Keywords
|
| Carbonic anhydrase, Ag55, larval cell, Anopheles gambiae, RNAi, gene silencing |
Introduction
|
| RNA interference (RNAi) is a powerful tool for manipulating mRNA levels and has been used in a variety of systems (for example, Elbashir et al, 2001; Zhao et al, 2007). One particular group of organisms in which RNAi is used extensively is arthropods, and the technique has been demonstrated in many arthropod species including mosquitoes (Lu et al, 2004). However, although RNAi has been adapted for use in adult mosquitoes (Blandin et al, 2002) it is not well established for mosquito larvae. Therefore, the demonstration of successful RNAi in a larval mosquito cell line will be an important step towards manipulating mRNA levels in vivo. The manipulation of larval gene expression is a crucial step in understanding the mechanisms responsible for their survival. Of particular interest to our laboratory is the regulation of genes involved in larval pH regulation. |
| Mosquito larvae generate a highly alkaline pH (~10.5) in a restricted area of their alimentary canal (AC), the anterior midgut (AMG) (Dadd, 1975), despite the absence of morphological barriers between the AMG and adjacent, more neutral regions of the AC. The alkaline environment is thought to aid in digestion and is crucial for larval survival (Corena et al, 2004). However, the molecular mechanisms responsible for the generation and maintenance of the pH gradient are not fully understood. A better understanding of these mechanisms could pave way for the discovery of novel targets for new and improved larvacides. |
| One group of proteins with a known role in AMG alkalization is the carbonic anhydrase (CA) family (del Pilar Corena et al, 2002). There are a predicted twelve genes belonging to the Anopheles gambiae CA family (www.ensembl.org), six of which have been cloned by members of our laboratory (Smith et al, 2007). Our laboratory has detected CA within the epithelial cells of the mosquito larval AC in both An. gambiae and Aedes aegypti using various methods (del Pilar Corena et al, 2002; Corena et al, 2004; Seron et al, 2004; Smith et al, 2007; Neira Oviedo et al, 2008). Additionally, we established that CA is necessary for mosquito larval alkalization and survival (Corena et al, 2004). |
| Currently, the specific roles of each CA member are unknown. A first step in determining if any one CA or combination of CAs is responsible for pH regulation is to silence them either individually or in concert. In order to determine whether CAs can be manipulated by RNAi, we used an An. gambiae larval cell line, Ag55, to demonstrate CA silencing. Here, we report the mRNA down-regulation of an abundant AC CA, AgCA9 (GenBank accession number DQ518576) in Ag55 cells and the downstream knockdown of the protein product. |
