Research Article - Biomedical Research (2016) Health Science and Bio Convergence Technology: Edition-I
Synthesis and biological evaluation of tetrahydrobenzo[b]pyran derivatives as potential anti-ovarian cancer agents
Chuan Wang*Department of Obstetrics and Gynecology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, China
- *Corresponding Author:
- Chuan Wang
Department of Obstetrics and Gynecology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, China
Accepted on May 10, 2016
Abstract
The synthesis and characterization of four novel pyran derivatives (1-4) have been done by means of IR, 1H NMR, HRMS, and single crystal X-ray crystallography. The antitumor activities of the four compounds were studied in order to inhibit three human ovarian cancer cells, which are OVCAR, HRA and COC1, growth by MTT assay. It was found that compound 4 exerted much more potent activities against all of these cell lines than other compounds did.
Keywords
Pyran, Crystal, Ovarian cancer.
Introduction
Oophoroma is one of the most common malignant tumours in the world [1]. Currently, the traditional therapies of surgery, chemotherapy and radiation therapy play an important role in the systemic treatment of ovarian cancer [2]. However, the treatment outcome is generally poor. Thus, it is very important to find an effective alternative treatment for oophoroma [3].
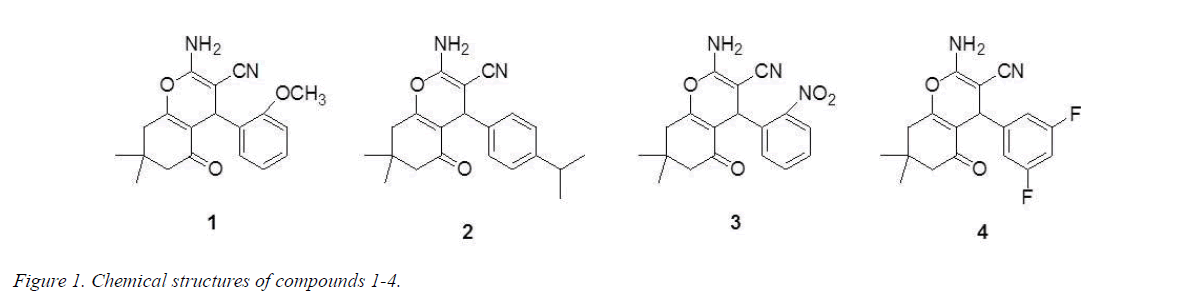
Much attention has been caught among many chemists in organic and medicinal filed on the synthesis of pyrans and their derivatives for many years as a great deal of natural and synthetic products contain this heterocyclic nucleus. Pyrans has a variety of pharmacological and biological activities including antitumor, analgesic and ulcerogenic, anti-inflammatory, anticoagulant, photo triggering, and fungicidal properties, moreover, it can be applied as anticoagulants in the production of pesticides [4-6]. In particular, the antitumor activity of pyran compounds has received considerable attention among researchers because of their cytotoxic activity against numerous types of cancers, including malignant melanoma, leukemia, renal cell carcinoma, prostate and breast cancer cell progression [7,8]. As mentioned above, we are here to report the synthesis of four new pyran derivatives (Figure 1) as well as their antitumor activity.
Experimental
Apparatus and materials
The way to obtain IR spectra (400-4000 cm-1) is using a Brucker Equinox-55 spectrophotometer, 1H NMR spectra using a Varian Inova-400 spectrometer (at 400 MHz), Mass spectra using a micrOTOF-Q II mass spectrometer. What is more, a XT-4 micro melting apparatus plays a role as taking on the melting points, and the thermometer remained uncorrected.
Three human ovarian cancer cells (OVCAR, HRA and COC1) were obtained by paying to Institute of Basic Medical Sciences (IBMS) of Chinese Academy of Medical Sciences (CAMS) (Beijing, China).
Synthesis and characterization of compounds 1-4
The synthesis of compounds 1-4 were on the basis of a reported procedure [9]. A mixture of 1,1-dimethyl-3,5- cyclohexanedione (10 mmol), aromatic aldehyde (10 mmol), malononitrile (10 mmol) and 4-(dimethylamino) pyridine (DMAP) (1 mmol) in ethanol (100 mL) was refluxed for 2-3 h and then cooled to room temperature. After filtering the sediments, they were orderly washed with ice-cooled water and ethanol finally becomes dried under a vacuum.
