Research Article - Current Pediatric Research (2021) Volume 25, Issue 8
Serological and epidemiological study on COVID-19 infections in Nineveh governorate.
KawkabIdrees Mahmood1*, Haitham Abdul-Malik Al-Nori2, Zainablssam Ali3
1Department of Anatomy, College of Medicine, University of Mosul, Mosul, Iraq
2Department of Surgery, College of Medicine, University of Mosul, Mosul, Iraq
3Department of Haematology, Ibn Al Ather Teaching Hospital, Mosul, Iraq
- Corresponding Author:
- KawkabIdrees Mahmood
Department of Anatomy
College of Medicine
University of Mosul
Mosul
Iraq
E-mail: abujamaljameel@gmail.com
Accepted date: 27th August, 2021
Abstract
Background: A novel coronavirus Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) discovered in China in December 2019 that has led to an unprecedented global pandemic. It causes coronavirus disease 19 (COVID-19), a respiratory illness with symptoms ranging from mild to severe with possible progression to pneumonia, multiple organ failure and even to death. Objectives: • To investigate the risk factors associated with COVID - 9 infections. • To investigate the occurrence and percentage of olfactory dysfunction in patients with COVID-19 infection. • To investigate the relationship between ABO and Rh blood groups and covid-19 infection. Methods: Blood samples were collected from 400 persons presenting with symptoms suggestive of COVID-19. These persons were admitted to Al Rabee hospital during a period from 1st June to 1st December 2020. ABO and Rh (D) blood groups test and covid-19 test were performed to all patients and control. COVID-19 test was confirmed by VIDAS methods. This study included 200 COVID-19 positive patients and 200 COVID-19 negative persons used as control. Results: Patients age ranged from 1 year to 80 years (mean age 54 years); the highest frequency of COVID-19 occurred in the age group (61-70) years. There were 120 (60%) males and 80 (40%) females with a ratio of 1.5:1. The most diffused risk factors associated with COVID-19 infection were older age, male sex and major comorbidities including (hypertension, cardiovascular disease, diabetes mellitus, chronic respiratory disease, obesity, smoking, chronic kidney disease, cancer, liver disease and malnutrition) 77% of patients reported olfactory dysfunction. The ABO blood group in 200 normal persons showed a distribution of 31%, 16%, 8% and 45% for A, B, AB and O, respectively vs. the ABO blood group in 200 COVID?19 positive patients showed a distribution of 46%, 18%, 9% and 27% for A, B, AB and O, respectively. ABO blood group was found to be significantly associated with COVID?19 status (P=0.001). The proportion of blood group A and O in COVID-19 patients were significantly higher and lower respectively than that in normal people (both P=0.001). No significant association was found between Rhesus negative (Rh-) blood group and occurrence of COVID-19 in present study. Conclusion: Our data found that the risk factors associated with COVID-19 infection were older age, male sex and comorbidities. And the risk of COVID-19 is higher with blood group A persons and lower with blood group O. Rhesus negative (Rh-) blood group associated with lower risk of COVID-19 illness.
Citation: Farvardin M, Johari MK, Nami M, et al. Annular choroidal detachment one year after argus-II retinal prosthes is implantation.
Ophthalmol Case Rep. 2020;4(1):23-25.
Keywords
COVID-19, SARS-COV-2, Risk factors, Olfactory dysfunction, ABO blood group, Rh blood group.
Introduction
A pandemic coronavirus SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) that causes the COVID-19(coronavirus disease 2019) syndrome can include upper respiratory infection symptoms, severe respiratory distress, acute cardiac injury, and death [1]. SARS-COV-2 infections were transmitted to Iraq in Najaf Al-Ashraf in 22 February 2020, and transmitted to Nineveh governorate in 22 March 2020 [2,3]. Classification of COVID-19 patients symptoms into asymptomatic, mild, moderate, severe and critical diseases [4]. SARS- COV-2 is a near sphere of protein inside a fatty membrane that protects a twisting single strand of RNA a molecule that holds the viruses’ genetic code [5].
