Research Article - Biomedical Research (2017) Volume 28, Issue 19
Study on fingerprint of Schisandra by high performance liquid chromatography
Yang Hui, Yuan Guangxin*, Sun Yunpeng, Cui Xinyuan and Li Yingnuo
College of Pharmacy, Beihua University, Jilin, Jilin, PR China
Accepted date: September 15, 2017
Abstract
To establish a fingerprint of Schisandra chinensis by HPLC. The chromatographic column was Agilent ZORBAX 300SB-C18 chromatographic column (4.6 mm × 250 mm, 5 μm). The mobile phase was methanol-water, a gradient elution was conducted and the detection wavelength was at 230 nm. The sample map was analysed by software and cluster analysis method using “Chinese medicine chromatographic fingerprint similarity evaluation system 2004A”. According to the comparative study of fingerprints, we found 10 communal peaks and identify 8 peaks. The similarity between the 20 batches of medicinal herbs was compared. It was suggested that the Similarity should be 0.950 and 0.850 for superior drugs and certified drugs. The HPLC fingerprint of Schisandra chinensis has good repeatability and strong characteristics, and it can provide scientific basis for the quality evaluation of Schisandra chinensis.
Keywords
Fingerprint, Schisandra, High performance liquid chromatography
Introduction
In traditional Chinese medicine, the dried ripe fruits of both Schisandra sphenanthera and Schisandra chinensis have long been used as Wuweizi, even though their chemical constituents and contents of the bioactive components are quite different. Since 2000, they have been accepted as two different crude drugs, Schisandrae chinensis Fructus and Schisandrae sphenantherae Fructus [1,2], respectively, by the Chinese Pharmacopoeia. The lack of reliable and effective quality control methods has become a major problem hindering the modernization of traditional Chinese Medicine. The technology of fingerprint has the characteristics of macroscopic, integrity and fuzziness. It can reflect the overall characteristics of the samples analysed. It is very suitable for the study of complex systems such as Chinese herbal medicine [3]. China has begun to use fingerprint technology to supervise and manage Chinese herbal medicines since 2004 [4]. In this paper, HPLC (HPLC) technique was used to analyse Schisandra chinensis and establish fingerprints of Schisandra chinensis to provide scientific and effective methods for the quality control and evaluation of Schisandra chinensis [5].
Materials and Methods
Instruments and reagents
SHIMADZU LC-20A High performance liquid chromatography(Shimadzu Corporation, Japan); AL204 Electronic balance (Mettler Toledo Corporation, Switzerland); RE-6000 Rotary evaporator (Yarong biochemical instrument factory, Shanghai); HHSI1-NI4thermostat water bath (Changan Scientific Instrument Factory, Beijing); KQ-50B Ultrasonic cleaner (Jiangdong Precision Instrument Corporation, Suzhou); DHG-9245A constant temperature drying oven (Yiheng Technology Co., Ltd, Shanghai); in addition, data analysis was carried out by using SPSS Statistics v19.0 (IBM Corporation, America) and traditional Chinese medicine chromatographic fingerprint similarity evaluation system 2004A (National Pharmacopoeia Committee).
Methanol (Chromatography pure) ethanol (analytical pure), ultrapure water.
Reference substances
Schisandrin (110857-200910); Schisantherin A (111529-200302); Anwulignan (111844-201002) are purchased National Institute for the control of pharmaceutical and biological products. Schi-sandrol B, deoxyschizandrin, γ- Schizandrin, pseudo-r-schizandrin are laboratory self-control, and were verified structure through 1H-NMR, 13C-NMR. They are all singlet after testing by HPLC. The purity of them is greater than 98%. Schisanhenol is provided by Schisandra chinensis Research Institute of Beihua University and its purity is 98.5%.