2-Amino-4-(2-methoxyphenyl)-3-cyano-7,7-dimethyl-5- oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran (1): 208-209°C. IR (KBr pellet cm-1): 3406, 2155, 1639, 1530, 1323, 1041, 761 cm-1. 1H NMR (DMSO-d6, δ, ppm): 7.143-7.186 (m, 1H), 6.944-7.008 (m, 2H), 6.819-6.873 (m, 3H), 4.484 (s, 1H), 3.755 (s, 3H), 2.431-2.574 (m, 2H), 2.233-2.274 (d, 1H), 2.049-2.089 (d, 1H), 1.050 (s, 3H), 0.974 (s, 3H). HRMS (ESI +): m/z: calcd for C19H20N2O3: 347.1366 [M+Na+]; found: 347.1327.
2-Amino-4-(isopropylphenyl)-3-cyano-7,7-dimethyl-5- oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran (2): 210-211°C. IR (KBr pellet cm-1): 3370, 3330, 3181, 2962, 2187, 1650, 1601, 1373, 1241, 1134, 1034, 846 cm-1. 1H NMR (DMSO-d6, δ, ppm): 7.149-7.169 (d, 2H), 7.037-7.057 (d, 2H), 6.964 (s, 2H), 4.135 (s, 1H), 2.822-2.856 (t, 1H), 2.503-2.521 (m, 1H), 2.237-2.277 (d, 1H), 2.139 (s, 1H), 2.092 (s, 1H), 1.191 (s, 3H), 1.174 (s, 3H), 1.046 (s, 3H), 0.977 (s, 3H). HRMS (ESI +): m/z: calcd for C21H24N2O2: 359.1730 [M+Na+]; found: 359.1788.
2-Amino-4-(2-nitrophenyl)-3-cyano-7,7-dimethyl-5- oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran (3): 239-240°C. IR (KBr pellet cm-1): 3446, 2360, 1666, 1546, 1353, 1091, 761 cm-1. 1H NMR (DMSO-d6, δ, ppm): 7.810-7.833 (q, 1H), 7.643-7.684 (m, 1H), 7.408-7.450 (m, 1H), 7.352-7.374 (q, 1H), 7.195 (s, 2H), 4.947 (s, 1H), 2.439-2.563 (m, 1H), 2.187-2.228 (d, 1H), 2.000-2.090 (t, 2H), 1.019 (s, 3H), 0.888 (s, 3H). HRMS (ESI+): m/z: calcd for C18H17N3O4: 362.1111 [M+Na+]; found: 362.1267.
2-Amino-4-(3,5-difluorophenyl)-3-cyano-7,7-dimethyl-5- oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran (4): 230-241°C. IR (KBr pellet cm-1): 3398, 3211, 2198, 1683, 1659, 1620, 1601, 1373, 1251, 1037, 992, 734 cm-1. 1H NMR (DMSO-d6, δ, ppm): 7.131 (s, 2H), 7.035-7.093 (m, 1H), 6.842-6.888 (m, 2H), 4.281 (s, 1H), 2.465-2.610 (m, 1H), 2.151-2.272 (q, 2H), 2.091 (s, 1H), 1.039 (s, 3H), 0.983 (s, 3H). HRMS (ESI+): m/z: calcd for C18H16F2N2O2: 353.1072 [M+Na+]; found: 353.1099.
Crystal structure determination
According to the evaporation of chloroform solution, Suitable single crystals of compound 1 come to hand. The diffraction data were acquired on a Bruker Smart Apex CCD area detector using a graphite monochromated Mo Kα radiation (λ=0.71073 Å) at room temperature. The structure was solved by using the program SHELXL-97 [10] and Fourier difference techniques, and refined by full-matrix least-squares method on F2. All hydrogen atoms were added academically. Crystallographic data for compound 1 have been showed in Table 1.
| Formula | C19H20N2O3 |
|---|---|
| Mr | 324.37 |
| Temperature/K | 293 (2) |
| Crystal system | Monoclinic |
| Space group | P21/n |
| a/Å | 8.8915 (4) |
| b/Å | 17.5552 (9) |
| c/Å | 11.0684 (5) |
| α/° | 90 |
| β/° | 97.750 (4) |
| γ/° | 90 |
| V/Å3 | 1711.91 (14) |
| Z | 4 |
| Dcalc/g·cm-3 | 1.259 |
| μ(Mo Kα)/mm-1 | 0.086 |
| θ range/° | 2.32 to 25.00 |
| Reflections collected | 6694 |
| No. unique data[R(int)] | 3020 [0.0227] |
| No. data with I ≥ 2σ(I) | 2257 |
| R1 | 0.0445 |
| ωR2(all data) | 0.1161 |
| CCDC | 1477404 |
Table 1. Crystal data, data collection and structure refinement of compound 1.
