- Biomedical Research (2016) Volume 27, Issue 2
Synergistic effects of ascorbyl palmitate and sodium ascorbyl phosphate loaded in multiple emulsions on facial skin melanin and erythema content.
| Hira Khan1, Naveed Akhtar1, Haji M Shoaib Khan1, Atif Iqbal Arshad1, Muhammad Naeem1, Muhammad Sohail1, Atif Ali2*, Fatima Rasool3, Zarqa Nawaz4 1Department of Pharmacy, Faculty of Pharmacy and Alternative Medicine, the Islamia University of Bahawalpur, Bahawalpur 63100,Pakistan 2Department of Pharmacy, COMSATS Institute of Information Technology, Abbottabad 22060, Pakistan 3University College of Pharmacy, the University of Punjab, Lahore, Pakistan 4Department of Chemistry, the Islamia University of Bahawalpur, Bahawalpur 63100,Pakistan |
| Corresponding Author: Atif Ali Department of Pharmacy COMSATS Institute of Information Technology Pakistan |
| Accepted: February 16, 2016 |
Abstract
Hyperpigmentation, such as melasma, postinflammatory melanoderma and dermatitis caused by increased production and accumulation of melanin are the major problems today. Ascorbyl Palmitate (AP) and Sodium Ascorbyl Phosphate (SAP), derivatives of ascorbic acid, having the inhibitory effect on skin melanin production and also has anti-inflammatory activity. Aim of this study was to investigate the synergistic effects of ascorbyl palmitate and sodium ascorbyl phosphate loaded in three multiple emulsion formulations i.e. ME1, ME2, and ME3 on facial skin melanin and erythema contents of Asian human females over 12-week treatment course. Thirty three female volunteers were enrolled to singleblinded, placebo-controlled, split-face trial, 3 groups of 11 volunteers each were treated with active treatments versus control/placebo for a period of 12 weeks. Evaluation was performed with non-invasive bioengineering techniques. Patch testing showed no side effects. Control multiple emulsion showed insignificant results while active multiple emulsion formulations showed a significant decrease in skin melanin and erythema content after statistically applied ANOVA (p <0.05). Ascorbyl palmitate and sodium ascorbyl phosphate are potent antioxidants. Treatments of human skin with active formulations; ME1, ME2, and ME3 containing Ascorbyl palmitate and sodium ascorbyl phosphate significantly reduces facial skin melanin and erythema thus could be explored further for the treatment of pigmentation disorders and dermatitis.
Keywords |
||||||
| Ascorbyl palmitate, Sodium ascorbyl phosphate, Multiple emulsions, Melanin, Erythema. | ||||||
Introduction |
||||||
| Excessive production and accumulation of melanin leads to many hyperpigmentation disorders such as melasma, postinflammatory pigmentation, solar lentigo, etc., which become prominent with aging [1]. Many modalities for acquired skin hyperpigmentation and inflammatory disorders of skin are available in the form of chemical agents or physical therapies [2]. | ||||||
| Commercially available skin lightening agents target the natural production of melanin and many of these agents are known as competitive inhibitors of tyrosinase, one of key enzymes in melanin production [3]. Vitamin C (L-Ascorbic acid) is the most potent antioxidant and natural controller of melanin formation. Anti-melanogenesis of Vitamin C were reported to result from its action as reducing agent at various oxidative steps of melanin formation. Decreased tyrosinase activity mediated by vitamin C seems to be caused by antioxidant activity and, not by the direct inhibition of tyrosinase activity [4]. Moreover, topical L-ascorbic acid has been shown to protect porcine skin from UVB induced erythema [5]. Although ascorbic acid has a range of physiological and pharmacological functions but its poor liposolubility limits the cumulative amount of ascorbic acid in the cells after permeating through the cell membrane [6]. | ||||||
