Research Article - Biomedical Research (2016) Volume 27, Issue 4
Synthesis and NAD(P)H: quinone oxidoreductase 1 inducer activity of acetamide and pyridine-3-carbonitrile derivatives
Mostafa M. Ghorab1,2*, Abdelaaty A. Shahat1,3, Mansour S. Alsaid1, Maureen Higgins4, Albena T. Dinkova-Kostova4,5 and Almoqbl A. Naser61Department of Pharmacognosy, College of Pharmacy, King Saud University, P.O. Box 2457, Riyadh 11451, Saudi Arabia
2Department of Drug Radiation Research, National Center for Radiation Research and Technology, Atomic Energy Authority, P.O. Box 29 Nasr City, Cairo, Egypt
3Phytochemistry Department, National Research Centre, 12311 Dokki, Cairo, Egypt
4Jacqui Wood Cancer Centre, Division of Cancer Research, Medical Research Institute, University of Dundee, Dundee DD1 9SY, United Kingdom
5Departments of Medicine and Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA
6Medicinal, Aromatic and Poisonous Plants Research Center (MAPPRC), College of Pharmacy, King Saud University, P.O. Box 2457, Riyadh 11451, Saudi Arabia
- *Corresponding Author:
- Mostafa M. M. Ghorab
Department of Pharmacognosy
College of Pharmacy
King Saud University
Riyadh 11451
Saudi Arabia
Accepted date: March 23, 2016
Abstract
Acetamide derivatives and one pyridone derivative were prepared. The structure of the synthesized compounds was verified through elemental analyses, 1H -NMR and 13C- NMR, IR spectra. The NQO1 inducer activity of the synthesized compounds was evaluated using a quantitative bioassay in Hepa1c1c7 murine hepatoma cells. The acetamide derivatives showed weak activity, with the 3-ethylphenyl being more potent than the 2-ethylphenyl derivative. The pyridone derivative was inactive.
Keywords
Acetamides, Pyridone, NQO1, Electrophilicity.
Introduction
The antioxidant activity is related with compounds competent of protecting a biological system against the potential harmful effects of oxidative processes. The antioxidant components in the last years have received enlarged care from medical researchers and nutritionists for their potential activities in stopping cancer, cardiovascular disorders, as well as aging [1]. Acetamide derivatives have been reported to possess a wide variety of important biological properties such as anti-malarial [2], antimicrobial [3-5], anti-proliferative [6], inhibitors of Pim-1 kinase [7], anti-inflammatory [8], anticonvulsant [9], antidyslipidemic, antioxidant [10], anti-HIV [11]. A large number of acetamide derivatives have been reported to show potential anticancer activity in vitro and in vivo [12-14]. In addition compounds containing heteroaromatic rings regularly are playing very important role as supports of bioactive substances. It is known that the pyridone and its derivatives are among the most popular N-heteroaromatic compounds integrated into the structures of many pharmaceutical compounds and their structural units occur in several molecules showing diverse biological activities [15-20]. Recently, a series of pyridine derivatives were reported to possess antioxidant, anticancer and angiotensin-I-converting enzyme (ACE-I) inhibitory activity [21-23]. As part of our continuing interest in this area, in the present work we synthesised acetamide derivatives (3, 5), and, dihydropyridine derivative (6) to evaluated their cytoprotective activity. We used induction of the cytoprotective enzyme NAD(P)H: quinone oxidoreductase 1 (NQO1) as a measure of cytoprotective activity.
Experimental
The melting points (°C, uncorrected) are determined in open capillaries on a Gallenkemp melting point apparatus (Sanyo Gallenkemp, Southborough, UK). Pre-coated with Silica gel plates (silica gel, 60 G F 254, 0.25 mm, Merck, Germany) are used for TLC, methanol/dichloromethane (0.5: 9.5 mL) mixture was used as a developing solvent system. IR spectra were recorded in KBr discs using IR-Shimadzu spectrometer (Shimadzu, Tokyo, Japan). NMR spectra in (DMSO-d6) were recorded on Bruker Ac-500 ultra-shield NMR spectrometer (Bruker, Flawil, Switzerland, δ ppm) at 500 MHz, using TMS as internal standard. Elemental analyses were performed on Carlo Erba 1108 Elemental Analyzer (Heraeus, Hanau, Germany). All compounds were within ± 0.4 % of the theoretical values.