Material And Methods
|
Polymerase chain reactions
|
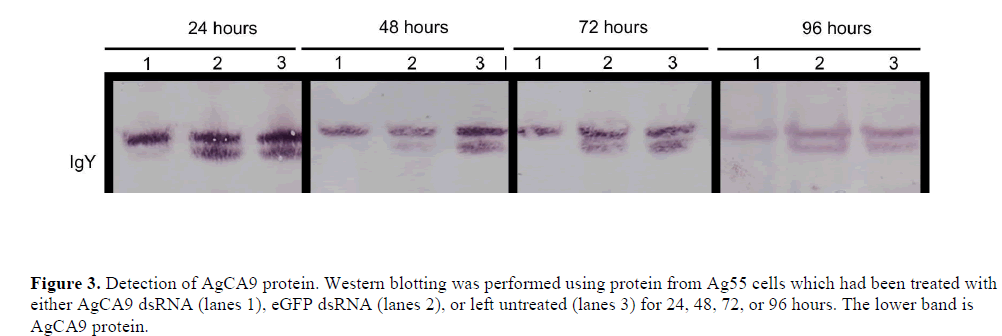
| Nucleotide sequence for primers and thermal cycling parameters used in the various polymerase chain reactions are given in Table 1. |
| Ag55 cells |
| Ag55 cell line (Pudney et al, 1979) was a gift from Kimberly Keene (Colorado State University). The cells were grown in 75 cm2 flasks (Fisher Scientific, Pittsburgh, PA) at 28°C in Leibovitz's L-15 media (Sigma-Aldrich, St Louis, MO) supplemented with 10% (v/v) fetal bovine serum (FBS, Atlantic Biologicals, Norcross, GA), 1% (v/v) Penicillin-Streptomycin solution (10,000U/ml and 10mg/ml, respectively) (Sigma-Aldrich). The cell culture medium was changed every other day. |
| Detection of AgCAs |
| RNA was extracted from 10 x 106 Ag55 cells or 20-30 whole An. gambiae fourth-instar larvae using TriZol Reagent (Molecular Research Center, Inc, Cincinnati, OH). Genomic contamination was removed using the TURBO DNA-free™ kit (Ambion, Austin, TX). RNA was reverse transcribed into cDNA using SuperScript® III Reverse Transcriptase (Invitrogen, Carlsbad, CA). All procedures were conducted according to manufacturer’s instructions. |
| cDNA from Ag55 cells or whole larvae was used as a template for PCR reactions with primers specific to one of the twelve predicted An. gambiae CA genes. Primers were designed to amplify whole mRNA, in the case of those fully cloned and sequenced genes, or a portion of the mRNA predicted by the An. gambiae genome (www.ensembl.org; February 2006 update). The PCR product was gel extracted (Qiagen Gel-extraction kit, Valencia, CA), ligated into the pCR4-TOPO vector (Invitrogen), and sequenced using the ABI Prism Big Dye Terminator Cycle Sequencing Kit (PE Biosystems, Foster City, CA). The reaction products were analyzed in an ABI Prism 310 Genetic Analyzer. |
| RNA production |
| RNA was produced by in vitro transcription in three steps: creation of double-stranded (ds)DNA flanked by the T7 promoter sequence on either the 5’ or 3’ end, transcription of sense and antisense strands into single-stranded RNA (ssRNA), and finally, annealing of the two single strands into dsRNA. Primers were designed to amplify either AgCA9 or eGFP DNA and to add a T7 promoter sequence at either the 5’ or 3’ end. eGFP cDNA was a gift from Lyric Bartholomay (University of Wisconsin). The PCR products were purified using the Qiagen PCR purification kit (Qiagen) according to manufacturer’s instructions. |