Antitumor activity
Viability of human ovarian cancer cells (OVCAR, HRA and COC1) was determined with the help of the MTT assay. Once the confluency of cells reaches to 70-80% that was treated with all kinds of concentrations of the synthesized compounds with 1% dimethyl sulfoxide (DMSO) as a negative control. And then the cells were incubated for 48 h, another 4 h incubation were done after adding 20 μL of MTT solution (5 mg/mL in PBS). What after, the medium was aspirated carefully, and added with 150 μL of DMSO. After taking 15 min to incubate, the FlexStation 3 benchtop multi-mode microplate reader (Molecular Devices, USA) measured the optical density which is 490 nm. Data were recorded and analyzed for the procedure of the effects of the test substances on cell viability and growth inhibition.
Results and Discussion
Molecular structure
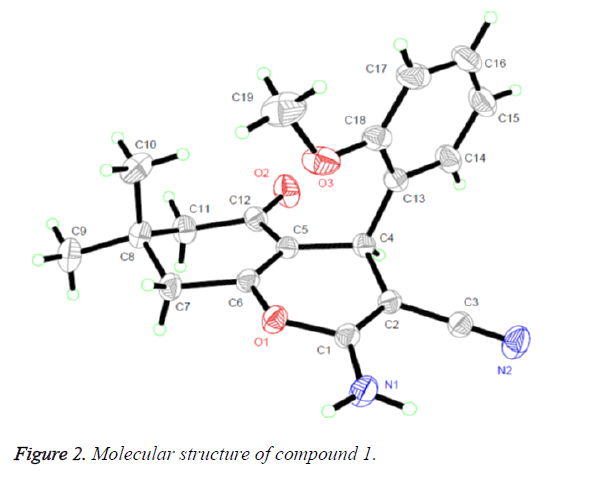
The 1H NMR, IR and MS for the products are suit for the title compounds. In the 1H NMR spectra of compounds 1-4, single peaks at δ 4.484, δ 4.135, δ 4.947 and δ 4.281 ppm characteristic of the CH group respectively. The single crystals of compound 1 were cultured for X-ray diffraction analysis so that can make the confirmation of the configuration of the product.
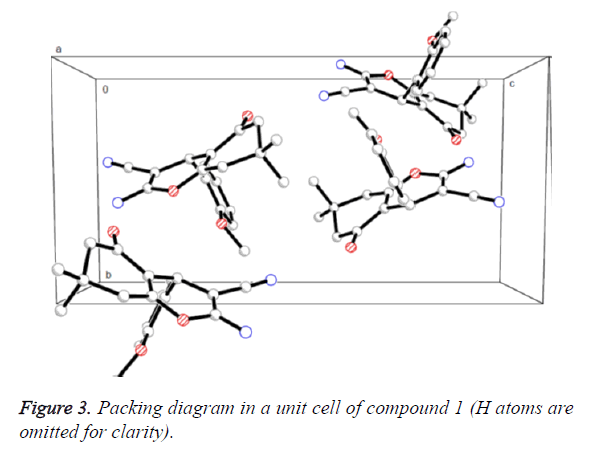
Figure 2 presented us the crystal structure of compound 1. The 4H-pyran ring of compound 1 is nearly planar [maximum deviation=0.0685 Å] and the adjoining ketone ring takes a flattened chair conformation [dihedral angle (C5-C6-C7- C8)=15.654°]. The 4H-pyran ring is nearly in vertical direction to the benzene ring [dihedral angle=86.324°] and is almost coplanar with the mean plane of the ketone ring [dihedral angle=8.767°]. In addition, the molecules were collected to form a three-dimensional structure through π-π stacking together with no intermolecular hydrogen bonding interactions, as illustrated in Figure 3.