2-Cyano-N-(2-ethylphenyl) acetamide (3)
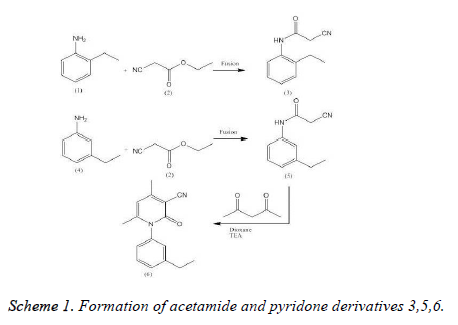
A mixture of 2-ethyl aniline 1 (1.21 g, 0.01 mol) and ethyl cyanoacetate 2 (1.13 g, 0.01 mol) was refluxed for 2 h, concentrated and cooled. The obtained solid was filtered andcrystallized from benzene/ether (3:2) give compound 3 [24].
Yield, 80 %, m.p. 130-132 °C; IR (KBr, max, cm-1): 3180 (NH), 2255 (CN), 1703 (CO). 1H- NMR spectrum of 3 in (DMSO-d6) δ : 1.09 [t, 3H, CH3], 2.23 [q, 2H, CH2], 3.85 [s, 2H, CH2CN], 7.3-7.5 [m, 4H, Ar-H], 9.45 [s, 1H, NH, exchangeable with D2O], “Anal. Calculated; for C11H12N2O C, 70.21; H, 6.38, 14.89; found: Carbon, 70.50; Hydrogen, 6.10; Nitrogen, 14.60.”
2- Cyano-N-(3-ethylphenyl) acetamide (5)
A mixture of 3-ethylaniline 4 (1.21 g, 0.01 mol) and ethyl cyanoacetate 2 (1.13 g, 0.01 mol) was fused at 220 °C (for 3 h). The concentrated reaction mixture was cooled. The gotten product was crystallized from the etahanol to afford compound 5 [25] (Scheme 1). The yield as 88 %, m. p. 86-88 °C; IR (KBr, nmax, cm-1): 3317 (NH), 3100 (CH. arom.), 2960, 2870 (CH aliph.), 2260 (CºN), 1670 (C=O). 1H NMR spectrum of 3 in (DMSO-d6)δ : 1.2 [t, 3H, CH3], 2.6 [q, 2H, CH2], 3.6 [s, 2H, CH2], 4.2 [s, 1H, NH, exchangeable with D2O], 7.03-7.7 [m, 4H, Ar-H]. “Anal. Calcd; for C11H12N2O: C, 70.21; H, 6.38, 14.89; found: C, 70.50; H, 6.10; N, 14.60.
1- (3-Ethylphenyl)-4, 6-dimethyl-2-oxo-1, 2- dihydropyridine-3 - carbonitrile (6)
Interaction of component 3 (1.88 g, 0.01 mol) with acetylacetone (1.00 g, 0.01 mol) in ethyl alcohol (50 mL), piperidine (0.5 mL) was refluxed for 5 h. The reaction mixture was triturated with ethyl alcohol and the obtained solid was recrystallized from dioxane to yield compound 6 [26] (Scheme 1). Yield: 86%. M.p.: 198200 °C. IR (KBr, ν, cm-1): 3100 (CH arom.), 2931, 2872 (CH aliph.), 2210 (C≡N), 1660 (C=O). 1H NMR (300 MHz, DMSOd6,δ, ppm): 1.2 (t, 3H, CH3, J=0.66 Hz), 1.5 (2s, 6H, 2CH3), 2.6 (q, 2H, CH2, J=0.66 Hz), 5.6 (s, 1H, CH pyridine), 7.27.7 (m, 4H, ArH). Anal. calcd. for C16H16N2O: C, 76.16; H, 6.39; N, 11.10. Found: C, 76.50; H, 6.70; N, 11.40%”.