| Sense (from dsDNA with 5’ T7) and antisense (from dsDNA with 3’ T7) RNA were transcribed from the above dsDNA using the MEGAscript T7 transcription kit (Ambion) and treated with TURBO DNase (Ambion) according to manufacturer’s instructions. Each ssRNA strand was then adjusted to equal concentrations for annealing. Equal volumes of each strand were combined and incubated in a 95°C water bath for five minutes; the heat was removed and the water bath (with tubes in it) was allowed to cool to room temperature overnight. |
| RNAi experiments |
| Ag55 cells were grown to 70% confluency in six well plates for experimental (AgCA9 dsRNA treated) and two control groups (eGFP dsRNA treated and untreated). The untreated group did not receive dsRNA, but was subjected to the same conditions as the other two groups. To treat the cells, media was replaced with 800μl serum-free medium plus 36.0μg dsRNA. The plate was rocked at room temperature for 30min and then 800μl medium plus 20% FBS was added. Cells were incubated at 28ºC and harvested at 24, 48, 72 or 96 hr post-treatment; after 96hr, the cells began to overgrow and die. To harvest, the cells were washed twice with 2.0 ml serum-free medium and resuspended in 0.4 ml Trizol Reagent to extract RNA and protein according to manufacturer’s instructions. |
| Quantitative PCR (q-PCR) |
| RNA from each set of cells was treated with DNase and reverse transcribed as described previously. In order to detect AgCAs, Q-PCR primers were designed to amplify ~50 bp fragments of both AgCA9 3’ UTR or an 18s ribosomal RNA control using ABI primer express software. The reactions were prepared and run using SYBR Green Master Mix (ABI) with 300UM of each AgCA9 primer or 100UM of each 18s primer in a 96 well plate; each reaction was run in triplicate. Q-PCR was performed using Applied Biosystems (ABI) 7000 Sequence Detection system and data were analyzed using the relative expression method described in Pfaffl (2001). |
| Northern blotting |
| To generate a radioactive northern probe, a pCR4-TOPO vector containing full-length AgCA9 was linearized by restriction enzyme digest with either PstI or NotI. This template was then reverse transcribed and radiolabeled with 32P-labeled dUTP (800Ci/mmol, 20mCi/ml; GE Healthcare Bio-Sciences Corp, Piscataway, NJ) using the Maxiscript kit (Ambion). The NorthernMax Kit protocol (Ambion) was used to run and analyze the northern blot. All procedures were conducted according to manufacturer’s instructions. The following amounts of RNA were loaded for each time point: 48hr - 10μg, 72hr - 6.3μg, and 96hr - 8.0μg. Less than 1.0μg RNA was extracted from 24hr time point cells, and could not be visualized on the northern blot. |