Experimental materials
15 batches of Schisandra chinensis samples from different habitats, 5 batches of Schisandrae sphenantherae Fructus samples. All of them are appraised by Wang Weili, a chief pharmacist, works in mesothecium of institute for food and drug control in Jilin, coming from Anguo, Hebei (No.1), Shangzhi, Hulin, Heilongjiang, (No.2~NO.4), Dandong, Benxi, Tieling, Liaoning (No.5~No.7), Dunhua, Zuojia, Jiaohe, Tonghua, Wangqing, Changchun, Jilin (No.8~No.13), Institute for food and drug control, Jilin (NO.14~NO.15), Hengshan, Hunan (No.16), Pingwu, Sichuan (No.17), Songxian, Henan (No.18), Yangcheng, Shanxi (No.19), Haozhou, Anhui (NO. 20).
Preparation of the reference substance solutions
Preparation of reference stock solution: weighing precisely reference substance of schizandrin, schisandrol-B, schisanhenol, anwuligan, schisantherin A, deoxyschizand-rin, γ-schizandrin, and pseudo-r-schizandrin. Add appropriate methanol to them and fix capacity in 10 ml capacity bottle by ultrasonic dissolution. Then get the preparation of reference stock solution, the concentration of them are 1.00, 1.00, 0.80, 0.80, 0.80, 1.00, 1.00 and 0.80 mg/ml. Stored at 4 degrees.
Preparation of mixed reference solution: Weighing precisely each reference substance solution for 1.0 ml. Put them into 10 ml measuring flask and add methanol to make mixed reference solution.
Preparation of test solution: Take 10 g of dry sample powder then add 300 ml methanol. After twenty-minutes’ ultrasound extraction, shake and rest for ten min. 150 ml methanol is added to filter residue after filtering. Ultrasonic extraction 20 min × 2 times. Merge the filtrate for 3 times, evaporated in vacuo. The residue is dissolved by methanol and fixed to 100 ml, and the filter is obtained after 0.45 μm filter film.
Chromatographic conditions
Agilent ZORBAX 300SB-C18 chromatographic column (4.6 mm × 250 mm, 5 μm), Shimadzu GVP protection column (4.6 mm), with methanol (A), water (B) as mobile phase gradient elution, elution procedure is 0~15 min, 60%~75% A, 40%~25% B; 16~20 min, 75% A, 25% B; 21~30 min, 75%~90% A, 25%~10% B; 31~40 min, 90%~100% A, 10%~0% B; 41~45 min, 100% A; 46~55 min, 100%~60% A, 0%~40% B. column temperature of 27 degrees, current speed; 0.5 ml.min-1; detection wavelength of 230 nm. Sample size; 10 μL.
Results
Blank test
Precision absorbing mobile phase 10 μl, analysing the samples according to the method of chromatographic condition in chromatographic conditions. The results showed that the blank solvent had no interference to the experiment.
Contrast test
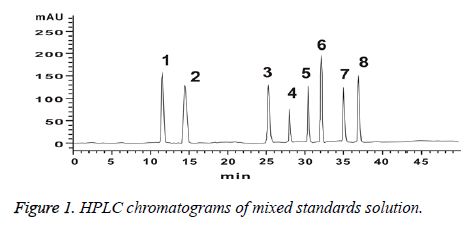
Precision absorbing mixed reference solution 10 μl, analysing the samples according to the method of chromatographic condition in chromatographic conditions. Recording chromatogram (Figure 1).
Repetitive study
Take the same batch of medicinal powder(No. 20), 5 sample solutions were prepared in parallel according to the methods in preparation of solution. Analyzing the samples according to the method of chromatographic condition in chromatographic conditions. The retention time and peak area of each characteristic peak are measured (the characteristic peaks in 2.6.1). The retention times of RSD are 0.11%, 0.12%, 0.12%, 0.15%, 0.15%, 0.28%, 0.24%, 0.32%, 0.36%, 0.48%; the peak area of RSD are 1.25%, 1.86%, 2.83%, 2.51%, 1.92%, 4.62%, 2.49%, 1.98%, 3.05%, 3.91%. The results show good repeatability of the test.
