- Biomedical Research (2015) Volume 26, Issue 3
Safety and efficacy of radiofrequency ablation with internally cooled electrode for perivascular hepatic malignancy.
Liping Wang , Liyu Chen* , Dong Xu , Chen Yang, Kaiyuan Shi, Chaowen Qian, Junping LiuDepartment of Ultrasonography, Zhejiang Cancer Hospital, HangZhou, China
- *Corresponding Author:
- Liyu Chen
Department of Ultrasonography, Zhejiang Cancer, Hospital,Hangzhou 310022, China
Accepted date: June 05 2015
Abstract
The objective of this study was to evaluate the therapeutic safety and efficacy of percutaneous radiofrequency ablation (RFA) with Internally Cooled Electrode for malignant hepatic tumors; which were in contact with blood vessels. A total of 297 patients with malignant hepatic tumors (358 nodules) who underwent RFA, by means of straight Internally Cooled Electrodes, were enrolled in this study. Seventy-seven of these patients had 79 perivascular nodules, which were situated within 5 mm of the intrahepatic vessels larger than 3 mm in diameter. While 220 patients had 279 non-perivascular nodules, which were more than 5mm away from the intrahepatic vessels with diameter lager than 3mm. The clinical data and outcomes were compared between the two groups. There were no treatment-related deaths, and the complication rates were similar (3.9% vs 2.7%, P>0.05) in the two groups. The disease-free 1-,2- ,and 3-year survival rates in the perivascular group were 54.3%, 48.8% and 35.5%, respectively while those in the nonperivascular group were 59.7%, 35.4% and 30.7%, respectively (P>0.05). There were no significant differences between perivascular and nonperivascular groups in local recurrence rate (22.8% vs 16.1%,P>0.05). It can therefore be concluded that percutaneous RFA with Internally Cooled Electrode is a safe and effective treatment for perivascular malignant hepatic tumors.
Keywords
Liver tumors, ultrasonography, risk factors, radiofrequency ablation.
Introduction
Percutaneous radiofrequency ablation (RFA) has been widely developed and practiced as a nonsurgical treatment for primary or secondary malignant hepatic tumors, because it has been shown to provide more consistent control of local tumors than other ablative techniques in several studies [1-4]. Nevertheless, RFA treatment for perivascular malignant hepatic tumors remains controversial. Several studies have shown that proximity of the target tumor to vital vascular structures results in attenuation of RF ablative energy due to ‘heatsink’ effect, which limits the ability to deliver sufficient heat for complete tumor cell destruction [5-8]; and RF ablation may also cause vascular occlusion, injury and thrombosis [9-12].
The local recurrence rates after radiofrequency, in some articles, were considered particularly high (47-53%) for zones of ablation adjacent to major vascular structures [13-14] while some studies considered that there was no significant correlation between tumor recurrences and proximity of the ablative tumors to the large hepatic vessels [15-16]. However, so far, the factors affecting ablative results, such as, the types and diameters of hepatic vessels have been rarely reported.
The present retrospective study aimed to evaluate the therapeutic safety and efficacy of ultrasound-guided radiofrequency ablation with Internally Cooled Electrodes for patients with perivasular malignant hepatic tumors. The clinical outcomes of these patients were compared with those with nonperivasular malignant hepatic tumors who had RFA in the same period.
Materials and Methods
Patients
The study was performed at Zhejiang Cancer Hospital, Hangzhou, China. Written informed consent was obtained from the patients and/or relatives, when appropriate, before treatment. Ethical approval was obtained from the Ethical Committees of Zhejiang Cancer Hospital.
Inclusion criteria of the study for performing R FA in patients with malignant hepatic tumors were as follows: (a) the tumor or tumors should be visualized with ultrasonography and accessible via the percutaneous route; (b) multiple tumors (n≤3); (c) a single tumor with no greater than 6 cm in the largest dimension; (d) liver malignant tumor was histologically confirmed by biopsy or diagnosed by the presence of a hypervascular liver mass in the arterial phase of a dynamic imaging (CT or MRI) with contrast washout during the portal or delayed phase plus angiographic confirmation of a hypervascular mass; (e) no portal venous thrombosis and extrahepatic metastasis; (f) prothrombin activity >50%, a platelet count greater than 5×109/L, and no refractory ascites; (g) Child-Pugh class A or B liver cirrhosis; (h) no other local treatment performed such as ethanol injection (PEI) or transcatheter arterial chemoembolization (TACE).