 |
| Western blotting |
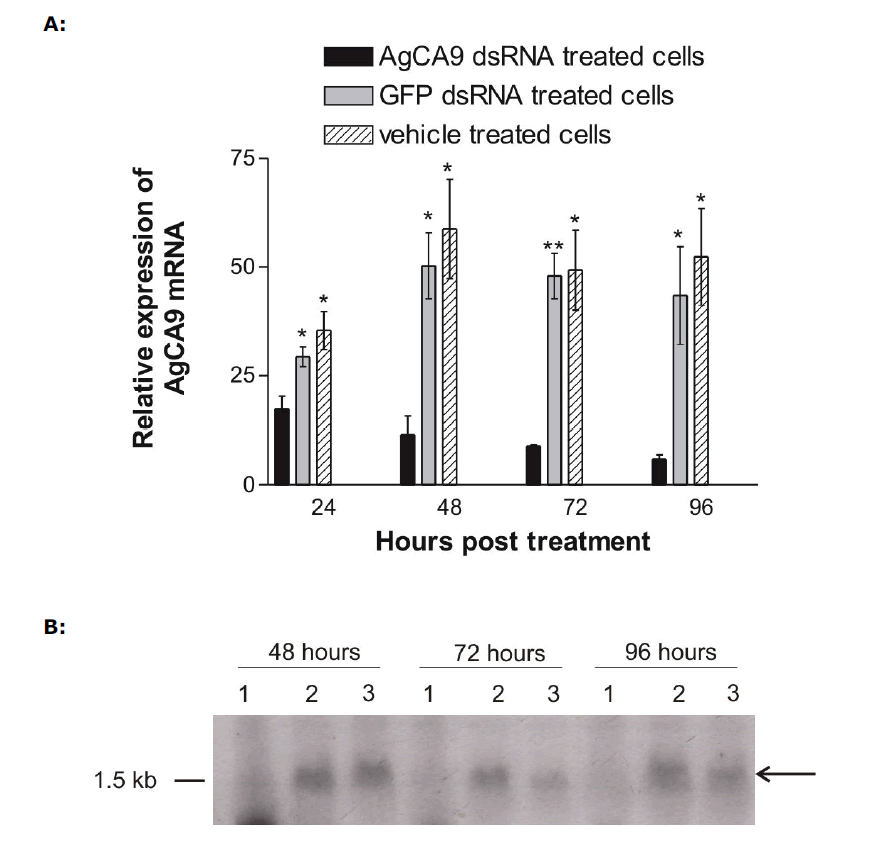
| Chicken antibodies were generated by Aves Labs, Inc (Tigard, OR) against the BSA-conjugated peptide: CZELGNRQLREVDSY and were used as purified IgY (Smith et al, 2007). To detect AgCA9 from each set of Ag55 cells (those treated with AgCA9 dsRNA or eGFP dsRNA, or those left untreated for 24, 48, 72 and 96 hr), western blotting was performed as described in Smith et al (2007). In addition to the total protein stain a second protein, which cross-reacted with the antibody, distinguishable by its slightly higher molecular weight, was used to support equal loading of each sample. |
Results And Discussion
|
| Expression of AgCAs |
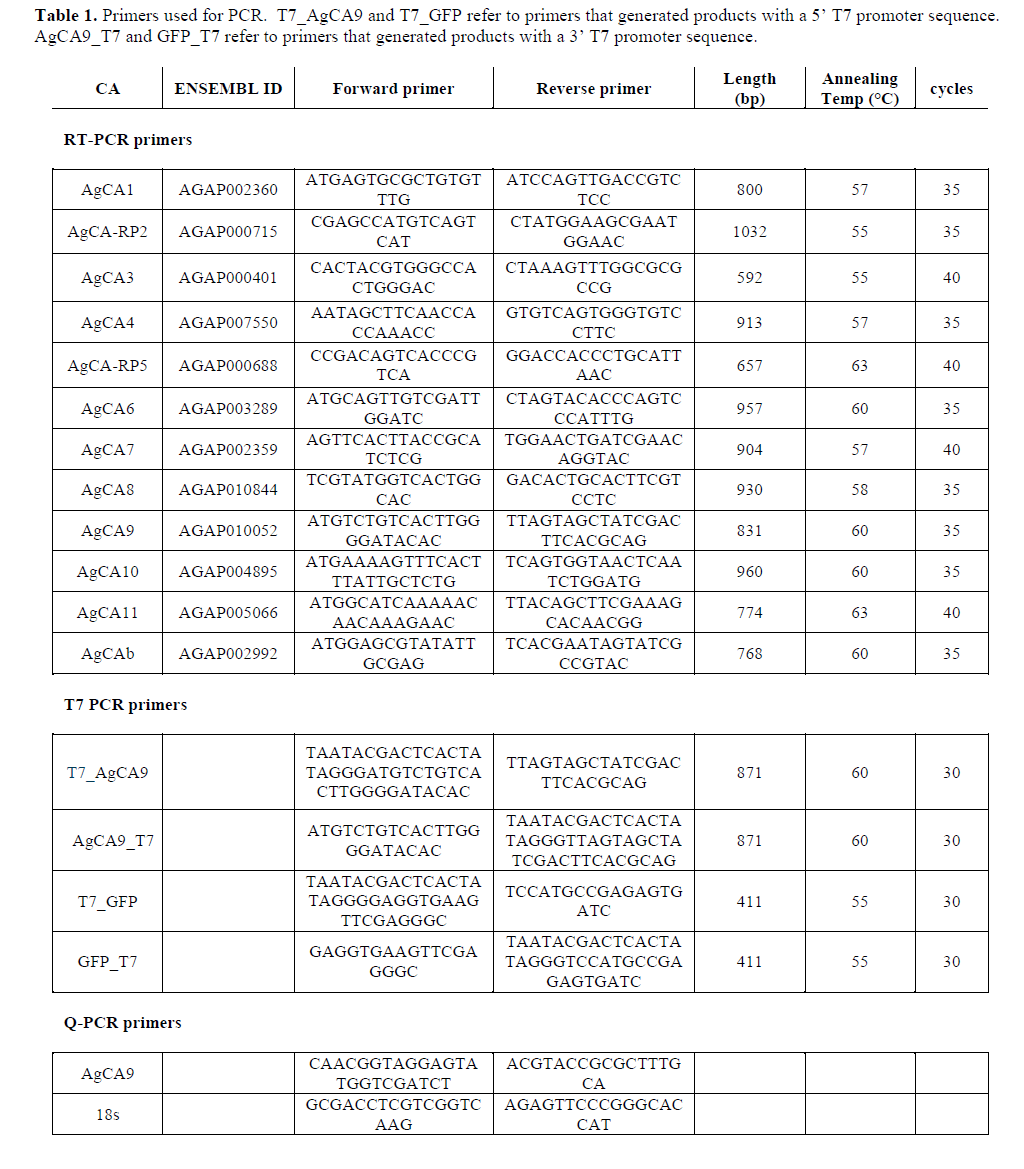
| The Ag55 cell line is an An. gambiae neonatal first-instar larval cell line which has been used by others for successful RNAi silencing (Konet et al, 2007). PCR was used to determine which CA genes were expressed in Ag55 cells, using whole larvae as a control template (Figure 1). All but AgCA1 and AgCA7 were detectable in the whole larvae controls using the indicated primers. It is possible that these CAs were expressed at a level too low to be detected using our methods. However, the expression of many CA genes is known to be highly-variable between larval and adult specimens (Dissanayake et al, 2006). Thus, it is also possible that these CAs might not be expressed at this particular developmental stage. Ag55 cells expressed AgCA3, AgCA9 and AgCAb. AgCA9 is a good candidate for a role in larval pH regulation due to the high-level mRNA expression in the gastric caeca and ectoperitrophic space (Smith et al, 2007). Therefore we chose to test whether this CA could be silenced in Ag55 cells using RNAi. |
 |
| Reduction of AgCA9 mRNA and protein levels by using dsRNA |
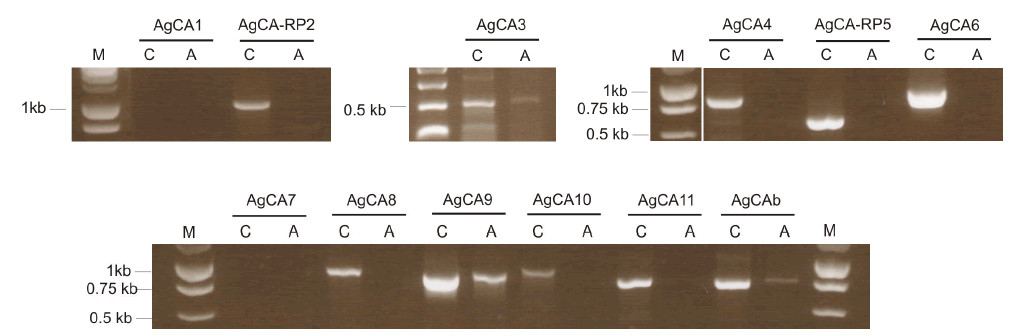
| Ag55 cells were treated with full length AgCA9 dsRNA, eGFP dsRNA or were left untreated for 24, 48, 72 or 96 hr. Treatment with AgCA9 dsRNA resulted in a statistically significant (P-value <0.05) knock-down of AgCA9 mRNA compared to the eGFP dsRNA or untreated cells as determined by q-PCR analyses (Figure 2A). This knock-down became evident after 24hr and persisted for at least 96hr, exhibiting 88% reduction in AgCA9 mRNA level at 96hr time-point. These time-point data are consistent with previous reports, which suggested RNAi silencing to occur in as little as 24hr (Blitzer et al, 2005) and to last for at least ten days (Keene et al, 2004). |