| Ascorbyl Palmitate (AP), an amphiphilic ascorbic acid derivative, has better stability and ability to penetrate the skin than ascorbic acid [7]. Ascorbyl palmitate acted as an antioxidant and as an anti-inflammatory agent. Sodium Ascorbyl Phosphate (SAP) is another strong and effective antioxidant which is cleaved by enzymes in the skin to release free ascorbic acid which protects cells against free radicals [8]. SAP acts on melanin formation process in the skin and prevents hyperpigmentation process. Thus it has skin lightening properties and therefore used in skin whitening preparations [9]. Our aim of this study was to investigate the synergistic effects of AP and SAP in three multiple emulsion formulations i.e. ME1, ME2, and ME3 on the human skin melanin and erythema contents of Asian human females over 12-weeks treatment course. | ||||||
Material and Methods |
||||||
| Ascorbyl palmitate (6-palmitoyl-L-ascorbic acid) with a CAS Number: 137-66-6, Empirical Formula: C22H38O7, Molecular Weight: 414.53 and sodium ascorbyl phosphate (Sodium Lascorbyl- 2-phosphate) with a CAS Number: 66170-10-3, Empirical Formula: C6H6Na3O9P × H2O, Molecular Weight: 322.05 (anhydrous basis) were purchased from Sigma Aldrich and identified through FTIR technique from the Islamia University Bahawalpur. | ||||||
| Multiple emulsions were developed using Cetyl Dimethicone copolyol as lipophilic emulsifier and polysorbate-80 as hydrophilic emulsifier. Multiple emulsions were of water-inoil- in-water emulsion (W/O/W) type. Four multiple emulsion formulations were tested in this study, having different composition and named ME1 (with 0.5% AP in oil phase and 0.25% SAP in internal and external water phase of W/O/W emulsion. ME2 (with 0.5% AP in oil phase and 0.5% SAP in external water phase of W/O/W emulsion). ME3 (with 0.5% AP in oil phase and 0.5% SAP in internal water phase of W/O/W emulsion) and a control emulsion without active ingredients. To find actual skin irritation potential of main ingredients in multiple emulsions, no preservative or any other agent was added except active compounds, emulsifiers and oil phase. Composition of tested multiple emulsions is given in table 1. | ||||||
| Two-step emulsification procedure was adopted for the preparation of multiple emulsions [10]. Primary W/O emulsion was prepared by emulsifying the oil phase with the aqueous phase in the presence of lipophilic surfactant. Both phases were preheated to 75°C in a digital water bath (Heidolph, Germany) before mixing. Mixing of W/O emulsion components was done using IKA Mixing Overhead Stirrer, Eurostar (IKA, Werke, Germany) at 2000 rpm to obtain small inner droplets. In secondary emulsification step, W/O emulsion was dispersed in external aqueous phase containing hydrophilic emulsifier to attain W/O/W multiple emulsion. This step was carried out under low speed (700 rpm) for 40 minutes to avoid rupturing of droplets. Formulated W/O/W emulsions were confirmed by microscopy (Figure 1). | ||||||
| A sample of 33 female volunteers with the age range of 22-25 years was recruited in this study. The sample was further divided into three groups, each having 11 volunteers. This monocentric study was carried out in Cosmetics Lab of the Islamia University of Bahawalpur and conducted according to the principles of Declaration of Helsinki. A written informed consent was taken from all the participants of the study. Participants were informed about possible adverse reactions, procedures, protocols and objectives of this study and they have the rights to quit study without informing about such reasons. Prior to the study, a cosmetic expert examined each volunteer for any type of skin reaction/sensitivity. Moreover, study participants were not informed about the contents of tested products to ensure blindness in study. The inclusion criteria were no history of hypersensitivity, every individual should sign the volunteer protocol and have to come to the laboratory for measurements on specific time intervals. The criteria for exclusion were: presence of any dermatitis/allergic diseases, smokers and previous treatment of forearms’ skin with sunscreens, moisturizers or anti-ageing cosmetics. | ||||||