Biological assay
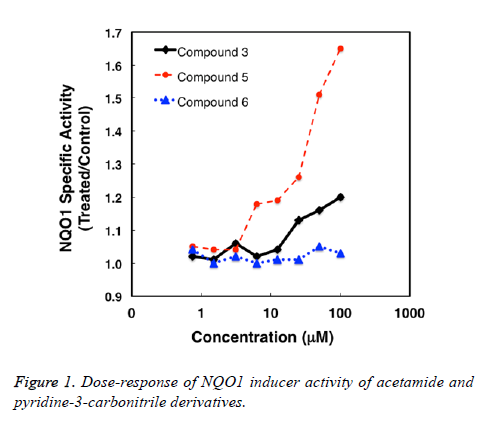
The NQO1 inducer activity was determined using a quantitative microtiter plate bioassay [27,28]. Compounds were prepared as stock solutions in DMSO, and then were diluted in the cell culture medium 1:1000. The final concentration of the solvent in the medium of the growing cells was 0.1% (v/v). Each compound was tested at eight replicates of 8 serial dilutions. The murine Hepa1c1c7 cell line was maintained at 37°C, 5% CO2, in α-MEM supplemented with 10% (v/v) fetal bovine serum that had been heat-and charcoalinactivated. Cells (104 per well) were plated in 96-well plates, and 24 h later, they were exposed to the compounds in fresh medium for a further 48 h. The cell culture medium was then removed; cells were washed 3 times with PBS, and lysed for 30 min at 25°C in digitonin (0.1 g/L, pH 7.8). To measure the enzyme activity of NQO1 the quinone menadione was used as a substrate. Protein concentrations were determined by the BCA assay (Thermo Scientific), and the values were used to calculate the specific NQO1 activity (Table 1 and Figure 1).
| Compounds | Induction Magnitude (Fold) |
| “2-Cyano-N-(2-ethylphenyl) acetamide (3)” | 1.2 |
| “2-Cyano-N-(3-ethylphenyl) acetamide (5)” | 1.65 |
| “1-(3-Ethylphenyl)-4,6-dimethyl-2-oxo-1,2-dihydropyridine- 3-carbonitrile (6)” | Inactive |
Table 1. NQO1 inducer activity of acetamide and pyridine-3- carbonitrile derivatives.
Results and Discussion
The aim of this work was to design and synthesis of a series of acetamides (3, 5) and pyridine (6) (Scheme 1) and evaluation of their potential NQO1 inducer activity. Interaction of 2-ethyl 1 or 3-ethylaniline 4 with ethyl cyanoacetate 2 furnished the corresponding acetamide derivatives 3 and 5, respectively. The structure of compounds 3 and 5 was proved in the basis of elemental analysis and spectral data. The corresponding pyridine derivative 6 was obtained via reaction of compound 5 with acetylacetone in the presence of a catalytic amount of piperedine. The structure of compound 6 was established by elemental analysis, spectral data.
NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1) is a cytoprotective enzyme which is activated by electrophilic compounds via the Keap1/Nrf2 pathway [29]. Numerous compounds which have been shown to induce NQO1 have been subsequently found to the broadly cytoprotective and to effectively inhibit tumor formation in animal models [30,31]. We found that the acetamide derivatives have weak NQO1 inducer activity. The 3-ethylphenyl (5) is more potent than the 2-ethylphenyl (3) derivative. The pyridone derivative (6) is inactive.
Conclusion
The present study objective to synthesize and evaluate the potential anticancer activities of some acetamide and pyridine derivatives. The acetamide derivative (5) was the most active.