| Northern blot analysis performed in parallel with q-PCR supported a considerable knock-down of AgCA9 mRNA in cells treated with AgCA9 dsRNA (Figure 2B). A distinct AgCA9 mRNA band was detected at the expected size (~1.5 kb), including un-translated regions, in the control lanes, which was absent from the cells treated with AgCA9 dsRNA. These data, in conjunction with q-PCR results, demonstrate that introduction of dsRNA is sufficient to silence AgCA9 mRNA in Ag55 cells. |
| When using RNAi to investigate protein function, the protein half-life must be considered. If the protein is stable, for example, with a half-life that exceeds the length of the experiment, protein levels may remain unchanged even if mRNA expression is decreased. However, there are no clear data to indicate the half-life of AgCA9 protein. We therefore, tested by western blotting whether we could reduce the AgCA9 levels parallel with the mRNA levels. Western blot analysis demonstrated that AgCA9 protein was indeed considerably down-regulated in cells treated with AgCA9 dsRNA compared with the controls (Figure 3). These results indicate that AgCA9 protein is capable of being manipulated by RNAi and suggests that this technique has the potential for in vivo silencing of CAs. By down-regulating CA protein in mosquito larvae, pH changes can be assessed and relative contributions of each CA to AMG pH regulation can be determined. |
| Several RNAi-based methods have been applied to silence genes in adult mosquitoes: These include direct injection of dsRNA (Boisson et al, 2006; Roy et al, 2007; Hansen et al, 2007), introduction of a transgene to deliver hairpin RNA (Franz et al, 2006), and infection with a virus that produces the dsRNA of interest (Adelman et al, 2001). The most straightforward approach to RNAi in mosquitoes appears to be direct injection of dsRNA into the hemocoel. Because the AC is only one cell thick, and mosquitoes have an open circulatory system, therefore, in principle, injected dsRNA has access to virtually every AC cell. Down-regulation of AgCA9 has proved difficult in our hands: We have attempted this by injecting full-length dsRNA, 300 bp dsRNA, or siRNAs into the hemolymph of An. gambiae for various time-points and at various concentrations with little success. Despite much work dedicated to larval RNAi on the part of these authors and others, to date there is only one report of successful RNAi in mosquito larvae via injection of dsRNA (Blitzer et al, 2005), and one report using transgenic larvae (Brown et al, 2003). |
