- Biomedical Research (2005) Volume 16, Issue 1
Supplementation with Alpha Lipoic Acid improves the in vitro development of embryos in nicotine-treated mice
Rozzana MS1*, Zaiton Z1, Rajikin MH2, Fadzilah S1 and Zanariyah A11Department of Physiology, Faculty of Medicine, Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, 53 000 Kuala Lumpur, Malaysia
2Faculty of Medicine, MARA University of Technology, 40 450 Shah Alam, Selangor, Malaysia
- *Corresponding Author:
- Dr. MS Rozzana
Dept. of Physiology, Faculty of Medicine
Univ. Kebangsaan Malaysia Jalan Raja
Muda Abdul Aziz 53000 Kuala Lumpu, Malaysia
E-mail: rozza_ms ( at ) yahoo.com
Accepted date: December 3 2004
Abstract
Alpha lipoic acid (ALA) is a known „universal? antioxidant which is particularly important in the treatment of diabetes-related complications. Nicotine, on the other hand, is a known pro-oxidant that has been shown to retard the development of preimplantation embryos. The objective of this study is to determine the effect of ALA supplementation on the in vitro development of embryo in nicotine-treated mice. Mus musculus mice aged between 4-6 weeks were co-treated with nicotine (5 mg/kg b.wt) and ALA (4 mg/kg b.wt) daily for thirty days. At the end of the treatment period, the mice were superovulated, mated, and the embryos were collected from the flushed fallopian tubes. All normal embryos were cultured in Whitten?s medium for 4 days. A total of 748 embryos were retrieved of which 65% were found normal. Nicotine treatment increased the number of abnormal embryos (13.0 ± 2.4) as compared to control (6.3 ± 2.0) [P<0.05], however, supplementation with ALA reduced this number to 8.5 ± 1.8. On subsequent culture, the embryos from the nicotine-treated mice failed to reach the stage of blastocyst, however, 83% embryos from the co-treatment group developed into blastocyst and even reached to the hatched stage as compared to control (89%). These results therefore, suggest that ALA is able to reverse the adverse effect of nicotine on the in vitro development of embryos. The reduction in plasma malondealdehyde (MDA) levels of the co-treated mice (0.54 ± 0.06 μmol/mg protein) as compared to nicotine treatment alone (1.66 ± 0.25 μmol/mg protein) [P<0.001] further supports the antioxidant effect of ALA. ALA is, therefore, found to be beneficial in reducing the deleterious effect of nicotine on the development of embryo in culture.
Keywords
Embryo, Alpha lipoic acid, Nicotine, Peroxidation, Malondealdehyde
Introduction
Alpha lipoic acid (ALA) has been dubbed as a “universal” anti-oxidant due to its ability to act as an antioxidant in both aqueous and lipid phase [1]. It is also able to regenerate other indigenous antioxidants and hence increases the protective mechanism of the body against free radicals [2]. Free radicals such as reactive oxygen and nitrogen species have been reported to cause structural damage to the membrane structure and even at the chromosomal levels [3,4]. Free radicals are continuously formed in the body because of cell metabolism. Their activities, however, are balanced by the presence of endogenous antioxidants. The cells are, therefore, protected from damages caused by the free radicals. However, when the levels of free radicals and other oxidizing agents which are known as reactive oxygen spe- cies overwhelm the antioxidants levels, oxidative stress occurs. This condition is manifested in our body in the form of patho-logical conditions and complications as found in diabetes, heart diseases and cancer [5,6,7].
Nicotine, one of the active components of tobacco smoke is known to be a prooxidant and found to be linked with the forma-tion of atherosclerotic plaque among smokers [8]. It has also been linked in a number of pregnancy complications including low birth weight, spontaneous abortion and fetal dysmorpho-genesis [9,10,11]. It has been reported that nicotine retards the development of preimplantation embryos [12,13]. Since ALA is a strong antioxidant, our study is, therefore, designed to deter-mine whether the detrimental effect of nicotine on the development of embryos can be minimized or completely reversed by ALA.