Establishment of HPLC fingerprint of Schisandra chinensis
Determination of characteristic peaks of HPLC fingerprints of Schisandra chinensis: Take Schisandra chinensis (No. 1~No. 15), the test sample solution was prepared according to the method in preparation of solution. Analyzing the samples according to the method of chromatographic condition in chromatographic conditions. By comparing the fingerprints of 15 batches of Schisandra chinensis, 10 common peaks were identified as characteristic peaks. 8 chromatographic peaks were identified as Schizandrin (Peak 1), schisandrol B (Peak 2), Schisantherin A (Peak 5), Schisanhenol (Peak 6), Anwuligan (Peak 7), Deoxyschizandrin (Peak 8), γ-Schizandrin (Peak 9), Pseudo-r-Schizandrin (Peak 10). Lignans are the main components of Schisandra, through the study on the fingerprints of 15 batches of Schisandra, we choose peak 8 (deoxyschisandrin) as a reference HPLC fingerprint of Schisandra chinensis cause its better retention time, peak area and the degree of separation [6].
Hierarchical cluster analysis
Using the national pharmacopoeia committee “similarity evaluation system of traditional Chinese medicine chromatographic fingerprint” 2004 A version, deals with 20 batches of samples, uses the sum of squares of deviations, and takes the square Euclidean distance as the measure [7-9]. The results show that the 20 batches of medicinal materials were divided into two groups: A (include No. 1~15) and B (include No. 16~20), and then divided into 3 groups by cluster analysis. It is easy to determine the class of Schisandra; Class A1 (include No.’s 2, 4, 5, 6, 8, 9, 11, 12, 14) is of high quality; class A2 (include No.’s 1, 3, 7, 10, 13, 15) is of conforming article; class B (include No. 16~20) is of Schisandrae sphenantherae Fructus. The results show this method can distinguish between Schisandra chinensis and Schisandrae sphenantherae Fructus, and can be effective for the quality evaluation of Schisandra chinensis.
Similarity comparison (Common mode)
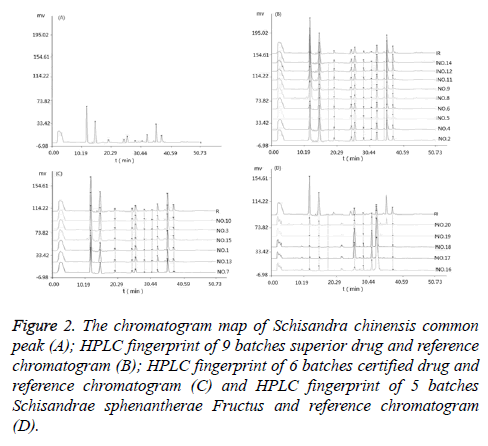
Establishment of HPLC fingerprinting common mode of Schisandra chinensis [10]: Take Schisandra chinensis (No. 1~No.15), the test sample solution was prepared according to the method in Preparation of Solution. Analysing the samples according to the method of chromatographic condition in chromatographic conditions. The HPLC fingerprints of the 15 batches of herbs were obtained. Using "Chinese medicine chromatographic fingerprint similarity evaluation system 2004A" combines Figure 1. HPLC chromatograms of mixed standards solution and sample peak condition and matching result. 10 common peaks are identified. A common mode map was generated as a contrast map (R) (Figure 2).
Figure 2: The chromatogram map of Schisandra chinensis common peak (A); HPLC fingerprint of 9 batches superior drug and reference chromatogram (B); HPLC fingerprint of 6 batches certified drug and reference chromatogram (C) and HPLC fingerprint of 5 batches Schisandrae sphenantherae Fructus and reference chromatogram (D).
Similarity calculation of Schisandra chinensis
Take samples of medicinal herbs of different habitats. The test sample solution was prepared according to the method in preparation of solution. Analysing the samples according to the method of chromatographic condition in chromatographic conditions. HPLC fingerprints of crude drug samples from different habitats were obtained. Comparing it with the established HPLC contrast map (R) and calculates the similarity. The results is as follows, the similarity of No.1 is 0.891; No.2 is 0.983; No.3 is 0.935; No.4 is 0.995; No.5 is 0.972; No.6 is 0.958; No.7 is 0.883; No.8 is 0.968; No.9 is 0.963; No.10 is 0.937; No.11 is 0.990; No.12 is 0.976; No.13 is 0.915; No.14 is 0.980; No.15 is 0.931; No.16 is 0.488; No.17 is 0.515; No.18 is 0.524; No.19 is 0.457; No.20 is 0.426. The HPLC fingerprint of Schisandra chinensis is shown in Figure 2.
