- Biomedical Research (2016) Volume 27, Issue 3
Studies on the structural characterization of compounds in Sinopodophyllum emodi and their inhibitory effect on K562 cell proliferation.
Chengli Yao1,2 and Zhiguo Ding1,2*
1Department of Surgery, Tongzhou TCM Hospital, Beijing 101121, PR. China
2Department of Surgery, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing 100078, PR. China
- *Corresponding Author:
- Zhiguo Ding
Department of Surgery
Tongzhou TCM Hospital, Beijing 101121, China
Department of Surgery
Dongzhimen Hospital
Beijing University of Chinese Medicine
Beijing 100078
China
Accepted date: March 25, 2016
Abstract
Sinopodophyllum emodi is a herbaceous perennial plant. It is used in the treatment of hemopathy, retained placenta and lochiorrhea. Its root contains podophyllotoxin, which has an anti-tumor effect. Compounds were isolated by column chromatography, and were analyzed by NMR chromatography; flow cytometry was used to determine the anticancer activity of ethanol extract of Sinopodophyllum emodi. The ethanol extract was subjected to column chromatography, and the isolated compounds were structurally identified by H-NMR and C-NMR techniques, three compounds were obtained from Sinopodophyllum emodi, namely podophyllotoxin, podophyllotoxone and 4'-demethyl podophyllotoxin; after 48 h action of Sinopodophyllum emodi ethanol extracts in each treatment group on K562 cells, they produced very significant effects on cell cycle distribution. The proportions of G0/G1 and S phase cells decreased, while the proportion of G2/M phase cells increased, all in an apparent dose dependent manner, indicating that the ethanol extract of Sinopodophyllum emodi arrested K562 cell cycle at the G2/M phase. Sinopodophyllum emodi ethanol extract can inhibit the proliferation of K562 cells.
Keywords
Sinopodophyllum emodi, Flow cytometry, Podophyllotoxin, Podophyllotoxone, 4'-demethyl podophyllotoxin.
Introduction
Sinopodophyllum emodi is a herbaceous perennial plant S. emodi Wall. var. Chinensis Sprague in the family Berberidaceae, it has a faint odor, bitter and slightly acrid taste, and is distributed mainly in Qinghai, Gansu, Shaanxi and Sichuan. It is used in the treatment of hemopathy, retained placenta and lochiorrhea [1]. Its root contains podophyllotoxin, which has an anti-tumor effect [2,3], as well as wind-damp eliminating, Qi and blood circulation activating, analgesic, antitussive and antiasthmatic efficacies, and is used in the treatment of rheumatism, traumatic injuries, wind-cold cough and other diseases [4]. In this paper, the chemical constituents of Sinopodophyllum emodi were extracted, isolated and purified, and the isolated compounds were structurally identified; meanwhile, the inhibitory effect of Sinopodophyllum emodi ethanol extract on human erythroleukemia K562 cells was studied, in order to provide data support for clinical anti-tumor application of Sinopodophyllum emodi.
Material and Methods
Cell lines and reagents
Human erythroleukemia cell line K562, purchased from Tianjin Institute of Hematology; ethanol extract of Sinopodophyllum emodi, self-made, prepared into the desired concentrations.
Instruments and reagents
RPMI 1640 culture medium, purchased from Gibco, USA; Dimethyl Sulfoxide (DMSO), trypsin and Propidium iodide (PI), purchased from Sigma-Aldrich, USA; Bruker AV 400 MHz superconducting NMR spectrometer; CO2 incubator, model CO-150, purchased from NBS, USA; clean bench, model SW-CJ-SF, purchased from Suzhou Purification Equipment Factory, Sujing Group; EPICS-XL flow cytometer, purchased from Beckman Coulter, USA.
Extraction and isolation
3 kg of Sinopodophyllum emodi medicinal material was taken, crushed, and repeatedly extracted with 95% ethanol and then ethanol was removed to give Sinopodophyllum emodi extract. The extract was loaded on the column by dry column method, subjected to column chromatography on silica gel, and gradient eluted with petroleum ether, chloroform, ethyl acetate and methanol, respectively. Compounds 1 and 2 were isolated from petroleum ether-chloroform eluent, and compound 3 from chloroform-ethyl acetate eluent.
Cell culture
K562 cells were cultured conventionally in RPMI 1640 medium containing 100 mL/L FBS, 100 ku/L penicillin and 100 mg/L streptomycin, and placed in a 37°C incubator containing 50 mL/L CO2, medium was replaced and cells were passaged every 2-3 days and set aside for later use.