Materials and Methods
Chemicals
Nicotine (98-100% pure), alpha lipoic acid, malondealdehyde (MDA), sodium chloride, potassium iodide, potassium dihydro-gen phosphate, magnesium sulphate, sodium carbonate, so-dium HEPES, glucose, lactic acid, sodium piruvate, penicillin, streptomycin, glutamic acid, taurine, ethylene-diamine-trichloro-acetic acid and bovine serum albumin were all purchased from Sigma Chemical Co. (USA). The chemicals used for the embryo culture were of cell culture grade. Tocopherol-free corn oil was obtained from ICN (USA). Both the pregnant mare serum go-nadotropin (PMSG) and human chorionic gonadotropin (hCG) were from Intervet (Holland).
Animal treatment
Twenty-four Mus musculus mice, aged between 4 – 6 weeks with an average body weight of 30g were randomly selected and divided into four groups of six each. Each group received different daily treatment for 30 days. The control group was given subcutaneous (s.c.) injection with normal saline while the second group was injected with nicotine (5 mg/kg b.wt). Both the groups were forced-fed with tocopherol-free corn oil. The third group was injected with the same dose of nicotine and supplemented with ALA (4 mg/kg b.wt). The fourth group had normal saline but supplemented with the same dose of ALA. At the end of the treatment period, mice were superovulated by using PMSG and hCG, mated, fallopian tubes were excised and flushed to collect embryos.
Embryo culture
Normal and abnormal embryos were categorized according to the criteria described by Ertzeid and Storeng [14]. Only normal embryos were cultured. The embryos were cultured in a four-hole Falcon dish filled with 50 μL Whitten's medium and covered with silicone oil. The cultures were maintained in a humidified atmosphere containing 5% CO2, at 37o C (IG150 Jouan) for 4 days. Evaluation of the embryo development was made by using the inverted microscope (KarlZeiss Vision Model Axiovert S100, Germany).
Determination of lipid peroxide in plasma and ovaries
Blood and ovarian samples were collected on the day of sacri-fice and processed accordingly for evaluation of the lipid perox-ide levels using the thiobarbituric acid reactive substances (TBARS) method [15]. The absorbance was measured photometrically at 532 nm and the concentrations are expressed as micromoles malondealdehyde (MDA) per milligram protein.
Statistical analysis
Data are expressed as means ± SEM. Statistical comparison between different groups were made by using one-way analysis of variance (ANOVA). Skewed data were analyzed with non-parametric test, the Kruskal-Wallis on ranks test. The results were considered significant at P<0.05. The rate of the in vitro embryo development is calculated as mean percent develop-ment of blastocyst at day 4 of culture.
Results
Effect of ALA on in vivo embryo development
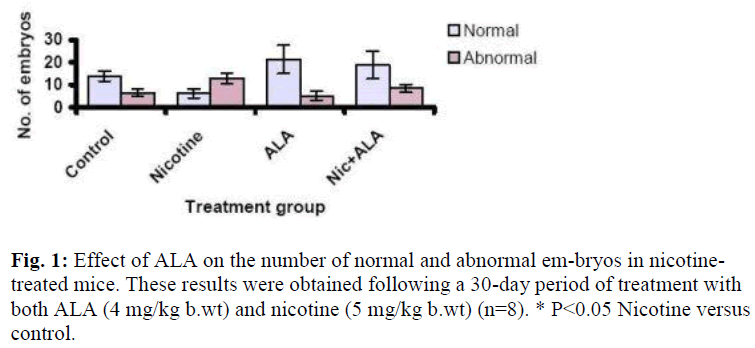
Figure 1 shows the effect of ALA on the number of normal and abnormal embryos from all the treatment groups. A total of 748 embryos were retrieved from all the groups of which 68% were normal. The remaining 32% were found abnormal. Most abnor-mal embryos were from the nicotine-treated mice. Nicotine treatment increased the number of abnormal embryos (13.0 ± 2.4) as compared to control (6.3 ± 2.0) [P<0.05], whereas sup-plementation with ALA reduced this number to 8.5 ± 1.8. Sup-plementation with ALA alone did not affect the number of normal (21.38 ± 6.35) and abnormal embryos (5.13 ± 1.95) compared to control.