| Before any measurement, all the volunteers were asked to have a rest in Cosmetic Lab, under constant environmental conditions of 22 ± 2°C and 50 ± 5% relative humidity, for at least 30 minutes. All the assessments recorded by single assessor to nullify person to person variations especially for objective assessments. Non-invasive bioengineering measurements were done. The melanin and erythema measurements (EI) were performed with a reflectance spectrophotometer, Mexameter from Courage and Khazaka Electronic GmbH, Cologne, Germany. The Mexameter was calibrated according to manufacturer’s guidelines. | ||||||
| A patch test was performed on forearms of each volunteer to find out any possible irritation/sensitivity of formulations. For single application closed patch test (48 hours), a specific area (5 cm × 4 cm) was marked on the right and left forearms of all human female volunteers. A small amount (~1g) of each active formulation was applied on the left forearms and respective vehicle control (without active ingredient) on right forearms of all volunteers. Volunteers were divided into three groups. In Group-I (11 volunteers), ME1 multiple emulsion was applied on left forearms and its vehicle Control (without active) was applied on right forearms. In Group-II (11 volunteers), ME2 multiple emulsion was applied on left forearms and its vehicle control was applied on right forearms. Similarly, in Group-III (11 volunteers) ME3 multiple emulsion was applied on left forearms and its vehicle control was applied on right forearms. This area of forearms was covered with semi-occlusive cotton bandage, fixed with adhesive tape (closed patch test). | ||||||
| Readings were taken on zero hour i.e. before application of any product on the marked sites. Then after 48 hours, the enclosed patches (for primary skin irritation testing) were removed and an experienced dermatologist observed forearms for any skin irritation. Mexameter was used for this purpose. In vivo investigations were carried out during the month of January to march. All instrumental measurements were completed by the author according to the manufacturer’s instructions. Volunteers were instructed not to use any other skin care product, two weeks before the study beginning and during the treatment period. Each volunteer was provided with two vessels with 50 g emulsion. The vessels with emulsions were marked as “right and left” which were specified for right and left cheeks respectively. The right cheek was specified for control emulsion while the left cheek was specified for test emulsion. Participants were instructed about proper application of the products and were reminded regularly about the use of product. They daily used the products at bedtime on respective half of the face. Measurements of skin melanin and erythema were made at baseline and during the week 2nd, 4th, 6th, 8th, 10th and 12th. | ||||||
| A questionnaire Performa containing seven questions (Panel test) was circulated in a duplicate to each volunteer for sensory assessment of ME1, ME2, and ME3 and respective Control/ Placebo at the end of study. Average points were calculated from the points assigned by each volunteer for each question for all of the Control and Active multiple emulsion formulations for 12-week study period. Parameters included were Ease of application, Spreadability, Sensation just after application, Sensation in the long term, Irritation, Shine on skin and Sensation of softness. Each of above parameter was assigned 11 values which ranged from –5 to +5 indicating very bad to very good, with 0 as baseline respectively. This form was completed independently by each volunteer on 12th week of study period. The melanin and erythema values of the right and left cheek of the volunteers were calculated at baseline (0 hr), in the week 2nd, 4th, 6th, 8th, 10th and 12th week. SPSS (version 20) was used for data analysis on the computer by using the two-way ANOVA for variation between different time intervals and the paired sample t-test for the variation between the three active formulations at the 5% level of significance. | ||||||
Ethical standards |
||||||
| This study was approved by the Board of Advance Studies and Research and Ethical Review Committee, The Islamia University of Bahawalpur (No. 975/AS & RB). The study was conducted in accordance with the Good Clinical Practice guidelines. | ||||||
