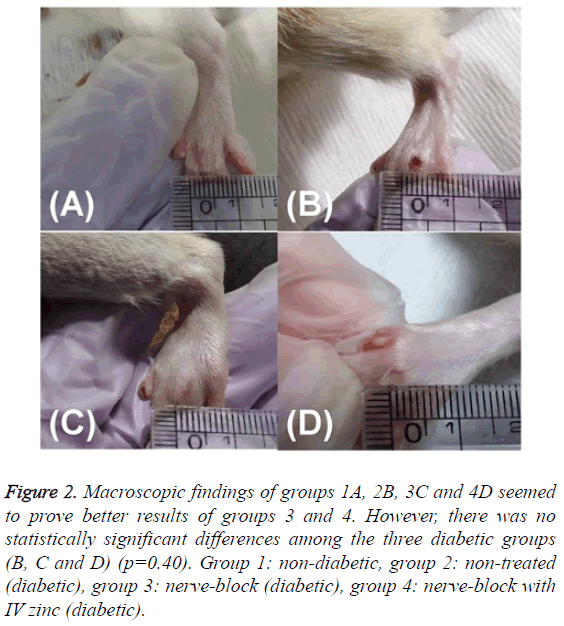
The first wound size was measured using a ruler on the day of making DFUs model (day 0) and photographed for the evaluation of wound healing. Additionally, the last wound size (Figure 2) was assessed in the same way immediately before obtaining the tissue of the wound (day 13).
Figure 2: Macroscopic findings of groups 1A, 2B, 3C and 4D seemed to prove better results of groups 3 and 4. However, there was no statistically significant differences among the three diabetic groups (B, C and D) (p=0.40). Group 1: non-diabetic, group 2: non-treated (diabetic), group 3: nerve-block (diabetic), group 4: nerve-block with IV zinc (diabetic).
Histological examination
Full-thickness specimens from the wound area were obtained two days after the third nerve block and IV zinc injection (day 13). The acquired skin tissue was fixed in neutral-buffered formalin 10% solution and sliced into about 5 μm thicknesses vertically on the surface of the skin. All of the tissue slides were prepared with haematoxylin and eosin (H & E; Figure 3) and Masson's trichrome (Figure 4) staining in order to observe the epidermal thickness and collagen fiber, respectively, using microscopy. One pathologist analysed the tissue slides without any specific notification or instruction.
Figure 3: Microscopic findings of skin sections from groups 2A, 3B and 4C showed no views with definite differences among groups. A, B and C were stained with haematoxylin and eosin (H & E). Group 1: non-diabetic, group 2: non-treated (diabetic), group 3: nerve-block (diabetic), group 4: nerve-block with IV zinc (diabetic).
Figure 4: Microscopic findings of skin sections from groups 2A, 3B and 4C showed no views with definite differences among groups. A, B and C were stained with Masson’s trichrome. Group 1: non-diabetic, group 2: non-treated (diabetic), group 3: nerve-block (diabetic), group 4: nerve-block with IV zinc (diabetic).
Statistical analysis
The experimental data were expressed as mean ± SD. A statistical analysis was performed using MedCalc (ver 14.8.1) with the nonparametric Mann-Whitney and Kruskal-Wallis tests. P values less than 0.05 were considered statistically significant.
Results
Macroscopic analysis
Wound size was measured twice (days 0 and 13). The wound surface areas in groups 1 and 2 were 0.40 ± 0.25 mm2 and 4.50 ± 0.50 mm2, respectively, on day 13. A significant difference in extent of wound healing between the control group (group 1) and diabetic group 2 was statistically confirmed (p=0.01). On gross findings, group 2 had not healed better than group 3 or 4 (Figure 2). However, a statistical comparison of the wound surfaces among groups 2, 3 and 4 (4.50 ± 0.50 mm2, 3.25 ± 0.75 mm2, and 4.50 ± 0.95 mm2, respectively) showed no significant differences (p=0.40, Table 1).
| Group | 1 | 2 | 3 | 4 | P value (2, 3, 4) |
|---|---|---|---|---|---|
| Wound surface area (mm2) | |||||
| Day 13 | 0.40 ± 0.25 | 4.50 ± 0.50* | 3.25 ± 0.75 | 4.50 ± 0.95 | 0.4 |
| Epidermal thickness (µm) | |||||
| Day 13 | 35.45 ± 1.25 | 28.75 ± 2.05 | 28.75 ± 1.25 | 32.50 ± 1.70 | 0.26 |
Whitney test.