Effect of ALA on in vitro embryo development
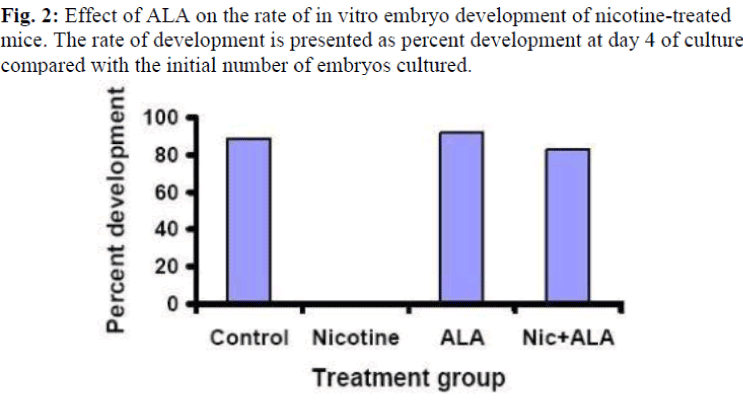
Figure 2 shows the effect of ALA on the rate of in vitro develop-ment of embryos in nicotine-treated mice. The embryos from the nicotine-treated mice failed to reach the blastocyst stage at day 4 of culture (0%), however, with ALA supplementation, 83% of the embryos developed into blastocyst and even reached to the hatched stage as compared to control (89%). Treatment with ALA alone increased the development rate by 21% as com-pared to control.
Effect of ALA on nicotine-induced lipid peroxides in plasma and ovaries
Table 1 shows the effect of supplementing ALA on lipid peroxide (measured as MDA) in plasma and ovaries of nicotine-treated mice. The MDA levels in both the plasma and ovaries of the nicotine-treated mice were high (plasma – 1.66 ± 0.25 μmol/mg protein, ovaries – 1.41 ± 0.63 μmol/mg protein) compared to the control (0.85 ± 0.14 for plasma and 0.28 ± 0.08 μmol/g protein for ovaries) [P<0.01]. Supplementation with ALA reduced the MDA level in the plasma (0.54 ± 0.06 μmol/g protein) [P<0.01] but not in the ovaries (1.24 ± 0.20 μmol/g protein). Supplemen-tation with ALA alone reduced the MDA levels in the plasma (0.79 ± 0.05 μmol/mg protein) as compared to control (P<0.01). The levels of MDA in the ovaries, however, was higher than in control (1.26 ± 0.30 μmol/g protein) [P<0.01].
Table 1: Effects of ALA on the levels of MDA in plasma and ovaries of nicotine-treated mice. ALA (4 mg/kg b.wt) was given together with nicotine (5 mg/kg b.wt) for a period of 30 days. *P<0.01 nicotine, ALA and Nic+ALA versus con-trol; #P<0.001 ALA and Nic+ALA versus nicotine, +P<0.05 Nic+ALA versus ALA. Results are expressed as mean±SEM
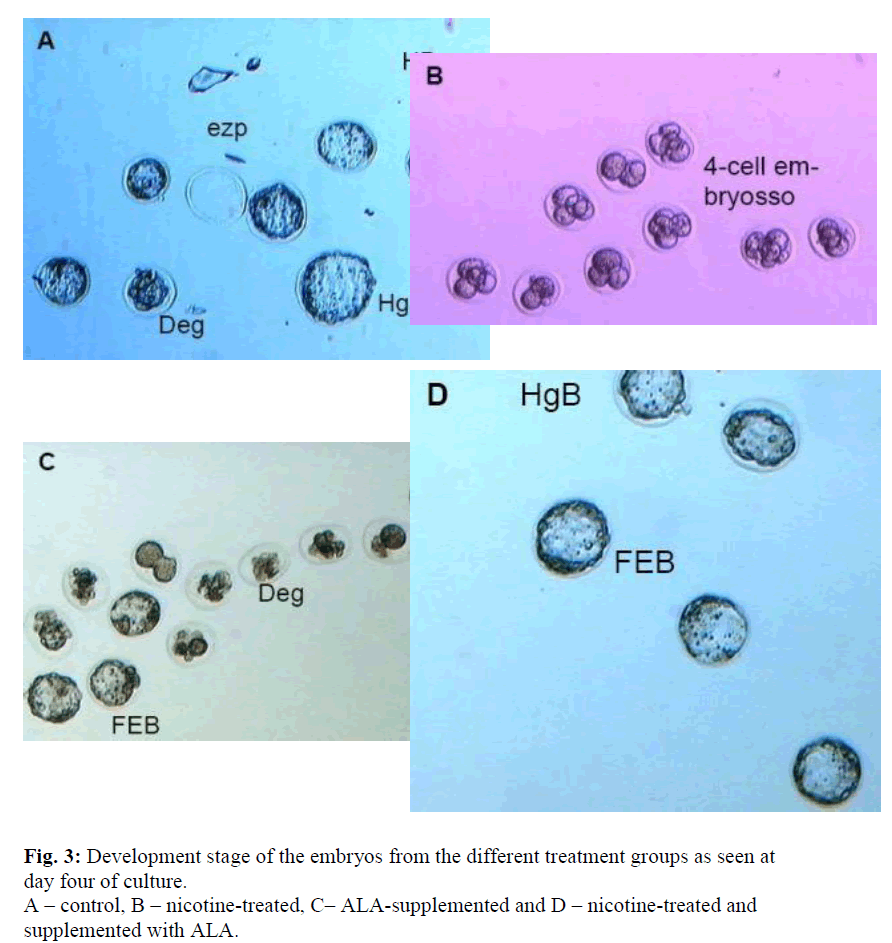
All treatments were done for 30 days. Ezp – empty zona pellucida, Deg – degenerating embryos, EB – expanded blastocyst, FEB – fully expanded blastocyst, HgB – hatching blastocyst, HB – hatched blastocyst
Discussion
Both ALA and its reduced form, dihydrolipoic acid (DHLA), are powerful antioxidants. As such, ALA has been largely used for the treatment of diabetic complications. In experimental and clinical studies, ALA markedly reduced the symptoms of diabetic pathologies, including cataract formation, vascular damage and polyneuropathy [2]. Packer et al [2] dubbed ALA as a universal antioxidant because of its water-soluble and lipid-membrane soluble characteristics, thus enabling it to reduce oxidized anti-oxidants at the interphase between lipid and water. Despite this fact, not much studies have been done to assess the effectiveness of ALA as an antioxidant against environmental pollutant especially regarding the reproductive toxicants. Amongst the reproductive toxicant that is gaining importance, nicotine, an active alkaloid, is normally found in the cigarette smoke and other tobacco products.