Between September 2007 and September 2011, a total of 297 cases with primary or secondary malignant hepatic tumors performed by ultrasound-guided RFA were enrolled in this study. All patients underwent routine ultrasound and contrast enhanced ultrasound (CEUS) before RFA. They were divided into two groups:a group of 77 patients (79 perivascular nodules) with perivascular tumors and a group of 220 patients (279 non-perivascular nodules) with non-perivascular tumors.
Perivascular tumor was defined as a tumor situated within 5 mm from intrahepatic vessels larger than 3 mm in diameter. A tumor would be regarded as non-perivascular if the distance between the tumor and the major intrahepatic vessels was more than 5 mm.
Instruments and Methods
The Cool-tip™ radiofrequency system (Radionics, Burlington, Massachusetts, USA) was used in all patients, the radiofrequency generator capable of delivering a maximum power of 200 W. Based on the distinct ablation zones of two types of RFA electrodes and our own experience, the principle of choosing radiofrequency electrodes was as follows: single radiofrequency electrodes with exposed length of 3 cm were used for tumors less than 2 cm in dimension; clustered electrodes (three parallel single electrodes close to each other) with a exposed length of 2.5cm were used to treat larger tumors ( ≥ 2 cm in dimension). Clustered electrodes are also more likely to be used in case of hepatic tumors close to larger vessels. The RF electrode was of 17 G, which contained internal dual channels for chilled water to be pumped through by a peristaltic pump.
The resulting cooling effect around the electrode tip could reduce charring of the surrounding tissue, which might otherwise decrease tissue conductivity and block the RF current.
Under intravenous anesthesia, all patients were treated by percutaneous RFA under real-time ultrasonographic guidance (model: LOGIQ 9, GE Health Care) with a 3.5- MHz probe. When cool-tip electrodes worked, RF was delivered at the maximum power of the generator in the impedance automatic mode for 12 minutes.
In all patients, single or overlapping ablations were performed on the basis of lesion size and geometry, as well as on findings from real ultrasonography (US) performed during ablation. Our aim was to make sure that the entire tumor was destroyed. The ablation zone was extended beyond the tumor margin by about 0.5 to 1.0 cm in a single session of RFA. After the ablation, the needle track was thermocoagulated by continuing the RFA current in a manual mode as the electrode was withdrawn slowly. For perivascular tumors, care was taken to avoid thermal injury to the nearby blood vessel by the RF current during each ablation process [7,15]. The patient was kept in the hospital overnight and was discharged the next day if found clinically well.
Data Collection And Outcome Measures
A complication was defined as any adverse event that required specific treatment, prolonged hospitalization or rehospitalization after RFA. Treatment-related mortality was defined as any death within 30 days of the RFA.
Local therapeutic efficacy of the patient was evaluated with dynamic CT scan or contrast-enhanced ultrasound (CEUS) 1 month after percutaneous RF ablation. Then dynamic CT or MR scan was performed every 2 to 3 months for follow-up. The absence of contrast enhancement within the original tumor was defined as complete ablation. Any contrastenhancing areas within the targeted tumor on post-ablation CT or MR scan indicated incomplete tumor ablation. Any new tumor that occurred in the liver outside the ablated area and showed arterial phase perfusion on focal liver lesion on dynamic CT or MR scans, was defined as distant intrahepatic recurrence.
When the indication of dynamic CT or MR scans failed to explicate, we comprehensively considered the diagnosis referring to further CEUS and dynamic serum alphafetoprotein (α-FP) level. During the postoperative follow-up process, continuity of the patient’s study was forced to be suspended and new interventions such as TACE, PEI or repeated RFA were performed if residual tumor or recurrence was found; the follow-up process continued automatically if no new malignant evidence was found. The shortest tumor-free survival time was 6 months in our study.
Statistical Analysis
Student's t test for quantitative data and Chi-square test for qualitative data were used to compare baseline characteristics of patients, and rank sum test was used to compare multiplicity of RFA procedures in the two groups. Recurrence risk and overall survival after RFA were calculated by the Kaplan-Meier method and the differences between two groups were determined with log-rank test. Data processing and analysis were performed by SPSS for Windows version 19 (SPSS, Chicago, IL). A value of P<0.05 was considered statistically significant.