| The apparent difference in RNAi susceptibility between mosquito adults and larvae may be due to the inability of larval cells to uptake dsRNA in a systemic manner, as suggested for Drosophila melanogaster (Miller et al, 2008). Our work indicates that, as in D. melanogaster, larval mosquito cells possess the RNAi machinery. However, for in vivo success delivery of intracellular dsRNA may be the critical step. We have demonstrated that the half-life of AgCA9 protein is likely to be short enough to facilitate RNAi-mediated down-regulation in An. gambie larvae. Further work in An. gambiae larvae, and possible generation of a transgenic line, will yield a greater understanding of this important family of genes in the crucial process of pH regulation. |
 |
| Figure 2. Analyses of AgCA9 mRNA expression. Quantitative PCR (A) and northern analysis (B) were used to determine endogenous AgCA9 mRNA levels in Ag55 cells which had been treated with either AgCA9 dsRNA, eGFP dsRNA, or left untreated for 24, 48, 72, or 96 hours. For northern analysis- lanes 1: AgCA9 dsRNA-treated cells; lanes 2: eGFP dsRNA-treated cells; lanes 3: untreated cells. *: P-Value < 0.05, **: P-Value < 0.005. |
 |
Conclusions
|
| In conclusion we demonstrate that AgCA9 dsRNA can down-regulate mRNA and protein levels in cultured larval cells. Furthermore, protein down-regulation occurs within 24 hours and lasts for at least 96 hours. |
Acknowledgements
|
| This research was supported by NIH grant: NIAID AI- 45098-10 (P. J. Linser). We thank Dr Bartholomay for providing eGFP DNA and Dr Keene for providing the Ag55 cell line. |
Competig Interests
|
| None declared. |
List Of Abbreviations
|
| AC; Alimentary canal |
| AMG; Anterior midgut |
| CA; Carbonic anhydrase |
| FBS ; Fetal bovine serum |
| q-PCR ; Quantitative PCR |
References
- Adelman ZN, Blair CD, Carlson JO, Beaty BJ, and Olson KE. 2001. Sindbis virus-induced silencing of dengue viruses in mosquitoes. Insect Mol Biol, 10, 265-273.
- Blandin S, Moita LF, Köcher T, Wilm M, Kafatos FC and Levashina EA. 2002. Reverse genetics in the mosquito
- Anopheles gambiae: targeted disruption of the Defensin gene. EMBO Reports, 3, 852-856.
- Blitzer EJ, Vyazunova I and Lan Q. 2005. Functional analysis of AeSCP-2 using gene expression knockdown in the yellow fever mosquito, Aedes aegypti. Insect Molec Biol, 14, 301-307.
- Boisson B, Jacques JC, Choumet V et al. 2006. Gene silencing in mosquito salivary glands by RNAi. FEBS Lett, 580, 1988-1992.