It has four structural proteins: S (spike); E (envelope); M (membrane); and N (nucleocapsid) [6]. SARS-CoV-2 infects cells through interactions between its Spike (S) protein and the Angiotensin-Converting Enzyme 2(ACE2) proteins on target cells. This interaction requires cleavage of the S protein, likely by the cell surface protease TMPRSS2 (trans-membrane serine protease 2) [1]. ACE2 is a cellular receptor expressed in the lungs (in type 2 pneumocytes), arteries, heart, kidneys, and the intestine [7]. A SARS-CoV-2 particle enters a person's nose or mouth and floats in the airway until it brushes against a lung cell that has an ACE2 receptor on the surface. The virus binds to that cell, slips inside and uses the cell's machinery to help make copies of it. They break out, leaving the cell for dead, and penetrate other cells. [5,8].
The virus induce a cytokine storm in the body, generate a series of immune responses, and cause changes in peripheral white blood cells and immune cells such as lymphocytes and increase in inflammatory cytokines including (IL-6). Some patients progressed rapidly with Acute Respiratory Distress Syndrome (ARDS) and septic shock, which was eventually followed by multiple organ failure [9].There are 34 recognized human blood group systems and hundreds of individual blood group antigens and alleles [10]. The ABO blood group system was discovered by Karl Landsteiner, who classified blood groups according to the presence of A and B antigens on the surface of red blood cells. An individual with blood group A has A antigen and anti-B antibodies; blood group B has B antigen and anti-A antibodies; blood group AB has both A and B antigens but no antibodies, whilst individuals with blood group O haveno antigen but both anti-A and anti-B antibodies [11].
In humans, besides their expression on red blood cells, A and B antigens a widely expressed on many other cell types, including vascular endothelial cells and epithelial cells of many organs [12]. An individual’s RBC surface may or may not contains Rh or D-antigen; accordingly, Rh-positive (D-antigen present) or Rh-negative (D-antigen absent) [11]. Since the discovery of the ABO blood group system, many studies have been undertaken to study the link between the ABO blood group system and diseases. Cheng et al. found that there is an association between ABO blood group and the risk of developing the Severe Acute Respiratory Syndrome (SARS caused by SARS?CoV?1 infection) and the severity of associated complications [13].
Objectives
To investigate the risk factors associated with COVID-19 infection, to investigate the occurrence and percentage of olfactory dysfunction in patients with COVID-19 infection and to investigate the relationship between ABO and Rh blood groups and covid-19 infection.
Methods
Blood samples were collected from 400 persons presenting with symptoms suggestive of COVID-19. These persons were admitted to Al-Rabee hospital during a period from 1st June to 1st December 2020.ABO and Rh (D) blood groups test and covid-19 test were performed to all patients and control. Blood group test was determined using (ABO & Rh) kit provided by (Biomerieux, France). COVID-19 test was confirmed by VIDAS methods using VIDAS-SARS-COV-2 IgM Kit and VIDAS-SARS-COV-2 IgG Kit provided by (Biomerieux, France) for detect the antibodies IgM and IgG respectively in human serum using (ELFA) technique Enzyme Linked Fluorescent assay [14,15].
This study included 200 COVID-19 positive patients and 200 COVID-19 negative persons used as control. In order to collect the information related to the study, a questionnaire form was used. The questionnaire form included: Age; sex; malnutrition; smoking; hypertension; cardiovascular disease; diabetes mellitus; chronic respiratory disease; obesity; chronic kidney disease; cancer and liver disease.Statistical analysis was performed by using chi-square test to find statistical association, and SPSS software (version 23). P-value of <0.05 was considered as a significant.