P<0.05 was considered statistically significant.
There was no statistical difference is found among groups (2, 3, and 4).
*: P<0.05 for compared with group 1.
Group 1: non-diabetic, group 2: non-treated (diabetic), group 3: nerve-block (diabetic), group 4: nerve block with IV zinc (diabetic).
Table 1: Histological analysis of control and three DFU groups.
Microscopic analysis
In the course of the study, three nerve blocks with or without IV zinc injection (on days 1, 3 and 10) were performed. Fullthickness skin-tissue samples were obtained two days after the final nerve block and IV zinc injection (day 13), and the histological changes were observed. The H & E and Masson's trichrome staining provided for a good view of the inflammatory cell infiltration and epidermal thickness (Figure 3) and collagen fiber (Figure 4), respectively. This enabled the one qualified pathologist to confirm that there were no histopathologically meaningful changes, and neither inflammatory cell infiltration nor significant epidermalthickness differences, among groups 2, 3 and 4: 28.75 ± 2.05 μm, 28.75 ± 1.25 μm, and 32.50 ± 1.70 μm, respectively (p=0.26, Table 1).
Discussion
One of the most common and serious chronic complications in diabetes is DFU, which in fact is the main cause of lower-limb amputation world-wide. Sadly, there is a 25% probability of a diabetic patient undergoing amputation in his or her lifetime [13]. Standard treatments of chronic wounds such as DFUs are debridement, minimal weight-bearing and vacuum dressing in cases of vascular insufficiency [14]. Nonetheless, DFUs often relapse, with poor prognosis and higher perioperative mortality. The five-year mortality rate after the first DFU attack ranges from 43 to 53%, and increases to 74% if the affected limb is amputated. This, significantly, is higher than the mortality rates of malignancies such as prostate and breast cancer [15]. In Europe, the survival rate following amputation is lower than in the United States: the reported three-year survival rates for Sweden and Italy are 59 and 50%, respectively [16]. In light of such dire statistics, a variety of diverse DFU treatments for prevention of lower-extremity amputation are currently being studied and reported on [3,14,17-19].
DFU pathogenesis, which is essential to DFU treatment research, is: 1) delayed cellular infiltration and granulationtissue formation; 2) decreased collagen organization; 3) decreased blood supply; 4) increased blood viscosity; 5) reduced angiogenesis. In general, wound healing manifests as a cascade of haemostasis, inflammation, proliferation, epithelialization, and scar maturation. Diabetes and its complications influence their interaction in the normal woundhealing process [16,17,20]. The pathogenesis-related factors impacting on the choice of peripheral nerve block and IV zinc in this study are as follows. 1) The beneficial effect of the nerve block in healing ulcers resides in the increased blood flow caused by its sympathectomy-like impact [7,8]. This effect of a single shot of nerve block is expected to be maintained over a relatively long span of time; research indicates, in fact, that the duration of action of peripheral nerve blocks is longer in diabetic patients than in non-diabetic individuals [21]. 2) Zinc, a cofactor in over 300 metalloenzymes, is an essential trace element in all organisms [22]. It is already known that zinc is required for wound healing and that topical application of zinc hyaluronate has a positive effect on DFUs [11]. Therefore, in this present study, we wanted to determine, through a DFU rat model, the practical wound-healing effects of nerve block alone and together with IV zinc.