Results
Patient Characteristics
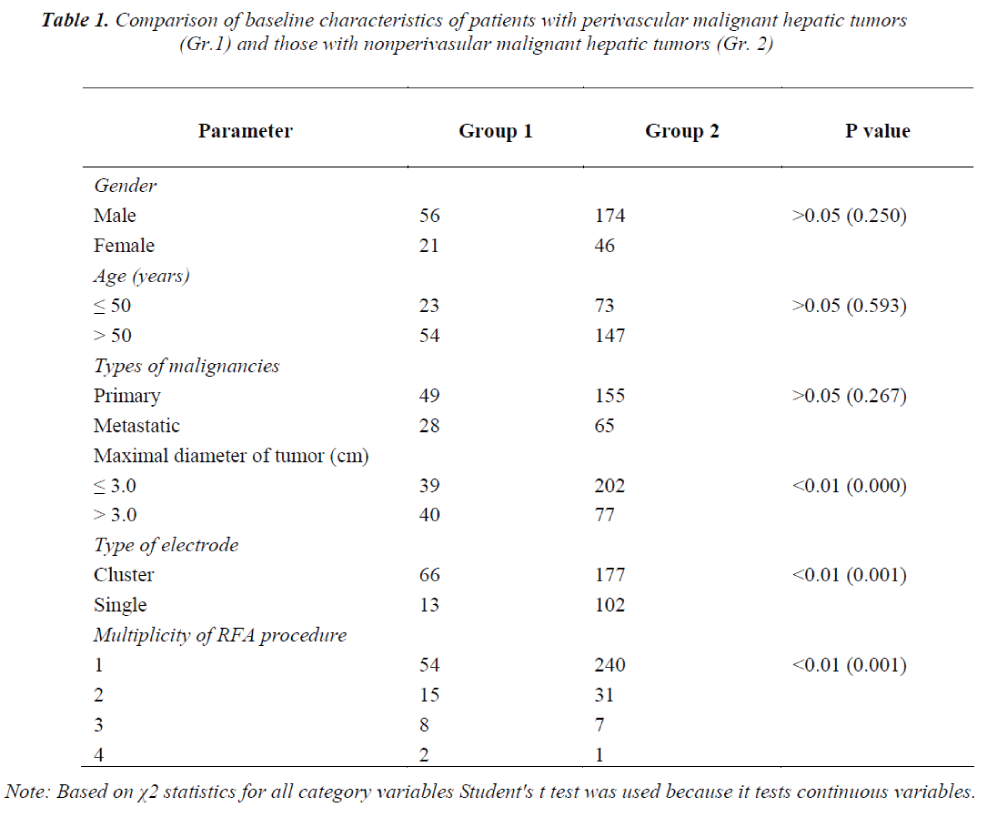
The baseline characteristics of patients with perivascular malignant hepatic tumors (Group 1) and those without perivascular malignant hepatic tumors (Group 2) are illustrated in Table 1.
There were no significant differences between these two groups in terms of age and types of malignancies (P>0.05). However, Group 1 had a higher proportion of patients with tumors ≥3 cm in dimension, cluster needles selections, and number of RFA sessions than Group 2 (P<0.01).
Treatment-related morbidity and mortality
The incidences of severe complications in Group 1 and Group 2 were 3.9% and 2.7% respectively, which are not statistically significant (P>0.05). The severe complications in Group 1 included 1 case of portal vein occlusion, 1 case of intra-abdominal infection and 1 case of gastrointestinal bleeding. Those in Group 2 included 2 cases of pleural hemorrhage, 1 case of chest infection, 1 case of biloma, 1 case of subarachnoid hemorrhage and 1 case of pericardial hemorrhage. None of the patients died as a direct result of a complication of RFA in any group.