- Brown AE, Crisanti A and Catteruccia F. 2003. Comparative analysis of DNA vectors at mediating RNAi in Anopheles mosquito cells and larvae. J Exp Biol, 206(Pt 11), 1817-1823.
- Corena M, Fiedler MM, Van Ekeris L, Tu C, Silverman DN and Linser PJ. 2004. Alkalization of larval mosquito midgut and the role of carbonic anhydrase in different species of mosquitoes.Comp Biochem Physiol Part C, 137, 207-225.
- Dadd RH. 1975. Alkalinity within the midgut of mosquito larvae with alkaline-active digestive enzymes. J Insect Physiol, 21,1847-1853.
- Dissanayake SN, Marinotti O, Ribeiro JM, and James AA. 2006. angaGEDUCI: Anopheles gambiae gene expression database with integrated comparative algorithms for identifying conserved DNA motifs in promoter sequences. BMC Genomics, 7, 116-127.
- del Pilar Corena M, Seron TJ, Lehman HK et al. 2002. Carbonic anhydrase in the midgut of larval Aedes aegypti: cloning, localization and inhibition. J Exp Biol, 205, 591-602.
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K and Tuschl T. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature, 411, 494-498.
- Franz AW, Sanchez-Vargas I, Adelman ZN et al. 2006. Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti. Proc Natl Acad Sci USA, 103, 4198-203.
- Hansen IA, Sieglaff DH, Munro JB et al. 2007. Forkhead transcription factors regulate mosquito reproduction. Insect Biochem Mol Biol, 37, 985-997.
- Keene KM, Foy BD, Sanchez-Vargas I, Beaty BJ, Blair CD and Olson KE. 2004. RNA interference acts as a natural antiviral response to O'nyong-nyong virus (Alphavirus; Togaviridae) infection of Anopheles gambiae. Proc Natl Acad Sci USA, 101, 17240-17245.
- Konet DS, Anderson J, Piper J, Akkina R, Suchman E and Carlson J. 2007. Short-hairpin RNA expressed from
- polymerase III promoters mediates RNA interference in mosquito cells. Insect Mol Biol, 16, 199-206.
- Lu R, Li H, Li WX, and Ding SW. 2004. RNA-based immunity in insects. In: S.H. Gillespie, GL Smith and A Osbourn, Editors, Society for General Microbiology Symposium 63:Microbe Vector Interactions in Vector-borne Diseases, Cambridge University Press, Cambridge, pp. 63-74.
- Miller SC, Brown SJ and Tomoyasu Y. 2008. Larval RNAi in Drosophila? Dev Genes Evol, 218, 505-510.
- Neira Oviedo M, Van Ekeris L, Corena-Mcleod MDP and Linser PJ. 2008. A microarray-based analysis of transcriptional compartmentalization in the alimentary canal of Anopheles gambiae (Diptera: Culicidae) larvae. Insect Mol Biol, 17, 61-72.
- Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res, 29, 2002-2007.
- Pudney M, Varma MGR and Leake CJ. 1979. Establishment of cell lines from larvae of culicine (Aedes species) and
- Anopheline mosquitoes. TCA Manual, 5, 997-1002.
- Roy SG, Hansen IA and Raikhel AS. 2007. Effect of insulin and 20-hydroxyecdysone in the fat body of the yellow fever mosquito, Aedes aegypti. Insect Biochem Mol Biol, 37, 1317-1326.
- Seron TJ, Hill J and Linser PJ. 2004. A GPI-linked carbonic anhydrase expressed in the larval mosquito midgut. J Exp Biol, 207(Pt 26), 4559-4572.
- Smith KE, Van Ekeris LA and Linser PJ. 2007. Cloning and characterization of AgCA9, a novel W-carbonic anhydrase from Anopheles gambiae Giles sensu stricto (Diptera: Culicidae) larvae. J Exp Biol, 210, 3919-3930.
- Zhao T, Li G, Mi S, Li S, Hannon GJ, Wang XJ, and Qi Y. 2007.A complex system of small RNAs in the unicellular green alga Chlamydomonas reinhardtii. Genes Dev, 21, 1190-1203.
|