Inhibitory effect of Sinopodophyllum emodi ethanol extracts on K562 cell proliferation
The inhibitory effect of Sinopodophyllum emodi ethanol extracts on K562 cells was determined by flow cytometry. The K562 cells were seeded in culture flasks at 5 × 104 cells/mL, and cultured at 37°C under 5% CO2 for 14 h, then the medium was changed and different concentrations of Sinopodophyllum emodi ethanol extracts (concentration relative to the Sinopodophyllum emodi crude drug of 25, 50 and 100 g/L) were added, and the cells were drug treated for 48 h. Then the cells were collected, prepared into a single cell suspension, centrifuged for 5 min, washed three times with PBS, fixed in 70% ice-cold ethanol, and allowed to stand overnight at 4°C. Ethanol was removed by centrifugation, and the remaining cells were washed twice with PBS, DNA stained with 50 mg/L Propidium iodide (PI), and their cell cycle distribution was determined by flow cytometry.
Results
Structural confirmation of compounds
Compound 1: mp.115-117°C. 1H-NMR (400 MHz, DMSO-d6) δ: 2.85 (1H, m, H-3), 7.23 (1H, s, H-5), 6.52 (1H, s, H-8), 6.43(2H, s, H-2',6'), 5.89 (2H, d, J=3.6 Hz, OCH2O), 4.95 (1H, d, J=7.6 Hz, H-4), 4.48 (1H, d, J=4.8 Hz, H-1), 4.62 (1H, dd, H-3α), 4.25 (1H, dd, J=8.8, 8.0 Hz, H-3β), 3.72 (3H, s, 4'- OMe), 3.75 (6H, s, 3', 5'-OMe), 3.13 (1H, dd, J=13.4, 4.6 Hz, H-2); 13C-NMR (75MHz, DMSO-d6) δ: 44.6 (C-1), 45.3 (C-2), 175.4 (C-2a), 41.4 (C-3), 71.6 (C-3a), 72.4 (C-4), 107.2 (C-5), 148.3 (C-6), 148.2 (C-7), 110.3 (C-8), 132.1 (C-9), 135.6 (C-10), 137.3 (C-1'), 109.6 (C-2'), 153.7 (C-3'), 138.4 (C-4'), 153.2 (C-5'), 109.2 (C-6'), 102.4 (OCH2O), 56.6 (OMe-3', 5'), 60.5 (OMe-4').
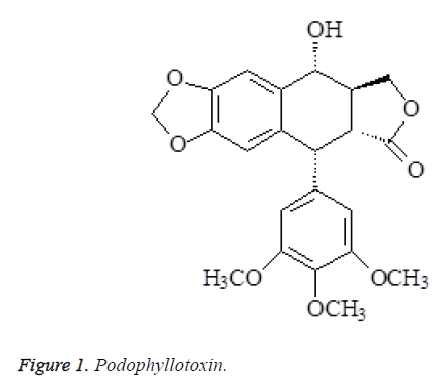
The above data were consistent with podophyllotoxin reported in the literatures [5,6], so the compound was identified as podophyllotoxin (Figure 1).
Compound 2: Colorless granular crystals, mp. 190-193°C. 1H-NMR (400 MHz, DMSO-d6) δ: 4.59 (1H, m, H-1), 3.22 (3H, s, J=15.4 Hz, H-2), 3.48 (1H, m, H-3), 4.59 (1H, d, J=9.0 Hz, 3a- αH), 4. 37 (1H, d, J=9.0 Hz, 3a-βH), 4.79 (1H, d, J=7.0 Hz, H-4), 7.56 (1H, s, H-5), 6.66 (1H, s, H-8), 6.34 (2H, s, H-2',6'), 6.12 (2H, d, J=8.5 Hz, OCH2O), 3.84 (3H, s, 4'-OMe), 3.69 (6H, s, 3', 5'-OMe); 13C-NMR (75 MHz, DMSO-d6) δ: 44.4 (C-1), 46.4 (C-2), 172.3 (C-2a), 43.2 (C-3), 67.2 (C-3a), 192.2 (C-4), 106.4 (C-5), 148.3 (C-6), 153.4 (C-7), 109.3 (C-8), 141.3 (C-9), 128.5 (C-10), 132.4 (C-1'), 107.2 (C-2'), 153.4 (C-3'), 137.3 (C-4'), 153.6 (C-5'), 107.3 (C-6'), 102.6 (OCH2O), 56.4 (OMe-3',5'), 60.3 (OMe-4').