Acknowledgements
Mostafa M. Ghorab1, Abdelaaty A. Shahat and Mansour S. Alsaid extend their truthful appreciation to the Deanship of Scientific Research at King Saud University for funding of this research through the Research Group Project no. RGPVPP- 262. Maureen Higgins and Albena T. Dinkova-Kostova are also grateful to Cancer Research UK (C20953/A10270) for financial support.
References
- Asif M, Singh A, Lakshmayya. Development of structurally diverse antitubercular molecules with pyridazine ring. Chronicles of Young Scientists 2013; 4: 1-8.
- Agarwal A, Srivastava K, Puri S, Sinha S, Chauhan P. A small library of trisubstituted pyrimidines as antimalarial and antitubercular agents. Bioorganic & medicinal chemistry letters 2015; 15: 5218-5221.
- Asif M, Singh A, Ratnakar. Antimicrobial Agents: Brief Study of Pyridazine Derivatives against Some Phathogenic Microrganisms. J Pharm Res 2011; 4: 664-667.
- Pedeboscq S, Gravier D, Casadebaig F, Hou G, Gissot A, De Giorgi F, Ichas F, Cambar J, Pometan JP. Synthesis and study of antiproliferative activity of novel thienopyrimidines on glioblastoma cells. European journal of medicinal chemistry 2010; 45; 2473-2479.
- Kassis P, Brzeszcz J, Bénéteau V, Lozach O, Meijer L, le Guével R, Guillouzo, C, Lewiński K, Bourg S, Colliandre L. Synthesis and biological evaluation of new 3-(6-hydroxyindol-2-yl)-5-(Phenyl) pyridine or pyrazine V-Shaped molecules as kinase inhibitors and cytotoxic agents. Eur. J. Med. Chem. 2011; 46: 5416-5434.
- Amir M, Javed S, Kumar H. Pyrimidine as antiinflammatory agent: A review. Indian journal of pharmaceutical sciences 2007; 69: 337-343.
- Asif M, Singh A. In-vivo anticonvulsant and in-vitro antimycobacterial activities of 6-aryl pyridazine-3(2h)-one derivatives. American Journal of Pharmacological Sciences 2014; 2, 1-6.
- Koneni V, Sashidhara, Gopala RP , Ranga P D, Ravi S, Khanna A K, Gitika B. Discovery of amide based fibrates as possible antidyslipidemic and antioxidant agents European Journal of Medicinal Chemistry 2012; 57: 302-310.
- Ölgen, S. Recent development of new substituted indole and azaindole derivatives as anti-HIVagents. Mini Rev. Med. Chem 2013; 13: 1700-1708.
- Xiang, Zhou PT, Wang L, Sun CY, Hu J, Zhao YL, Yang L. Novel Benzothiazole, Benzimidazole and Benzoxazole Derivatives as Potential Antitumor Agents: Synthesis and Preliminary in Vitro Biological Evaluation. Molecules 2012; 17: 873-883.
- Ahmed RA, El-Bendary E R, Ghaly MA, Shehata IA. Novel acetamid othiazole derivatives: Synthesis and in vitro anticancer evaluation. European Journal of Medicinal Chemistry. 2013; 69: 908-919.
- Situ Xue, Linlin Ma, Rongmei Gao, Yuhuan Lin , Zhuorong Lin. Synthesis and antiviral activity of some novel indole-2-carboxylate derivatives. Acta Pharmaceutica Sinica B. 2014; 4: 13–321.
- Ghorab MM, Ragab FA, Heiba HI, M. Agha H. Synthesis of Some Novel Sulfonamides Containing Biologically Active Alkanoic Acid, Acetamide, Thiazole, and Pyrrole Moieties of Expected Antitumor and Radiosensitizing Activities. J. Basic. Appl .Chem.,2011; 1: 8-14.
- Ghorab MM, Al-Saidand MS, El-Hossary EM. In vitro cytotoxic evaluation of some new heterocyclic sulfonamide derivatives. Journal of Heterocyclic Chemistry 2011; 48: 563–571.