Results |
||||||
Patch test for skin erythema |
||||||
| Percentage change of skin erythema content for all the 33 female volunteers after the application of control and active multiple emulsions; ME1, ME2 and ME3 for 48 hours were found to be -3.33+5.71 and -3.77+6.90, respectively. Two-way ANOVA test was applied, active multiple emulsion formulations showed insignificant results in skin erythema with respect to control. All values were measured in triplicates (n=3) Results showed that erythema content decreased with the use of active multiple emulsions (ME1, ME2 and ME3) while increased slightly with the use of control multiple emulsion. | ||||||
Melanin content |
||||||
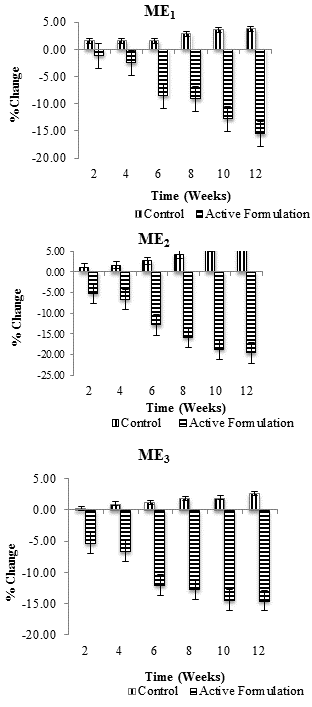
| Percentage change (average) in skin melanin values in each individual after application of Control and ME1, ME2 and ME3 were measured for 3 months at specific time intervals i.e., at 2nd, 4th, 6th, 8th, 10th and 12th week of study period. Combined result for percentage change in melanin with respect to time is shown in figure 1. All values were measured in triplicates (n=3). Melanin content was found to decrease with active multiple emulsions while increased with the use of control multiple emulsion. | ||||||
Erythema content |
||||||
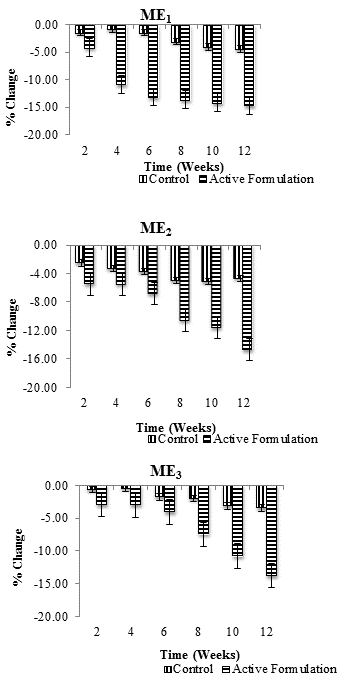
| Percentage change (average) in skin erythema values in each individual after application of control and ME1, ME2 and ME3 were measured for 3 months at specific time intervals i.e., at 2nd, 4th, 6th, 8th, 10th and 12th week of study period. Combined result for percentage change in erythema with respect to time is shown in Figure 2. All values were measured in triplicates (n=3). Erythema content was found to decrease with both active multiple emulsions as well control but this decrease was more pronounced for active multiple emulsions (Figure 3). | ||||||
Panel test |
||||||
| Results for panel test for control and active multiple emulsions ME1, ME2, and ME3 are given in table 2. All measurements were monitored in triplicates (n=3). Panel test results showed the acceptability and appropriateness of control and active multiple emulsions for topical use. | ||||||
Discussion |
||||||
| Patch testing is a widely used procedure to evaluate acute irritant reactions of any formulation [11]. No erythema was observed after the application of ME1, ME2, and ME3. However, a very slight erythema was observed after the application of control multiple emulsion which was ignorable. Finally, from the patch test of 48 h, it was found that both, the active formulations and control produced no skin irritation, so both can be applied safely on human skin for in vivo evaluation. | ||||||
| Melanin, a pigmented polymer, provides photo-protection of the skin against UV radiation. Melasma and other hyperpigmented disorders are caused by excessive production of melanin [4]. Vitamin C is broadly used for the treatment of hyper-pigmented disorders because of its inhibitory action on melanogenesis [5]. Although ascorbic acid has a range of physiological and pharmacological functions but its poor liposolubility limits the cumulative amount of ascorbic acid in the cells after its permeation through the cell membrane. Modification of the hydroxyl groups of ascorbic acid with long-chain fatty acids has been developed to improve its liposolubility [6]. There are three forms commonly available in cosmetics: ascorbyl palmitate, magnesium ascorbyl phosphate, and L-ascorbic acid [12]. | ||||||