Results
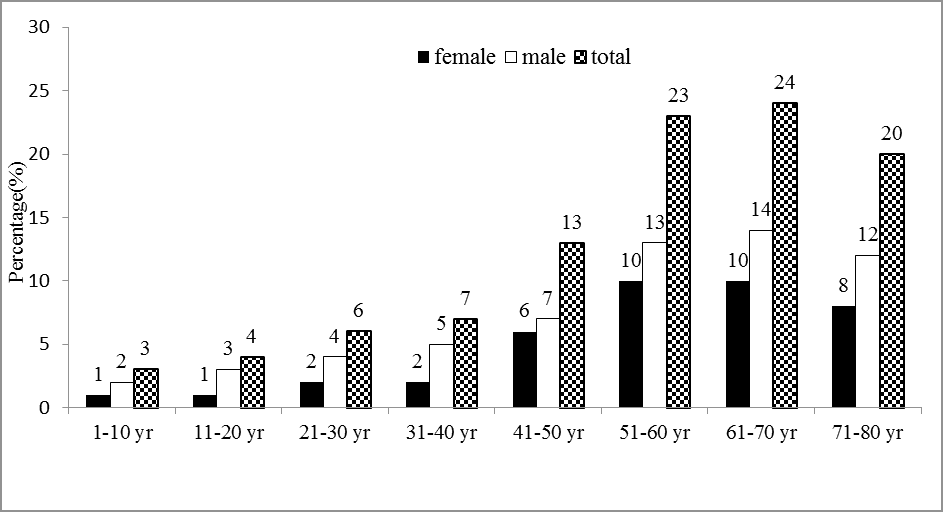
Patient’s age ranged from 1 year-80 years (mean age 54 years). The present study showed that the highest frequency of COVID-19 was found in the age group 61 years-70 years 48 patients (24%) followed by age group 51 years-60 years 46 patients (23%) and the lowest frequency of COVID-19 was observed in the age group 1 year-10 years 6 patients (3%) shown in Figure 1.
The results were statistically significant regarding the differences in the age groups 1 years-10 years and 61 years-70 years ( both p=0.001) as it shown in the Table 1.
| Age groups (Year) | Covid-19 (n=200) | Control (n=200) | OR | CI 95% | P* value | ||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| 01-Oct | 6 | 3 | 30 | 15 | 1.14 | 1.07-1.23 | 0.001 |
| Nov-20 | 8 | 4 | 17 | 8.5 | 1.06 | 1.00-1.11 | 0.05 |
| 21-30 | 12 | 6 | 15 | 7.5 | 1.17 | 0.54-2.52 | 0.42 |
| 31-40 | 14 | 7 | 26 | 13 | 1.05 | 0.98-1.12 | 0.05 |
| 41-50 | 26 | 13 | 46 | 23 | 1.13 | 1.03-1.24 | 0.01 |
| 51-60 | 46 | 23 | 22 | 11 | 0.87 | 0.79-0.95 | 0.001 |
| 61-70 | 48 | 24 | 24 | 12 | 0.85 | 0.79-0.95 | 0.001 |
| 71-80 | 40 | 20 | 20 | 10 | 1.01 | 0.96-1.07 | 0.004 |
| Total | 200 | 100 | 200 | 100 | |||
Table 1. Distribution of COVID-19 patients according to age groups. *: Chi square test; CI: Confidence Interval; OR: Odds Ratio.
Table 2 illustrates the association between risk factors and the occurrence of COVID-19 patients. There were 120 (60%) males and 80 (40%) females with a male to female ratio of 1.5:1. The presence of COVID-19 was significantly associated with older age more than 50 years old (p=0.001) and male sex (p=0.04).
The most common major comorbidities were: hypertension 22% (p=0.001), cardiovascular disease 15% (p=0.001), diabetes mellitus 14% (p=0.001), chronic respiratory disease 12% (p=0.004), obesity 10% (p=0.006), chronic kidney disease 7% (p=0.007), cancer 6% (p=0.007) and liver disease 4% (p=0.005) 7% of COVID-19 patients were smokers (p=0.02) and 3% of patients have malnutrition (0.01).
| Risk factors | Covid-19 (n=200) | Control (n=200) | OR | CI 95% | P value | |||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | |||||
| Gender | Male | 120 | 60 | 102 | 51 | 0.964 | 0.467-1.031 | 0.04 |
| Female | 80 | 40 | 98 | 49 | ||||
| Age | >50 | 118 | 59 | 134 | 67 | 0.24 | 0.18-0.71 | 0.001 |
| ≤ 50 | 82 | 41 | 66 | 33 | ||||
| Hypertension | 44 | 22 | 11 | 5.5 | 0.226 | 0.115-0.443 | 0.001 | |
| Cardio vascular d. | 30 | 15 | 10 | 5 | 0.298 | 0.142-0.628 | 0.001 | |
| Diabetes mellitus | 28 | 14 | 9 | 4.5 | 0.323 | 0.153-0.685 | 0.001 | |
| Chronic respiratory d. | 24 | 12 | 8 | 4 | 0.306 | 0.134-0.698 | 0.004 | |
| Obesity | 20 | 10 | 6 | 3 | 0.278 | 0.109-0.709 | 0.006 | |
| Smoking | 14 | 7 | 4 | 2 | 0.271 | 0.088-0.839 | 0.02 | |
| Chronic kidney d. | 14 | 7 | 3 | 1.5 | 0.271 | 0.088-0.839 | 0.007 | |
| Cancer | 12 | 6 | 2 | 1 | 0.158 | 0.035-0.716 | 0.007 | |
| Liver d. | 8 | 4 | 0 | 0 | 0.96 | 0.933-0.988 | 0.005 | |
| Malnutrition | 6 | 3 | 0 | 0 | 0. 970 | 0.947-0.994 | 0.01 | |
Table 2. Distribution of risk factors among patients with COVID-19 and control group.