- Taha NMH, Boray A AA, Saleh NM, Fouad SA. Synthesis and antitumor screening of some novel pyrrolo, pyrrolo[2,3-d] pyrimidinone and pyrrolo [2,3-b] pyridinone derivatives of sulfaquinoxaline. Journal of Applied Sciences Research. 2013; 9: 3108-3117.
- Akhtar T, Hameed S, Al-Masoudi NA, Lacolla RL. In vitro antitumor and antiviral activities of new benzothiazole and 1,3,4-oxadiazole-2-thione derivatives. Acta Pharm. 2008; 58: 135–149.
- El-Henawy AA, Mohamed S, Ma T, Ibrahim GAM, El-Hag A. Synthesis of New Sulfonamide Scaffolds Acting As Anticancer Targeting CAII Protein Based Docking Studies. New York Science Journal.2011; 4: 20-29.
- Mohammadi-Farania A, Heidarianb N, Aliabadi A. N-(5-Mercapto-1,3,4-Thiadiazol-2-yl)-2-Phenylacetamide Derivatives: Synthesis and In-vitro Cytotoxicity Evaluation as Potential Anticancer Agents. Iranian Journal of Pharmaceutical Research. 2014; 13: 487-492.
- El-Henawya AA, Mohamedb SI, Hassana AA, Halawaa AH, Elnassaga MA, Elhagea GA. Discovery Potent of Novel Peptide Derivatives Containing Sulfonamide Moiety As Inhibitors of CA Using Structure Based Virtual Screening and Binding Assays. New York Science Journal. 2011; 4: 19-25.
- Chegwidden WR, Spencer IM. Sulphonamide inhlbitors of carbonic anhydrase inhibit the growth of human lymphoma cells in culture. InflammoPharmacology. 1995; 3: 231-239.
- Miriana M, Zarghib A, Sadeghia S, Tabarakic P, Tavallaeec M, Dadrassc O, Sadeghi-aliabadi H. Synthesis and Cytotoxic Evaluation of Some Novel Sulfonamide Derivatives Against a Few Human Cancer Cells. Iranian Journal of Pharmaceutical Research 2011; 10: 741-748.
- Scozzafava A,Owa T,Mastrolorenzo A,Supuran CT. Anticancer and antiviral sulfonamides. Curr Med Chem, 2003; 10 : 925-53.
- Ren Y, Zhang L, Zhou C-He, Geng R-X. Recent Development of Benzotriazole-based Medicinal Drugs. Med chem 2014; 4: 640-662.
- Zareef M, Iqbal R, Mirza B, Khan KM, Manan A, Asim F, Khan SW. Synthesis and antimicrobial activity of some derivatives of acylhydrazine including novel benzenediaza sulfonamides ARKIVOC, 2008; 141-152.
- Magda MF, Noaman E. Novel pirfenidone analogs as antifibrotic agents: Synthesis and antifibrotic evaluation of 2-pyridones and fused pyridines. Med Chem Res 2005; 14: 382-403.
- Al‐Saida MS, El‐Gazzarb MG, Ghorab MM. In‐vitro cytotoxic and radiosensitizing evaluation of novel 2‐pyridone, isoquinoline, chromene and chromenopyridone derivatives. Eeu J Chem 2012; 3: 228‐234.
- Prochaska HJ, Santamaria AB. Direct measurement of NAD(P)H:quinone reductase from cells cultured in microtiter wells: a screening assay for anticarcinogenic enzyme inducers. Anal Biochem 1988; 169: 328-336.
- Fahey JW, Dinkova-Kostova AT, Stephenson KK, Talalay P. The "Prochaska" microtiter plate bioassay for inducers of NQO1. Methods Enzymol 2004; 382: 243-258.
- Dinkova-Kostova AT, Talalay P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys 2010; 501: 116-123.
- Liby KT, Sporn MB. Synthetic oleananetriterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease Pharmacol Rev 2012; 64: 972-1003.
- Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer 2007; 7: 357-369.