Although streptozotocin-induced diabetic rats are commonly used in the field of diabetes studies [13], Streptozotocin, with its direct toxicity to pancreatic β-cells, leads to type 1 diabetes in experimental animals [23]. However, a genetically induced diabetic OLETF rat model, with LETO rats as a control group, seemed to be more suitable to the present DFU study than chemically induced one; the specific reason is that the OLETF rat model, with characteristics such as expression of diabetes after 18 weeks of age, obesity and diabetic nephropathy, is more representative of type 2 diabetes [24-27]. All such rats can suffer renal complications [27], which necessitated the present study’s lesser dosage of intraperiotoneal tiletaminezolazepam (Zoletil 50, Virbac, France), 60 mg/kg, for anesthesia [28]. Even so, two of the rats died. And because renal nephropathy possibly had further progressed in the 37- week-old rats, and also because only a minimally invasive procedure was planned, the present study’s rats were safely and adequately anesthetized by a reduced, 40 mg/kg dose of tiletaminezolazepam for the peripheral nerve block.
Conventionally, sciatic nerve block in rats has been done by surgical or percutaneous methods [29-33]. Based on recent ultrasound-based sciatic nerve block research in animals [34] as well as the related anatomy of the rat’s sciatic nerve [35-39], we performed ultrasound-guided sciatic nerve block as a minimally invasive method. The volume of local anaesthetics with reference to the preceding studies on sciatic nerve block in rats [29-34], was set to 0.2 ml 0.5% bupivacaine. The dose of IV zinc was calculated according to a human-to-animal dose-conversion formula [22,39]. The IV-administered zinc had to be diluted maximally due to the possible phlebitis complication. Even though the maximum IV injection volume for rats is less than 0.5 ml, the OLETF-rat weight makes 1.0 ml acceptable [39,40]. In our experimental design, we had planned to perform nerve block and administer IV zinc every day for one week, but the 37-week-old diabetic rats, with their expected nephropathy, did not show good enough recovery from the anesthesia to do so. In consideration of the condition of the rats with regard to anesthesia, the treatment intervals were set at two and seven days (days 1, 3 and 10) and woundtissue samples were obtained on day 13.
As far as the authors are aware, this is the first study to use peripheral nerve block and IV zinc for DFU healing. When we hypothesized that these two treatment means, both of which are well established, would be helpful for DFU healing, and we were confident of positive results; however, we were unable to achieve such results. As DFU wound healing involves multiple complex factors, it is actually very difficult to identify all possible cases associated with them. Therefore, in this study, there were some experimental limitations associated with variables such as the age of the rats, the number of nerve blocks and IV zinc injections, and the zinc dose, any or all of which could have influenced the results of this study. The outcomes might have been different, for example, if the nerve block and IV zinc injection had been more frequently performed or if continuous block by a catheter had been done and a constant blood level of zinc been maintained in some way or another.
Notwithstanding, we are convinced that this research will offer significant insight into DFU treatment helpful to subsequent DFU-management studies that doubtless will confirm the effects reported herein through the application of modified versions of the present experimental model.
Conclusion
Nerve block and/or IV zinc injection seemed to promote wound healing at first glance; however, the gross findings indicated no statistically significant differences in the wound surface areas among the three DFU groups. There were also no special findings upon histological examination. In light of these consistent results-statistical insignificance macroscopically, and non-specificity microscopically-it was determined that intermittent (three-time) nerve block and IV zinc injection did not have any significant effect on DFU healing.
Acknowledgements
This study was supported by a grant from Kosin University College of Medicine in 2014.
References
- Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention 2011.
- Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014; 103: 137-149.
- Singla S, Singla S, Kumar A, Singla M. Role of epidermal growth factor in healing of diabetic foot ulcers. Indian J Surg 2012; 74: 451-455.
- Alavi A, Sibbald RG, Mayer D, Goodman L, Botros M. Diabetic foot ulcers: Part II. Management. J Am Acad Dermatol 2014; 70: 21.