| In this study, effects of three active multiple emulsion formulations (ME1, ME2, and ME3) containing ascorbyl palmitate plus sodium ascorbyl phosphate on facial skin melanin and erythema contents of female volunteers were evaluated. Active formulation ME1 (with 0.25% SAP in internal and external water phases of W/O/W emulsion) produced a continuous decrease in the melanin content throughout the study period of 12 weeks. Decrease in melanin was more pronounced at 4th, 6th, 8th, 10th and 12th week of study. Average percentage change in skin melanin content was -2.47%, -8.51%, -9.14%, -12.73%, and -15.56%, respectively; while the respective control increased the melanin content up to 3.77%. After application of ME2 (with 0.5% AP in oil phase and 0.5% SAP in external water phase of W/O/W emulsion), a gradual reduction in melanin content was observed throughout the study period of 12 weeks. Effects were more pronounced during 6th (-12.86%), 8th (-15.88%), 10th (-18.74%) and after 12th weeks (-19.64%); whereas the control multiple emulsion produced about 6.2% increase in melanin content from 2nd to 12th week of study. ME3 (with 0.5% AP in oil phase and 0.5% SAP in internal water phase of W/O/W emulsion) showed 12-14% decrease skin melanin content during the study period of 12 weeks. More pronounced decrease in skin melanin values was observed at 6th (12.02%), 8th (12.73%), 10th (-14.43%) and 12th week of study (-14.55%); while the control slightly increased the melanin content (Figure 2). | ||||||
| When two-way ANOVA (analysis of variance) test was applied, ME1, ME2, and ME3 showed significant reduction in skin melanin content of human (females’) skin with respect to time (P ≤ 0.05) while their respective, control showed insignificant results. With the help of paired sample t-test, significant differences were observed between the melanin effects of control and active multiple emulsions ME1, ME2, and ME3 throughout the study period of 12 weeks. | ||||||
| The decrease in skin melanin content after the application of ME1, ME2, and ME3 loaded with AP and SAP may be attributed to their strong antioxidant action. Ascorbyl palmitate has amphiphilic nature as it molecules are orientated in the lipid bilayers with the palmitic residue in the lipophilic phase and the lactone ring in the lipid–water interphase. Only the 3- hydroxy group of the lactone ring is responsible for the reaction with free radicals and therefore scavenging is possible in hydrophilic compartments of the skin [7]. Sodium ascorbyl phosphate is a strong and effective antioxidant. It protects the cell against the oxidative damage caused by free radicals, produced from UV radiations. Sodium ascorbyl phosphate is cleaved by the enzymes in the skin and produced free vitamin C. SAP also acts on melanin formation process in the skin and prevents hyperpigmentation process. Thus it has skin lightening properties and therefore used in skin whitening preparations [9]. | ||||||
| After topical application of ME1, erythema content was found to be gradually decreased. The decrease was more pronounced during the week 4th (-10.85%), 6th (-13.18%), 8th (-13.67%), 10th (-14.21%) and 12th (-14.67%) respectively while control multiple emulsion caused a 4.41% decrease in erythema content after 12 weeks. After application of ME2, cutaneous erythema level was reduced at constant level from 2nd to 12th week. More pronounced decrease was observed during 6th (-6.84%), 8th (-10.62%), 10th (-11.64%) and 12th (-14.74%) week of study, while respective control slightly decreased (4.66%) skin erythema content during the study period of 12th week of study. ME3 caused gradual reduction in erythema content. Maximum reduction was observed during 8th (-7.45%), 10th (-10.78%) and 12th week (-13.75%), while respective control caused only -3.48% decrease in erythema content at the end of study (Figure 3). When ANOVA test (two-way) was applied, it was found that ME1, ME2, and ME3 produced significant decrease in cutaneous erythema level, while control produced insignificant decrease with respect to time. With paired sample t-test it was evident that there was significant variation (P ≤ 0.05) in skin erythema/irritation results with respect to control and active multiple emulsions throughout the study period of 12 weeks. | ||||||