Treatment outcome after RFA
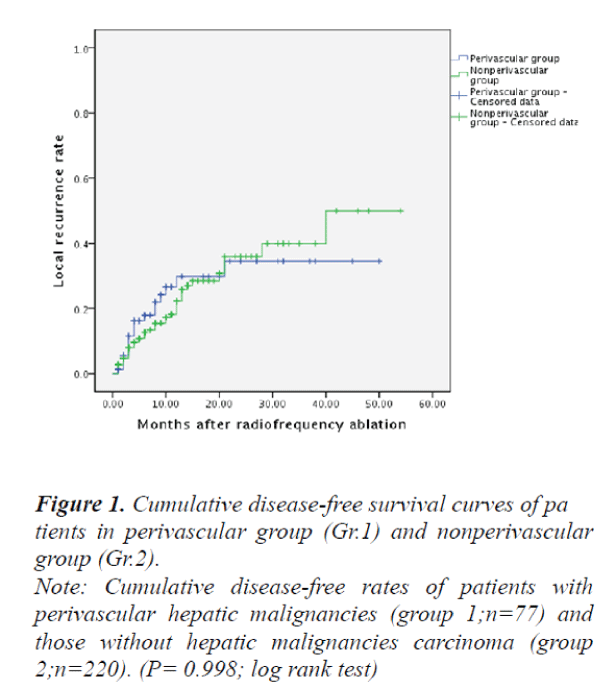
The therapeutic outcomes after RFA treatments in patients with perivascular malignant hepatic tumors (Group 1) and those without perivascular malignant hepatic tumors (Group 2) were summarized with an overall mean follow-up of 13.4±10.5 months as follows: The disease-free 1-, 2-, and 3 year survival rates in patients with perivascular malignant hepatic tumors (Group 1) were 54.3%, 48.8%, and 35.5%, respectively;
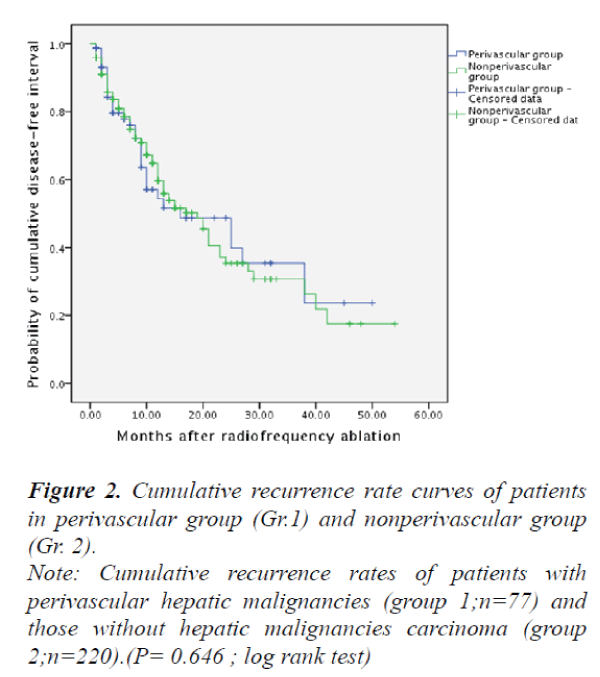
and the survival in those patients without perivascular malignant hepatic tumors (Group 2) were 59.7%, 35.4% and 30.7%, respectively. However, there were no significant differences between the two groups in terms of disease-free survival rates (P>0.05) (Fig. 1). The local recurrence rates of Group 1 and Group 2 were 22.8% versus 16.1% respectively, no significant differences were found between the two groups (P>0.05) (Fig. 2).
Note: Cumulative disease-free rates of patients with
perivascular hepatic malignancies (group 1;n=77) and
those without hepatic malignancies carcinoma (group
2;n=220). (P= 0.998; log rank test)
Figure 1. Cumulative disease-free survival curves of pa
tients in perivascular group (Gr.1) and nonperivascular
group (Gr.2).
Note: Cumulative recurrence rates of patients with
perivascular hepatic malignancies (group 1;n=77) and
those without hepatic malignancies carcinoma (group
2;n=220).(P= 0.646 ; log rank test)
Figure 2. Cumulative recurrence rate curves of patients
in perivascular group (Gr.1) and nonperivascular group
(Gr. 2).