- Shemesh D, Raikhinstein Y, Orkin D, Goldin I, Olsha O. Anesthesia for vascular access surgery. J Vasc Access 2014; 15: 38-44.
- Shemesh D, Olsha O, Orkin D, Raveh D, Goldin I, Reichenstein Y, Zigelman C. Sympathectomy-like effects of brachial plexus block in arteriovenous access surgery. Ultrasound Med Biol 2006; 32: 817-822.
- Aguirre J, Del Moral A, Cobo I, Borgeat A, Blumenthal S. The role of continuous peripheral nerve blocks. Anesthesiol Res Pract 2012; 2012: 560879.
- Melsom H, Danjoux G. Perioperative care for lower limb amputation in vascular disease. Contin Educ Anaesth Crit Care Pain 2011; 11: 162-166.
- Khan SA, Qianyi RL, Liu C, Ng EL, Fook-Chong S, Tan MG. Effect of anaesthetic technique on mortality following major lower extremity amputation: a propensity score-matched observational study. Anaesthesia 2013; 68: 612-620.
- Dinh T, Tecilazich F, Kafanas A, Doupis J, Gnardellis C. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes 2012; 61: 2937-2947.
- Tankova T, Dakovska G, Koev D. Zinc hylauronate in the treatment of diabetic foot ulcers: a controlled randomized open-label study. Diabetol Croat 2001; 30: 93-96.
- Maldonado MA, Gil EB, Fernández SM, RM, González JA, Guijarro MA, de DLCJ. Zinc levels after intravenous administration of zinc sulphate in insulin-dependent diabetes mellitus patients. Klin Wochenschr 1991; 69: 640-644.
- Mendes JJ, Leandro CI, Bonaparte DP, Pinto AL. A rat model of diabetic wound infection for the evaluation of topical antimicrobial therapies. Comp Med 2012; 62: 37-48.
- Mehrannia M, Vaezi M, Yousefshahi F, Rouhipour N. Platelet rich plasma for treatment of nonhealing diabetic foot ulcers: a case report. Can J Diabetes 2014; 38: 5-8.
- Robbins JM, Strauss G, Aron D, Long J, Kuba J, Kaplan Y. Mortality rates and diabetic foot ulcers: is it time to communicate mortality risk to patients with diabetic foot ulceration? J Am Podiatr Med Assoc 2008; 98: 489-493.
- Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet 2003; 361: 1545-1551.
- Babaei S, Bayat M, Nouruzian M, Bayat M. Pentoxifylline improves cutaneous wound healing in streptozotocin-induced diabetic rats. Eur J Pharmacol 2013; 700: 165-172.
- Moura LI, Dias AM, Suesca E, Casadiegos S, Leal EC, Fontanilla MR, Carvalho L, de Sousa HC, Carvalho E. Neurotensin-loaded collagen dressings reduce inflammation and improve wound healing in diabetic mice. Biochim Biophys Acta 2014; 1842: 32-43.
- Elsharawy MA, Naim M, Greish S. Human CD34+ stem cells promote healing of diabetic foot ulcers in rats. Interact Cardiovasc Thorac Surg 2012; 14: 288-293.
- Kesici U, Kesici S, Ulusoy H, Yucesan F, Turkmen AU5. Effects of glutamine on wound healing. Int Wound J 2015; 12: 280-284.
- Cuvillon P, Reubrecht V, Zoric L, Lemoine L, Belin M, Ducombs O, Birenbaum A, Riou B, Langeron O. Comparison of subgluteal sciatic nerve block duration in type 2 diabetic and non-diabetic patients. Br J Anaesth 2013; 110: 823-830.
- Yasuno T, Okamoto H, Nagai M, Kimura S, Yamamoto T, Nagano K, Furubayashi T, Yoshikawa Y, Yasui H, Katsumi H, Sakane T, Yamamoto A. The disposition and intestinal absorption of zinc in rats. Eur J Pharm Sci 2011; 44: 410-415.