Antitumor activity
Three human ovarian cancer cells (OVCAR, HRA and COC1) ,which is the representative three tumor types respectively, were used in the systematic analysis of the antitumor activities of the newly synthesized compounds 1-4 in vitro. For the sake of comparing, the evaluation of the cytotoxicity of cyclophosphamide, a standard antitumor drug, was set in the same condition. The results represent that the four compounds possessed a set number of antitumor activities against the three tumor cell lines and their inhibitory action become intensify with the corresponding higher concentration. The relevant half maximal inhibitory concentration (IC50) and IC90 values (dose of the compound which cause a different reduction of survival values, one is 50% and the other one is 90%) are listed in Table 2.
| Drugs | SMMC-7721 | Hep G2 | HB611 | |||
|---|---|---|---|---|---|---|
| IC50 | IC90 | IC50 | IC90 | IC50 | IC90 | |
| Compound 1 | 286.21 | 567.44 | 312.32 | 601.22 | 296.01 | 610.31 |
| Compound 2 | 301.26 | 612.36 | 298.31 | 589.43 | 356.32 | 623.62 |
| Compound 3 | 311.34 | 656.33 | 334.11 | 612.55 | 412.12 | 654.23 |
| Compound 4 | 26.11 | 50.21 | 23.44 | 45.23 | 71.23 | 132.34 |
| Cyclophosphamide | 55.30 | 110.99 | 58.89 | 98.45 | 76.07 | 145.67 |
Table 2. IC50 and IC90 values of compounds 1-4 and cyclophosphamide against three tumor cell lines (μg/mL).
As can be seen in Table 2, there is great difference in the antitumor activity among the four compounds. Compound 4 represented much stronger antitumor activity against the three human ovarian cancer cells with IC50 and IC90 values of 23.44-71.23 μg/mL and 45.23-132.34 μg/mL, respectively, which is much lower than the IC50 and IC90 values (55.30-76.07 μg/mL and 98.45-145.67 μg/mL) of the standard antitumor drug cyclophosphamide. However, compounds 1-3 showed lower antitumor activity with relatively higher IC50 and IC90 values.
Conclusion
In this study, four new pyran compounds were synthesized and were found to exhibit cytotoxicity towards three human ovarian cancer cells (OVCAR, HRA and COC1). It showed that the potential of pyran compounds to be developed into the new antitumor agent. However, the exact target is still unknown, whether it has an exact target or is just a cytotoxic agent, the detailed mechanisms of the inhibitory effects need to be further investigated.
References
- Douillard S, Olivier D, Patrice T. In vitro and in vivo evaluation of radachlorin(R) sensitizer for photodynamic therapy. PhotochemPhotobiolSci 2009; 8: 405-413.
- Xia CH, Wang BQ, Wang Y. Damaging effects of nanosized TiO2 on bel-7402 human liver cancer cell under photoinduce. J Inorg Mater 2006; 21: 1467-1471.
- Lim SH,Thivierge C, Nowak-Sliwinska P. In vitro and in vivo photocytotoxicity of boron dipyrromethene derivatives for photodynamic therapy. J Med Chem 2010; 53: 2865-2874.
- Farag AM, Mayhoub AS, Barakat SE. Synthesis of new N-phenylpyrazole derivatives with potent antimicrobial activity. Bioorg Med Chem 2008; 16: 4569-4578.
- Insuasty B, Tigreros A, Orozco F. Synthesis of novel pyrazolic analogues of chalcones and their 3-aryl-4-(3-aryl-4,5-dihydro-1H-pyrazol-5-yl)-1-phenyl-1H-pyrazole derivatives as potential antitumor agents. Bioorg Med Chem 2010; 18: 4965-4974.
- Farag AM, Mayhoub AS, Barakat SE. Regioselective synthesis and antitumor screening of some novel N-phenylpyrazole derivatives. Bioorg Med Chem 2008; 16: 881-889.
- Wang Z, Shi XH, Wang J. Synthesis, structure-activity relationships and preliminary antitumor evalutation of benzothiazole-2-thiol derivatives as novel apoptosis inducers. Bioorg Med ChemLett 2011; 21: 1907-1101.
- Capdeville R, Buchdunger E, Zimmermann J. Glivec (STI571, imatinib), a rationaly developed, targeted anticancer drug. Nat Rev Drug Discov 2002; 1: 493-502.
- Ramiz MMM, El-Sayed WA, El-Tantawy AI. Antimicrobial activity of new 4,6-disubstituted pyrimidine, pyrazoline, and pyran derivatives. Arch Pharm Res 2010; 33: 647-654.
- Sheldrick GM. SHELXL-97, Program for Solution Crystal Structure and Refinement, University of Göttingen, Germany1997.