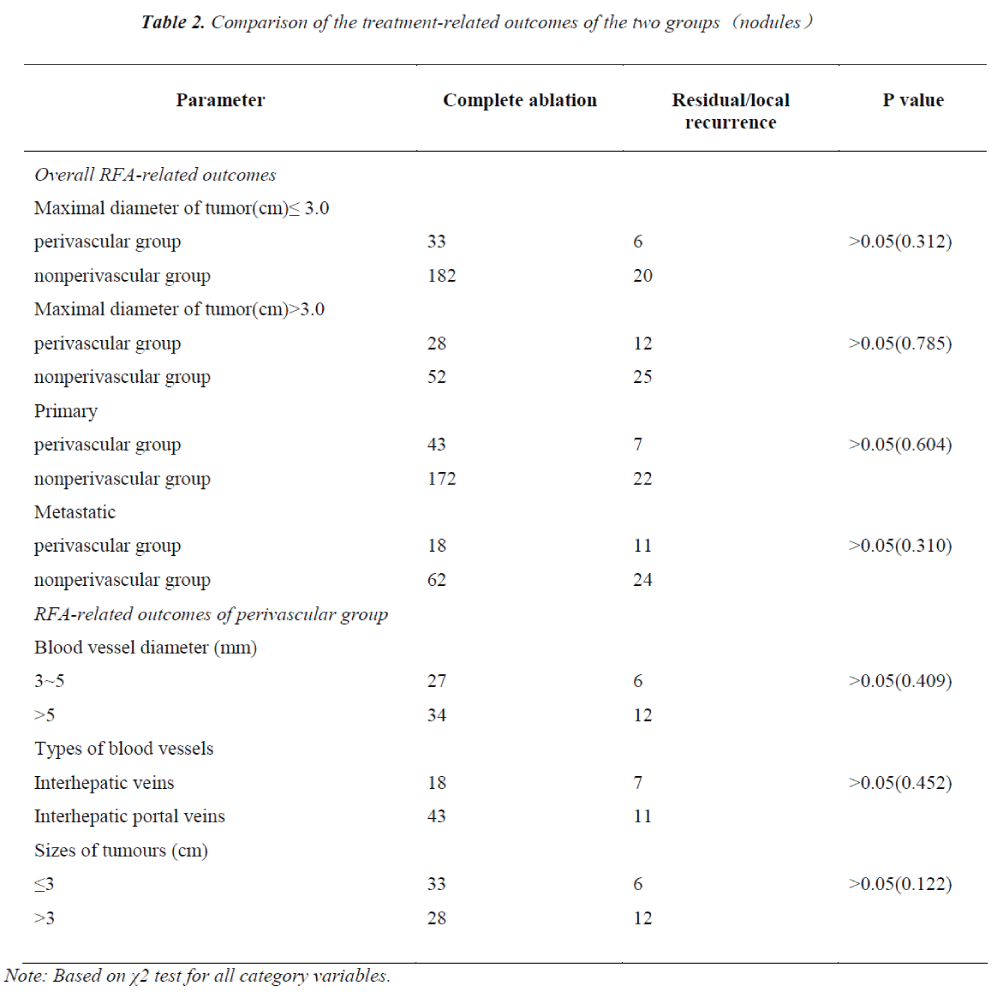
The comparison of therapeutic outcomes based on the multi-factors between inter-groups and intra-Group 1 are illustrated in Table 2.
The RFA outcomes did not differ in the sizes and types (Primary or Metastatic) of the malignant hepatic tumors between Group 1 and Group 2. Similarly, the ablation outcomes had no significant difference in vessel sizes, vessel types and tumor sizes in intra-Group 1 (P>0.05).
Discussion
Primary liver carcinoma is one of the most prevalent types of malignancies, worldwide whereas, the liver is the second most common site of metastasis [18-20]. The circulatory system of the liver is unlike that seen in any other organ. It has dual sources of blood supply and one hepatic venous system. A great number of patients with liver malignancies cannot be radically resected because of the close proximity to major hepatic vessels. Surgical resection is quite difficult and risky and the outcome is still unsatisfactory for those patients [21-22].
Therefore, a more safe and effective treatment option for those liver tumors is always a clinical concern. At present, RFA is well established as the standard local ablative therapy for various liver malignancies, and can be used as an alternative to surgery because of its superior local control rates and high survival rate relative to other local treatments [1,2]. In recent years, a series of animal experiments and clinical studies on the perivascular malignant hepatic tumors have been conducted, but the therapeutic efficacy and safety of ultrasound-guided radiofrequency ablation for those tumors remains controversial [9,11].
Metealfe et al [9] performed radiofrequency zone of ablation adjacent to hepatic veins in large white pig with RITA™ electrodes. The zones of ablation were examined histologically 72 hours after RFA. The result showed that zones of ablation necrosis adjacent to the vessel wall were incomplete in three of seven cases. All zones of ablation were associated with intimal necrosis and most with mural thrombosis. The study concluded that ablation of hepatic tumors by radiofrequency was unreliable adjacent to hepatic vein and the modality of radiofrequency ablation was not recommended for those zones of ablation.