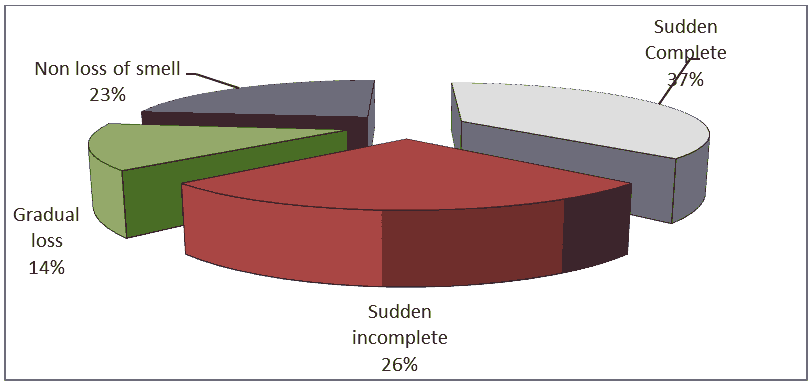
Regarding the clinical presentation, the study showed that 154 patients (77%) out of 200 patients reported olfactory dysfunction including: (37%) reported sudden complete olfactory loss, (26%)reported sudden incomplete loss and 14% reported gradual loss, While (23%) reported non loss of olfactory as shown in Figure 2.
Table 3 manifests the association between olfactory dysfunction and the occurrence of COVID-19 and the result was statistically highly significant (p=0.001).
| Olfactory dysfunction | Covid-19 (n=200) | Control (n=200) | OR | CI 95% | P value | ||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| Sudden Complete | 74 | 37 | 8 | 4 | 2.58 | 2.15-3.03 | 0.001 |
| Sudden incomplete | 52 | 26 | 4 | 2 | 2.35 | 1.96-2.74 | 0.001 |
| Gradual loss | 28 | 14 | 2 | 1 | 16.1 | 15.86-16.38 | 0.001 |
| Non loss of smell | 46 | 23 | 186 | 93 | 16.1 | 15.63-16.60 | 0.001 |
| Total | 200 | 100 | 200 | 100 | |||
| P value | 0.001 | ||||||
Table 3. Distribution of olfactory dysfunction in patients with COVID-19 and control group.
Females with COVID-19 were significantly more affected by olfactory dysfunction than males (p=0.05) as reported in Table 4.
| Olfactory dysfunction | Covid-19 | Control | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | % | Female | % | Male | % | Female | % | ||
| Sudden Complete | 34 | 28.3 | 40 | 50 | 4 | 3.9 | 4 | 4 | 0.001 |
| Sudden incomplete | 26 | 21.7 | 26 | 32.5 | 2 | 2 | 2 | 2 | 0.001 |
| Gradual loss | 14 | 11.7 | 14 | 17.5 | 2 | 2 | 0 | 0 | 0.01 |
| Non loss of smell | 46 | 38.3 | 0 | 0 | 94 | 92.1 | 92 | 94 | 0.001 |
| Total | 120 | 80 | 102 | 98 | |||||
| P value | 0.05* | ||||||||
Table 4. Distribution of olfactory dysfunction according to sex among COVID-19 patients and control group.