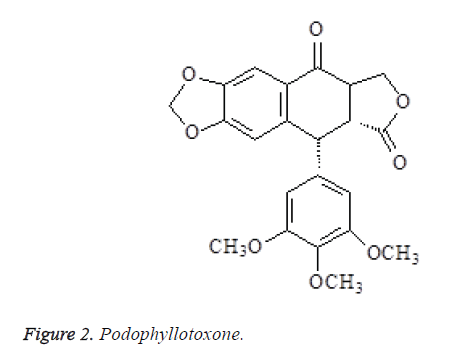
The spectral data of compound 2 were consistent with podophyllotoxone in the literatures [7,8], so the compound was identified as podophyllotoxone (Figure 2).
Compound 3: mp. 248-251°C. 1H-NMR (400 MHz, DMSO-d6) δ: 7.13 (1H, s, H-5), 6.53 (1H, s, H-8), 6.36 (2H, s, H-2',6'), 6.11 (1H, s, OCH2O), 6.02 (1H, s, OCH2O), 5.44 (1H, 4-OH), 4.69 (1H, d, J=8.6 Hz, H-4β), 4.61 (2H, m, H-1, H-3aα), 4.16 (1H, t, J=9.5 Hz, H-3aβ), 3.77 (6H, s, 3', 5'-OCH3), 2.84 (1H, dd, J=8.5, 2.1 Hz, H-2), 2.81 (1H, m, H-3); 13C-NMR (75 MHz, DMSO-d6) δ: 44.6 (C-1), 45.7 (C-2), 174.3 (C-2a), 41.6 (C-3), 71.3 (C-3a), 72.6 (C-4), 106.7 (C-5), 147.5 (C-6), 147.5 (C-7), 109.6 (C-8), 131.5 (C-9), 133.5 (C-10), 132.4 (C-1'), 108.5 (C-2'), 146.5 (C-3'), 133.3(C-4'), 146.5 (C-5'), 108.4 (C-6'), 101.6 (OCH2O), 56.4 (OMe-3',5').
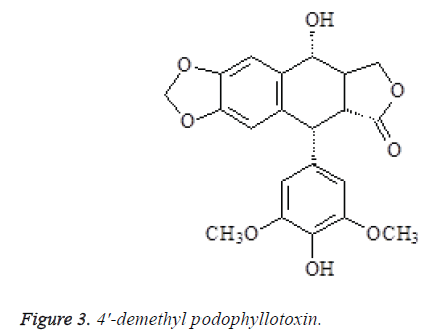
The spectral data of compound 3 were consistent with 4'- demethyl podophyllotoxin in the literature [9], so the compound was identified as 4'-demethyl podophyllotoxin (Figure 3).
Flow cytometric determination of the effects of Sinopodophyllum emodi ethanol extracts on cell cycle distribution and apoptosis rate
Flow cytometry was used to observe the effect on cell cycle distribution after 48 h action of different concentrations of Sinopodophyllum emodi ethanol extracts on K562 cells. The proportions of G0/G1 and S phase cells decreased, while the proportion of G2/M phase cells increased, all in a certain dose dependent manner, indicating that the ethanol extract of Sinopodophyllum emodi arrested K562 cell cycle at the G2/M phase (table 1).
| Group | Concentration (g/L) | G0/G1 phase | S phase | G2/M phase |
|---|---|---|---|---|
| Control group | - | 64.34 ± 0.13 | 27.46 ± 0.16 | 8.20 ± 1.02 |
| Sinopodophyllumemodi ethanol extract | 25 | 53.57 ± 0.14 | 24.58 ± 0.11 | 21.85 ± 0.63 |
| 50 | 46.67 ± 0.02 | 22.43 ± 0.12 | 30.90 ± 0.32 | |
| 100 | 36.85 ± 0.07 | 19.51 ± 0.03 | 43.64 ± 0.21 |
Table 1: Effects of Sinopodophyllum emodi ethanol extracts on cell cycle distribution and apoptosis rate of K562 cells (n = 4, x̅ ± s).
Discussion
The root, rhizome and fruit of Sinopodophyllum emodi all have relatively high medicinal values; its roots mainly contain lignans and flavonoids, as well as saponins, polysaccharides, tannins, etc. This species is poisonous, and its toxicity comes from the podophyllum resin contained in the roots and rhizomes. Toxic symptoms are usually manifested as vomiting, respiratory excitation, ataxia and coma [10]. Experiments have shown that after gastrointestinal and non-gastrointestinal administration to rats, most animals had dyspnea, diarrhea, comet tails, and then excitation, spasm, exhaustion and coma until death within 8 h. In the present study, the rhizome of Sinopodophyllum emodi was extracted, the ethanol extract was subjected to column chromatography, and the isolated compounds were structurally identified by 1H-NMR and 13CNMR techniques, three compounds were obtained from Sinopodophyllum emodi, which were podophyllotoxin, podophyllotoxone and 4'-demethyl podophyllotoxin, respectively.