Discussion
From the above results, we can learn that the HPLC fingerprints of Schisandra chinensis from different habitats (No.1~15) are similar to those of the control plots, and are all greater than 0.850, indicating that the chemical constituents of Schisandra chinensis vary little with the origin. The HPLC fingerprints of Schisandra sphenanthera Rehd. etWils (No. 16~20) showed a low similarity with the control map, both of which were less than 0.750, indicating that there was a big difference between the components of Schisandra chinensis and Schisandrae sphenantherae Fructus.
Set 0.950 and 0.850 as the threshold respectively, the similarity of high quality of Schisandra chinensis is more than 0.950, qualified product is between 0.949~0.850, adulterants (Non Schisandra) is less than 0.850. The result is as follows:
High quality: No.2, No.4, No.5, No.6, No.8, No.9, No.11, No. 12, No.14;
Qualified product: No.1, No.3, No.7, No.10, No.13, No.15;
Adulterants: No.16, No.17, No.18, No.19, No.20.
Conclusion
HPLC fingerprints of Schisandra chinensis are established by High Performance Liquid Chromatography (HPLC). 10 common peaks are identified as characteristic peaks, and 8 components of them are figured. The HPLC fingerprints of 20 batches of samples were obtained. Cluster analysis and common mode analysis were used to analyse the fingerprint of the samples. The results of the two methods are consistent, which can distinguish Schisandrae sphenantherae Fructus and Schisandra chinensis effectively, and evaluate the quality and authenticity of Schisandra chinensis. It further proves the specificity of the fingerprint and provides a new idea for the study of the fingerprint of Chinese Medicine.
Conflict of Interest
The authors have no competing interests to disclose.
Acknowledgements
This work was financially supported by the Jilin province Science and technology development project (201603095YY) and Beihua University young teacher promotion program (Beihua University (2016) 43).
References
- Liu Y, Fu S, Fan L. Research progress in the differences of chemical constituents and pharmacological actions between Schisandra chinensis and Fructus schisandrae chinensis. Chin J Exp Tradit Med Form 2017; 12: 228-234.
- Ke H, Li H, Su J. Comparison of lignans in Schisandra chinensis and Fructus schisandrae chinensis. Chin J Exp Tradit Med Form 2015; 17: 40-43.
- Du Y, Li Y, Zhang X. HPLC fingerprinting of hawthorn leaves in Chengde. Chinese J Mod Appl Med 2012; 10: 906-910.
- Wang P, Li L, Yang H. Chromatographic fingerprinting and quantitative analysis for the quality evaluation of Xinkeshu tablet. J Pharm Anal 2012; 6: 422-430.
- Shao J. Research progress of Chinese traditional medicine fingerprint. Chinese Herb Med 2009; 6: 994-998.
- Li X, Liu Y, Yi J. South schisandra berry and study on HPLC fingerprint of lignans and determination of medicine. Sci Dir 2011; 6: 920-924.
- Xie Q, Chen Z, Hong P. HPLC fingerprint based clustering analysis method to evaluate the quality of different andrographitis. Spectros Lab 2012; 4: 2392-2397.
- Wang Y, Wang B, Xu H. Cluster analysis and principal component analysis of the mussel extracts infrared fingerprint based on. J Fisheries China 2012; 7: 1146-1152.
- Ding P, Wang B, Li X. Different origin of Schisandra chinensis by principal component analysis and clustering analysis. Liaoning J Trad Chinese Med 2013; 10: 2088-2091.
- Yin F, Chen B, Lu T. Schisandra RP-HPLC fingerprint and total model. Chinese Med 2005; 8: 872-875.