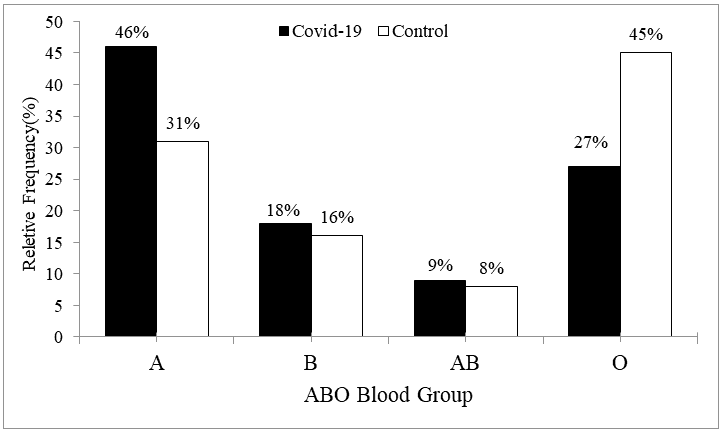
The ABO blood group in 200 normal persons showed a distribution of 31%, 16%, 8% and 45% for A, B, AB and O, respectively versus, the ABO blood group in200 COVID?19 positive patients showed a distribution of 46%, 18%, 9% and 27% for A, B, AB and O, respectively as shown in Figure 3.
Table 5 represents the association between ABO blood group and COVID-19 infection.ABO blood group was found to be significantly associated with COVID?19 status (P=0.001). The proportion of blood group A in patients with COVID-19 was significantly higher than that in normal persons being 46% in the patients versus 31% in the normal persons (P=0.001). The proportion of blood group O in patients with COVID-19 was significantly lower than that in normal persons, being 27% in the patients versus 45% in the normal persons (P=0.001).
| ABO Grouping | Covid-19 (n=200) | Control (n=200) | OR | CI 95% | P value | ||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| A | 92 | 46 | 62 | 31 | 1.896 | 1.260-2.854 | 0.001 |
| B | 36 | 18 | 32 | 16 | 1.152 | 0.683-1.943 | 0.345 |
| AB | 18 | 9 | 16 | 8 | 1.137 | 0.563-2.299 | 0.429 |
| O | 54 | 27 | 90 | 45 | 0.452 | 0.297-0.687 | 0.001 |
| Total | 200 | 100 | 200 | 100 | |||
| P value | 0.001 | ||||||
Table 5. ABO blood group distribution in patients with COVID-19 and control group.
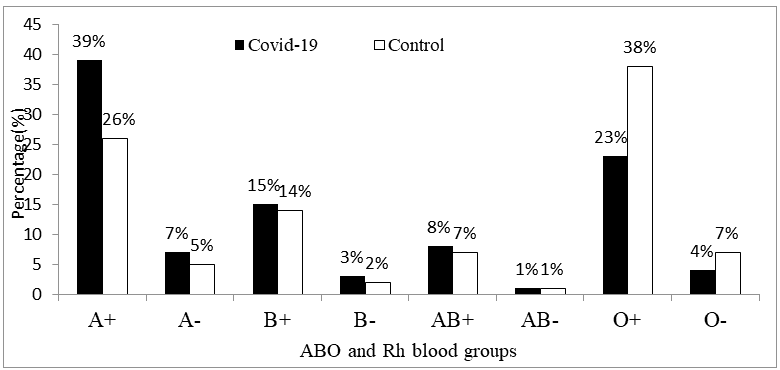
The ABO and Rh blood groups in 200 normal persons showed a distribution of 26%, 5%, 14 %, 2%, 7%, 1%, 38% and 7% for A+,A-, B+, B-, AB+, AB-, O+ and O-respectively vs. the ABO and Rh blood groups in 200 COVID?19 positive patients showed a distribution of 39%, 7%, 15%, 3 %, 8%, 1%, 23%, and 4% for A+, A-, B+, B-, AB+, AB-, O+ and O-respectively; as shown in Figure 4 and Table 6.
| ABO Grouping | Covid-19 (n=200) | Control (n=200) | OR | CI 95% | P value | ||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| A+ | 78 | 39 | 52 | 26 | 1.819 | 1.360-2.279 | 0.02 |
| A- | 14 | 7 | 10 | 5 | 1.43 | 1.120-1.660 | 0.41 |
| B+ | 30 | 15 | 28 | 14 | 1.084 | 0.740-1.430 | 0.79 |
| B- | 6 | 3 | 4 | 2 | 1.515 | 1.360-1.670 | 0.52 |
| AB+ | 16 | 8 | 14 | 7 | 1.155 | 0.900-1.414 | 0.71 |
| AB- | 2 | 1 | 2 | 1 | 1 | 0.902-1.098 | 1 |
| O+ | 46 | 23 | 76 | 38 | 0.487 | 0.036-0.940 | 0.006 |
| O- | 8 | 4 | 14 | 7 | 0.554 | 0.330-0.777 | 0.2 |
| P value | 0.001 | ||||||
Table 6. ABO and Rh blood groups distribution in patients with COVID-19 and control group.