Nicotine has been implicated in hastening the formation of atherosclerotic plaque in smokers with cardiovascular problems [8]. It is also the culprit behind some of the pregnancy compli-cations such as spontaneous abortion, low birth weight and delayed conception [16,17]. Our studies have demonstrated that the nicotine also affects the normal development of oocytes in mice. Although some of the normal embryos retrieved were normal, yet these embryos were unable to develop beyond the morula stage (Figure 3-B). This thus indicates that nicotine and possibly some of its metabolites perturb the normal metabolism of oocytes and changed the protein structures rendering them to degeneration and cell apoptosis.
Supplementation with ALA, however, reduced the number of abnormal embryos to approximately 50% (Figure 1) which sug-gests that the effect of nicotine has been successfully reversed by ALA. Moreover, more than 80% of these embryos developed into blastocysts as compared to none in the nicotine-treated group. This antioxidant effect of ALA could be mediated through the removal of the radical species formed from the metabolism of nicotine. The low plasma level of MDA has certainly indicated this activities. However, a high level of ovarian MDA in both the supplemented and non-supplemented samples as well as in the ovaries of the mice which were not treated with nicotine but supplemented with ALA were evident. MDA is normally used to indicate the level of peroxidation process, therefore, our results may suggest that ALA possibly causes some peroxidation in the ovaries. This could possibly happen due to the low ovarian perfusion. The resultant accumulation of ALA in the ovaries consequently acts as a pro-oxidant. According to Bast et al [18], the antioxidant function of DHLA which also functions as a pro-oxidant is probably mediated by the reduction of transition met-als. Nevertheless, this prooxidant activity does not affect the development of oocyte and thus the higher number of normal embryos retrieved as compared to the control even though the difference was not significant (Figure 1). Furthermore, these embryos were able to develop to the hatched blastocyst stage at a good rate of 92% which is slightly higher than that of the control (89%) (Figure 3).