- Hue JJ, Kim JS, Kim JH, Nam SY, Yun YW, Jeong JH, Lee BJ. Anti-glycemic effect of L-carnosine in streptozotocin-induced diabetic mice. Korean J Vet Res 2012; 50: 105-111.
- Kawano K, Hirashima T, Mori S, Natori T. OLETF (Otsuka Long-Evans Tokushima Fatty) rat: a new NIDDM rat strain. Diabetes Res Clin Pract 1994; 24: 317-320.
- Kim JH, Chung HS, Kang M, Kim Y, Kim BS, Kim YS, Bae H. Anti-diabetic effect of standardized herbal formula PM021 consisting of Mori Folium and Aurantii Fructus on type II diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Diabetes Res Clin Pract 2011; 93: 198-204.
- King A, Bowe J. Animal models for diabetes: understanding the pathogenesis and finding new treatments. Biochem Pharmacol 2016; 99: 1-10.
- King AJ. The use of animal models in diabetes research. Br J Pharmacol 2012; 166: 877-894.
- Spinella G, Vilar JM, Anastasi C, Santana A, Prati U, Roveda L, Ricciardi G, Britti D. Three combinations of clonidine in association with tiletamine-zolazepam for anaesthesia induction in rats: evaluation of reflexes and pain sensibility. Veterinarni Medicina 2012; 57: 536-542.
- Kolarczyk LM, Williams BA. Transient heat hyperalgesia during resolution of ropivacaine sciatic nerve block in the rat. Reg Anesth Pain Med 2011; 36: 220-224.
- Brummett CM, Norat MA, Palmisano JM, Lydic R. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology 2008; 109: 502-511.
- Sousa AM, Ashmawi HA, Costa LS, Posso IP, Slullitel A. Percutaneous sciatic nerve block with tramadol induces analgesia and motor blockade in two animal pain models. Braz J Med Biol Res 2012; 45: 147-152.
- Kroin JS, Buvanendran A, Tuman KJ, Kerns JM. Effect of acute versus continuous glycemic control on duration of local anaesthetic sciatic nerve block in diabetic rats. Reg Anesth Pain Med 2012; 37: 595-600.
- Kroin JS, Buvanendran A, Williams DK, Wagenaar B, Moric M, Tuman KJ, Kerns JM. Local anaesthetic sciatic nerve block and nerve fiber damage in diabetic rats. Reg Anesth Pain Med 2010; 35: 343-350.
- Nijhuis TH, Smits ES, van Neck JW, Visser GH, Walbeehm ET, Blok JH, Hovius SE. Ultrasound-guided needle positioning near the sciatic nerve to elicit compound muscle action potentials from the gastrocnemius muscle of the rat. J Neurosci Methods 2011; 194: 283-286.
- Maciel FO, Viterbo F, Chinaque Lde F, Souza BM. Effect of electrical stimulation of the cranial tibial muscle after end-to-side neurorrhaphy of the peroneal nerve in rats. Acta Cir Bras 2013; 28: 39-47.
- Hillerup S, Bakke M, Larsen JO, Thomsen CE, Gerds TA. Concentration-dependent neurotoxicity of articaine: an electrophysiological and stereological study of the rat sciatic nerve. Anesth Analg 2011; 112: 1330-1338.
- Breshah MN, Sadakah AA, Eldrieny EA, Saad KA. Functional and histological evaluation of rat sciatic nerve anastomosis using cyanoacrylate and fibrin glue. Tanta Dent J 2013: 10; 67-74.
- Kochi T, Imai Y, Takeda A, Watanabe Y, Mori S, Tachi M, Kodama T. Characterization of the arterial anatomy of the murine hindlimb: functional role in the design and understanding of ischemia models. PLoS One 2013; 8: e84047.
- Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 2016; 7: 27-31.
- Ponrasu T, Suguna L. Efficacy of Annona squamosa on wound healing in streptozotocin-induced diabetic rats. Int Wound J 2012; 9: 613-623.