Table 7 shows the association between Rh blood group and COVID-19 infection. No significant association was found between Rhesus negative (Rh-) blood group and occurrence of COVID-19 in present study (p=0.556). The proportion with Rh- status was 15%.
| Rh Grouping | Covid-19 (n=200) | Control (n=200) | OR | CI 95% | P value | ||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| Rh- | 30 | 15 | 30 | 15 | 1 | 0.578-1.731 | 0. 556 |
| Rh+ | 170 | 85 | 170 | 85 | 1 | 0.578-1.731 | 0.556 |
| Total | 200 | 100 | 200 | 100 | |||
| P value | 0.001 | ||||||
Table 7. Rh blood group distribution among COVId-19 patients and control group. >0.05: Non-significant; <0.05: Significant; <0.001: Highly significant.
Discussion
The present study showed that the highest frequency of COVID-19 patients was found in the age group 61 years-70 years (26%), which was comparable with Lechien et al. [16] found that the maximum frequency of COVID-19 observed in age group 61 years-70 years. The percentage of COVID-19 patients increased in the age group 61-70 due to that older age patients had weaker immune functions [17] and had stronger host innate responses to virus infection than younger adults [18].
The present study showed that the lowest frequency of COVID-19 was observed in the age group 1 year-10 years (3%), which was similar to Yuki et al. [4] that concluded that the children are less susceptible to COVID-19 due to less intense immune response to the virus in children compared to the adults (cytokine release syndrome). viral interference in the respiratory tract of young children leads to lower viral load in children and the expression level of the receptor of COVID-19 Angiotensin Converting Enzyme-2 (ACE2) may be lower in children [19].
The present study showed that males formed (60%) of cases of COVID-19 while females formed (40%), which was similar to Docherty et al. findings. The reduced susceptibility of females to SARS-COV-2 infections could be attributed to a the differences in immune response to COVID-19 infection, sex hormones, protection from X chromosome [4,16].
Our study found that SARS COV-2 is more likely to infect older adult males with chronic comorbidities due to weaker immune functions of these patients. In this study the chronic underlying diseases were (hypertension, cardiovascular diseases, diabetes mellitus, chronic respiratory diseases, obesity and cancer) our data were in line with Li et al., Chen et al., Huang et al., Huang et al. [21-24]. D’Errico et al. found relationship between COVID-19 severity outcomes and cardiovascular disease [25]. Guo et al. found that diabetes mellitus is a risk factor for rapid progression and bad prognosis of COVID-19 due to COVID-19 patients with diabetes have weak immune system and are more susceptible to a cytokine storm that lead to rapid deterioration of COVID-19 [26]. Brake et al. found that smoking has the potential to up regulate the ACE2 receptor, making smokers more vulnerable to Covid-19 [27].
Altered olfactory function is a common symptom of COVID-19. The proportion of COVID-19 patients affected by olfactory dysfunction in our study(77%) was close to that reported by Lechien et al. (70.2%) and Amer et al. (83%) and higher than Cazzolla et al. (65.7%) and lower than Lechien et al. (85.5%) [16,28-30].The present study showed that the clinical symptom olfactory dysfunction was significantly influenced by gender. Females are more affected by olfactory dysfunction than males due to gender-related differences in the inflammatory reaction process [28-30].
Brann et al. found that SARS-CoV-2 infection of non-neuronal cell types, the support cells known as sustentacular cells leads to olfactory dysfunction in patients with COVID-19 [31]. Brann et al. focused on sustentacular cells because the SARS COV-2 attacks by targeting the Angiotensin Converting Enzyme 2 (ACE2) receptor on cell surfaces, and Sustentacular cells have many of these receptors, unlike olfactory neurons. This indicates that the virus is infecting the support cells, leaving the nerve cells weak and starved of nutrients found that olfactory support cells, not neurons, are vulnerable to novel coronavirus infection. But there may be other ways that Covid-19 causes a loss of smell. For example, Cazzolla et al. in Italy showed the key role of interleukin-6 in the pathogenesis of chemosensitive disorders in COVID-19 patients [29,32].