The prooxidant effect of ALA may not be seen in the oocytes and embryos because the oocytes may be well-protected due to the presence of other antioxidants including vitamin E and glutathione. There is also the possibility that the vitamin E in the oocytes neutralizes the free radicals generated by nicotine in the oocytes, and this used vitamin E is then being recycled by ALA through synergistic action of other antioxidants including glutathione and vitamin C [19]. As ALA and DHLA are used for the regeneration process, they themselves are then become oxidized to a prooxidant. These effects are then manifested in the ovaries but not in the oocytes/embryos. Although the exact mechanism could not be ascertained by this experiment, our study has shown the beneficial effect of ALA in protecting oocytes or embryos against free radical activities. Further study on the mechanism of action of ALA is warranted to confirm our findings.
Acknowledgement
This work was funded by a short-term research grant (FP38/2001) from the Medical Faculty of Universiti Kebangsaan Malaysia.
References
- Packer L, Witt EH, Tritschler HJ. α-lipoic acid as biological antioxidant. Free Rad Biol Med 1995; 19: 227-250.
- Packer L, Kraemer K, Rimbach G. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition 2001; 17: 888-895.
- Diplock AT. Antioxidant nutrients and disease prevention: an overview. Am J Clin Nutri 1991; 198S-193S.
- Wang X, Falcone T, Attaran M, Goldberg JM, Agarwal A, Sharma RK. Vitamin C and vitamin E supplementation reduce oxidative stress-induced embryo toxicity and improve the blastocysts development rate. Fert Steril 2002; 78: 1271-1277.
- Evans P, Halliwell B. Micronutrients: antioxidant status. Bri J Nutr 2001; 85: S67.
- Qureshi AA, Salser, WA, Parmar R, Ameson EE. Novel tocotrienols rice bran inhibit atherosclerotic lesions in C57BL/6 ApoE-deficient mice. J Nutrition 2001; 131: 2606-2618.
- Tomeo AC, Geller M, Watkins TR, Gapor A, Bierenbaum ML.Antioxidant effects of tocotrienols in patients with hyperlipidemia and carotid stenosis. Lipids 1995; 30: 1179-1183.
- Ashakumary J, Vijayammal PL. Additive effect of alcohol and nicotine on lipid peroxidation and antioxidant defence mechanism in rats. J App Toxicol 1996; 16: 305-308.
- Gocze PM, Szabo I, Freeman DA. Influence of nicotine, cotinine, anabasine and cigarette smoke extract on human granulose cell progesterone and estradiol synthesis. Gynecol Endoc: the Official Journal of the International Society of Gynecological Endocrinology 1999; 13: 266-272.
- Nelson E, Jodscheit K, Guo Y. Maternal passive smoking during pregnancy and fetal developmental toxicity. Part 1: gross morphological effects. Hum Exp Toxicol 1999; 18: 252-256.
- Jacqz-Aigrain E, Zhang D, Maillard G, Luton D, André J, Oury JF. Maternal smoking during pregnancy and nicotine and cotinine concentrations in maternal and neonatal hair. Bri.J Ob-stet.Gynecol 2002; 109: 909-911.
- Balling R, Beier HM. Direct effects of nicotine on rabbit pre-implantation embryos. Toxicology. 1985; 34: 309-313.
- Baldwin KV, Racowsky C. Nicotine and cotinine effects on development of two- cell mouse embryos in vitro. Reprod Toxicol 1988; 1: 173-178.
- Ertzeid G, Storeng R. Adverse effects of gonadotrophin treatment on pre- and postimplantation development in mice. J Reprod Fert 1992; 96: 649-655.
- Ledwozyw A, Micha; ak J, Stepien A, Kadziolka A. The relationship between plasma triglycerides, cholesterol, total lipids and lipid peroxidation products during human atherosclerosis. Clin Chem Act 1986; 155: 271-284.
- Taylor CT. Antioxidants and reactive oxygen species in human fertility. Env Toxicol Pharmacol 2001; 10: 189-198.
- Zenzes MT. Smoking and reproduction: gene damage to human gametes and embryos. Hum Reprod Update 2000; 6: 122-131.
- Bast A, Haenen GRMM. The toxicity of antioxidants and their metabolites. Env. Toxicol Pharmacol 2002; 11: 251-258.
- Biewenga GP, Haenen GRMM, Bast A. The pharmacology of the antioxidant lipoic acid. Gen Pharmac 1997; 29: 315-331.