| It was concluded that all the active multiple emulsion formulations (ME1, ME2, and ME3) significantly decreased cutaneous erythema level during the study period of 12-week. So the active multiple emulsions containing ascorbic acid derivatives (ascorbyl palmitate and sodium ascorbyl phosphate) can be used safely and effectively on human skin; as all three active multiple emulsions produced significant antiinflammatory effects (P ≤ 0.05) with respect to time. The role of ascorbic acid as an antioxidant and in the protection of skin against UVB light has been recognized by many workers [7,13]. Derivatives of ascorbic acid have been synthesized which have similar but long lasting effects than ascorbic acid. In this study, after the application of active multiple emulsion formulations containing ascorbyl palmitate and sodium ascorbyl phosphate, a significant anti-erythemic potential was observed. No skin irritation/sensitization was observed which indicated that these derivatives are safe and therefore can be used in cosmetic and dermatological products. | ||||||
| The reduction in skin erythema level after topical application of ME1, ME2, and ME3 may be attributed to anti-inflammatory effect of ascorbyl palmitate and sodium ascorbyl phosphate. In a study, ascorbyl palmitate when applied after UV burning, reduced redness 50% sooner than untreated areas on the same patient. The supposed mechanism was the antioxidant and as an anti-inflammatory activity of ascorbyl palmitate. Other dermatologic conditions with inflammation such as psoriasis and asteototic eczema, have shown clinical improvement when treated with ascorbyl palmitate salt [12]. Sodium ascorbyl phosphate, a water soluble derivative of ascorbic acid, significantly reduces the inflammatory reactions and follicular keratinization by reducing lipid oxidation in vivo. SAP strongly improves the inflammatory and non-inflammatory lesions of acne vulgaris in an open human study [14]. | ||||||
| Although composition of ME1, ME2, and ME3 was different, they produced slight variation in effects on skin melanin and erythema content. A few possible mechanisms were suggested behind this phenomenon. In formulation ME1, 0.25% of SAP from external water phase of W/O/W phase made the release and penetration of SAP was easy. In formulation ME2, AP was present in oil phase while SAP was present in external water phase of W/O/W emulsion, so release as well as penetration SAP into the skin was easy also. While in ME3, as SAP was present in internal water phase of W/O/W emulsion; it was considered that oil phase may act as barrier for its release as well as penetration into the skin so it might produce somewhat little and delayed effects. But results were almost similar because, in this case AP first released from middle oil phase and produced its effects. | ||||||
| It was also obvious that synergistic activity of ascorbyl palmitate and sodium ascorbyl phosphate produced the strongest effect of all three active multiple emulsion formulations irrespective of amount and location of active compounds in these formulations. In this particular study, the concentration of the active compounds did not matter in facial hypopigmenting effect; the results rather suggest synergistic effects of the two actives. In the subjective analysis by using panel test at the end of three months (Table 2), it was found that active multiple emulsions (ME1, ME2, and ME3) containing combination of ascorbyl palmitate and sodium ascorbyl phosphate proved to be acceptable and appropriate for topical use. | ||||||
Conclusion |
||||||
| Treatments of human skin with active multiple emulsion formulations; ME1, ME2, and ME3 containing ascorbyl palmitate plus sodium ascorbyl phosphate significantly reduces facial skin melanin and erythema. Furthermore, combined treatment with AP plus SAP in all the three active formulations is superior to placebo for its effectiveness as it produce synergistic effects on the skin‘s melanin and erythema. Also, patch test and panel test reinforces their accepted awareness as topical antioxidants. Thus, we believe that combination of ascorbyl palmitate and sodium ascorbyl phosphate in topical formulations could be a good treatment option for patients with hyperpigmentation, dermatitis and its associated diseases. | ||||||
Tables at a glance |
||||||
|
||||||
Figures at a glance |
||||||
|
||||||
References |
||||||
|