In this study ABO blood group was found to be significantly associated with COVID?19 status (P=0.001) and the risk of COVID-19 is higher with blood group A persons and lower with blood group O, which was comparable with AL-Khikani et al., Robinson et al., Ahmed et al., Li et al. [10,11,33,34]. Zhao et al. in China concludes that blood group A is associated with an increased risk of SARS-CoV-2 infection and severe COVID-19, while blood group 0 is associated with a reduced risk compared to non-blood group O [35]. Gerard et al. in UK analyzed Zhao et al. data and found that people with anti A in serum (B and O) are significantly less represented in the COVID-19 patients than those lacking anti A (A and AB blood groups) and anti A from O Individuals is more protective than anti A from B suggesting that IgG anti A may play important role [36].
SARS COV-2 replicates in epithelial cells of the respiratory and digestive tracts that have the ability to synthesize A and/or B glycan antigens depending on individuals ABO phenotype. The spike proteins produced in A, B or A/B individuals could be decorated with A, B and A/B glycan antigen respectively. Anti A, anti B and anti A, B antibodies could bind to the A, B and A/B antigens on the S proteins. Respectively and block the interaction between S and ACE2 proteins [12,37].
In the genetic European study by Ellinghaus et al. a genome-wide association analysis was performed with severe COVID-19 patients with respiratory failure from the epicenters of the European pandemic in Italy and Spain [38]. The researchers were able to identify gene variants on chromosomes 3 and 9 that were associated with severe COVID-19 courses. A strikingly large number of carriers of these two gene variants were people with blood group A. The data confirm that blood group A is associated with a higher risk than the non-A blood groups and the risk for blood group 0 are lower compared to the non-0 blood groups [11].
Nevertheless the present study demonstrate No significant association between Rhesus negative Rh- blood group and the occurrence of COVID-19 (p=0.556) and the proportion with Rh- status was (15%), this was similar to that found by Ray et al., who found the Rh-blood group associated with a slightly lower risk for SARS-CoV-2 infection and the proportion with Rh- status was (13.1%) [39,40,41].
Conclusion
Risk factors associated with COVID-19 infection were older age, male sex and comorbidities. The most important symptom in COVID-19 patients is sudden olfactory dysfunction. The risk of COVID-19 is higher with blood group A persons and lower with blood group O. Rhesus negative (Rh-) blood group associated with lower risk of COVID-19 illness. Our study recommended that older age males and individuals with comorbidities (such as diabetes mellitus, obesity and cancer) are at higher risk of severe or critical COVID-19 infection that needs more health care and avoid public gathering. Individuals with blood group A might require strict hygiene measures to lower the infection and extra attention from clinicians.
References
- Brann DH, Tsukahara T, Weinreb C, et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv 2020; 6(31): 1-19.
- Mouhamad RS, Al-Khafaji KA, Allami RH, et al. The epidemiological plateau of Corona virus in Gulf countries: A descriptive statistics study. Lat Am J Biotechnol Life Sci 2020; 5(2): 1-8.
- https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200430-sitrep-101-covid-19.pdf?sfvrsn=2ba4e093_2
- Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin Immunol 2020; 215: 108427.
- https://www.scientificamerican.com/article/a-visual-guide-to-the-sars-cov-2-coronavirus/
- In vivo Gen. Spotlight on COVID-19: Infection. The infection cycle of SARS-CoV-2. 2020.
- Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020; 367: 1260-3.
- https://www.visualcapitalist.com/visualizing-what-covid-19-does-to-your-body
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020; 395(10223): 507 - 13.
- AL-Khikani FH. The role of blood group in COVID-19 infection: More information is needed. J Nat Sci Med 2020; 3(3): 225-6.
- Robinson J, Banerjee I, Sathian B, et al. ABO blood groups as determinants to patient outcomes in SARS-CoV-2. Int J Biomed Sci 2020; 7(1): 28-32.
- Breiman A, Ruve¨n-Clouet N, Le Pendu J. Harnessing the natural anti-glycan immune response to limit the transmission of enveloped viruses such as SARS-CoV-2. PLoS Pathog 2020; 16(5): 1-4.