Apoptosis is a physiological death process which ends cell life under certain physiological or pathological conditions following its own program. Tumor cells are characterized by concentrated distribution in the S phase where DNA synthesis is active. Flow cytometry results have shown that the nontreated cells are mostly present in the G0/G1, G2/M and S phases of the cell cycle. While the decrease of G1 phase cells, which are in an early stage of DNA replication, will cause inhibition of cell proliferation and easy phenotypic changes, which will affect cell metabolism and function and lead to cell apoptosis, thereby achieving the purpose of inhibiting cell proliferation. After 48 h action of Sinopodophyllum emodi ethanol extracts in each treatment group on K562 cells, they produced very significant effects on cell cycle distribution. The proportions of G0/G1 and S phase cells decreased, while the proportion of G2/M phase cells increased, all in an apparent dose dependent manner, indicating that the ethanol extract of Sinopodophyllum emodi arrested K562 cell cycle at the G2/M phase. It can be considered that the ethanol extract of Sinopodophyllum emodi achieved the induction of K562 cell apoptosis by blocking the G2/M phase.
Podophyllotoxin compounds have good anti-cancer activity, many scholars have conducted researches on these compounds, and anti-cancer agent ("Tian Fu Xing" III) with "Sinopodophyllum emodi" as the main drug has come into the market. However, although these compounds have significant anti-cancer effects, they are also highly toxic, which greatly limits their clinical application. How to reduce toxicity through various means, such as through the study of podophyllotoxin derivatives and the use of lipid nanoparticle technology, while not reducing the clinical efficacy is the focus and difficulty of future studies on these compounds.
Conclusion
Experiments have shown that after gastrointestinal and nongastrointestinal administration to rats, most animals had dyspnea, diarrhea, comet tails, and then excitation, spasm, exhaustion and coma until death within 8 h. In the present study, the rhizome of Sinopodophyllum emodi was extracted, the ethanol extract was subjected to column chromatography, and the isolated compounds were structurally identified by 1H-NMR and 13C-NMR techniques, three compounds were obtained from Sinopodophyllum emodi, which were podophyllotoxin, podophyllotoxone and 4'-demethyl podophyllotoxin, respectively.
After 48 h action of Sinopodophyllum emodi ethanol extracts in each treatment group on K562 cells, they produced very significant effects on cell cycle distribution. The proportions of G0/G1 and S phase cells decreased, while the proportion of G2/M phase cells increased, all in an apparent dose dependent manner, indicating that the ethanol extract of Sinopodophyllum emodi arrested K562 cell cycle at the G2/M phase. It can be considered that the ethanol extract of Sinopodophyllum emodi achieved the induction of K562 cell apoptosis by blocking the G2/M phase.
References
- Xiong WY, Wei SN, Yue M. HPLC analysis of podophyllotoxin content in Sinopodophyllum emodi. Chinese Trad Patent Med 2010; 32: 875-878.
- We R. DNA to Poisomerases as targets for cancer therapy. Biochem Pharmacol 1985; 34: 4191-4195.
- Shang MY, Xu LS, Wang QX. Study on pharmacodynamics of Chinese herbal drug Guijiu and its lignan. Chinese Trad Herbal Drugs 2002; 33: 722-724.
- Liu CJ, Hou SS. Current research status of Podophyllum Lignans. Nat Product Res Dev 1997; 9: 81-88.
- Jackson , Dewick PM. Aryltetralin lignans from podophyllum hexandrum and podophyllum peltatum. Phytochemistry 1984; 23: 1147-1152.
- Imbert TF. Discovery of podophyllotoxins. Biochimie 1998; 80: 207-222.
- Raffaele S, Fiani C, Belfiore MC, Gualandi G, Penna S, Mosesso P. Methyltrioxorhenium catalysed synthesis of highly oxidised aryltetralinlignans with anti-topoisomerase II and apoptogenic activities. Bioorg Med Chem 2005; 13: 5949-5960.
- Fonseca SF, Ruveda EA, Chesney JD. 13C-NMR analysis of podophyllotoxin and some its derivatives. Phytochemistry 1980; 19: 1527-1530.
- Jackson DE, Dewick PE. Aryltetralin lignans from Podophyllum Huxandrum and Podophyllum peltatum. Phytochemistry 1984; 23: 1147-1152.
- Wang BX, Wang RS. Extraction and HPLC analysis of podophyllotoxin in Sinopodophyllum emodi. Chinese J Pharmaceuticals 1999; 30: 484-486.