- Cheng Y, Cheng G, Chui CH, et al. ABO blood group and susceptibility to severe acute respiratory syndrome. JAMA 2005; 293(12): 1450-1.
- https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance
- Kubina R,Dziedzic A.Molecular and serological tests for COVID-19. A comparative review of sars-cov-2 coronavirus laboratory and point-of-care diagnostics. Diagnostics (Basel) 2020; 10(6): 434.
- Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420European patients with mild-to-moderate coronavirus disease 2019. J Intern Med 2020; 288(3): 335-44.
- Aly MH, Rahman SS, Ahmed WA, et al. Indicators of critical illness and predictors of mortality in COVID-19 patients. Infect Drug Resist. 2020; 13: 1995-2000.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020; 395(10229): 1054-62.
- Lee PI, Hu YL, Chen PY, et al. Are children less susceptible to COVID-19?. J Microbiol Immunol Infect 2020; 53(3): 371-2.
- Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: Prospective observational cohort study . BMJ 2020; 369: 1-12.
- Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol 2020; 146(1): 110-118.
- Chen R, Liang W, Jiang M, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest 2020; 158(1): 97-105.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395(10223): 497-506.
- Huang S, Wang J, Liu F, et al. COVID-19 patients with hypertension has more severe disease: A multicenter retrospective observational study. Hypertens Res 2020; 43: 824-31.
- D’Errico S, Zanon M, Montanaro M, et al. More than pneumonia: Distinctive features of SARS-Cov-2 infection. From autopsy findings to clinical implications: A systematic review. Microorganisms 2020; 8(11): 1642.
- Guo W, Li M, Dong Y, et al. Diabetesis a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev 2020; 36(7): 1-9.
- Brake SJ, Barnsley K, Lu W, et al. Smoking upregulates angiotensin-converting enzyme-2 receptor: A potential adhesion site for novel coronavirus SARS-CoV-2 (Covid-19). J Clin Med 2020; 9(3): 1-7 .
- Amer MA, Elsherif HS, Abdel-Hamid AS, et al. Early recovery patterns of olfactory disorders in COVID-19 patients; a clinical cohort study. Am J Otolaryngol 2020; 41(6): 102725.
- Cazzolla AP, Lovero R, Muzio LL, et al. Taste and smell disorders in COVID-19 patients: Role of interleukin-6. ACS Chem Neurosci 2020; 11(17): 2774-81.
- Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur Arch Otorhinolaryngol 2020; 277(8): 2251-61.
- Kosugi EM, Lavinsky J, Romano FR, et al. Incomplete and late recovery of sudden olfactory dysfunction in COVID-19. Braz J Otorhinolaryngol 2020; 86(4): 490-6.
- https://hms.harvard.edu/news/how-covid-19-causes-loss-smell
- Ahmed I, Quinn L, Tan KB. COVID?19 and the ABO blood group in pregnancy: A tale of two multiethnic cities. Int J Lab Hematol 2021; 43(1): e45-7.
- Li J, Wang X, Chen J, et al. Association between ABO blood groups and risk of SARS?CoV?2 pneumonia. Br J Haematol 2020; 190(1): 24-7.
- Makhlouf A-MA, Mahmoud AM, Ibrahim RG, et al. Effects of vitamin D and simvastatin on inflammatory and oxidative stress markers of high-fat diet-induced obese rats. J Sci Res Med Biol Sci 2021; 2(3): 39-50.
- Zhao J, Yang Y, Huang H, et al. Relationship between the ABO Blood Group and the COVID-19 susceptibility. Med Rxiv 2020; pp:1-18.
- Gérard C, Maggipinto G, Minon J-M. COVID?19 and ABO blood group: Another viewpoint. Br J Haematol 2020; 190(2): e93-4.
- Yamamoto F. ABO blood groups and SARS-CoV-2 Infection. 2020.
- Ellinghaus D, Degenhardt F, Bujanda L, et al. Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med 2020; 383: 1522-34.
- Ray JG, Schull MJ, Vermeulen MJ, et al. Association between ABO and Rh blood groups and SARS-CoV-2 infection or severe COVID-19 illness: A population-based cohort study. Ann Intern Med 2021; 174(3): 308-15.
- Zietz M, Tatonetti NP. Testing the association between blood type and COVID-19 infection, intubation, and death. Med Rxiv 2020; pp:1-27.