Another experiment with RFA using a porcine model; was reported by Ng et al [23]. The procedure using a cooled-tip electrode was performed 5 mm from the left main portal vein branch under ultrasonographic guidance for 12 min. The results indicated that there was no significant change in mean portal vein flow velocity before RFA and after the procedure. Gross and histological examination of the portal vein branches showed no damage without (n = 10) a Pringle maneuver. In another study by Lu et al [24], radiofrequency lesions targeted to tissues adjacent to a variety of vessels were created in vivo in the liver of 10 Yorkshire pigs. Postablation contrast-enhanced CT and then histopathologic analysis of the vessels and lesions were performed after sacrificing the pigs. At CT, 42 (95%) of 44 veins greater than 3 mm remained patent without vascular occlusion. On histopathology, the extent of vessel wall injury decreased with increasing vessel diameter in 24 vessels greater than 3 mm. The authors concluded that the heatsink effect was seen consistently and substantial vascular injury was rare in hepatic vessels beyond 4 mm.
Even though in existing literatures of clinical studies there are evidences that perivascular RFA could lead to portal vein occlusion, most clinical studies are inclined to support the safety of perivascular RFA procedure [14-15]. Similarly, in our study, no RFA-related death occurred in any of the 77 patients, and no significant difference of serious complications was seen between perivascular group and nonperivascular group. Of course, it should be highlighted that the type of straight Internally Cooled Electrodes, which have been used in this study are different from the Expandable Multitined Electrodes usually used in other similar studies.
The features of the latter make it more difficult to precisely display and position each tine under the circumstance of its full deployment. Further, the ultrasound image quality is severely impaired by RFAinduced hyperechogenicity. In our country, the majority of primary liver carcinomas are associated with severe cirrhosis infected with chronic hepatitis B. In such cases it is almost impossible to achieve the theoretically umbrellashaped array with the electrode tines and the actual positions of the tines deviate from the required design points. The above-mentioned concerns motivated our team to choose Internally Cooled Electrodes instead of Expandable Multitined Electrodes for RF ablation of the tumors near the major intrahepatic structures.
A study conducted by Lu et al [14] concluded that the presence of vessels at least 3 mm in size contiguous with hepatic tumors was the dominant predictor of incomplete tumor destruction by RFA. In their study, the overall local recurrence rate was 19 per cent in 105 malignant liver tumors. Up to 48 per cent of perivascular tumors recurred locally at previous ablated sites, whereas only 7 per cent of nonperivascular tumors had local recurrence after RFA.
It is recommended by Huang [11] that avoiding the RFA cool-tip electrode placement next to the large parallel blood vessel would have a better heat treatment during RFA heating, and reducing blood flow rate could help reduce significant cooling by large blood vessel. Nevertheless, another study by Ng and his colleagues [15] reported an opposite finding. The outcome of 52 RFA treated patients with perivascular HCC was compared with 90 RFA treated patients with non-perivascular HCC, at the same time. Their study showed no significant difference in the rates of complete ablation and local recurrence in the two groups. They speculated that the choice of RFA approaches might affect the final ablation results.
To obtain a sufficient margin of safety, in our study we used more clustered electrodes to perform the RFA procedures. The volume of coagulation necrosis of clustered electrodes fixed with an exposed length of 2.5cm, which can theoretically reach 1.25cm from the front tip and 2.0cm in a direction perpendicular to the electrode. So we preferred to apply them for ablations of perivascular or subcapsular hepatic tumors. Results of a number of studies have demonstrated that the short-axis diameters of RF ablation zones adjacent to the vessels were shorter than those of the non-vascular side [8,25], therefore, in order to ensure sufficient coagulated coverage in intended areas of nonperivascular sides, we deflected the RFA electrodes to the targeted locations near major intrahepatic blood vessels as far as possible. At the same time, relatively large volumes of ablation zones produced by cluster electrodes rendered us more degrees of freedom for the introduction of the RFA electrode .
In addition, we hypothesized another important factor in incomplete ablation may be attributable to unsatisfactory RFA electrode deployment hampered byblood vessel. A straight Internally Cooled RFA electrode employed in a session of RFA, which produces a relatively large size of coagulation, is an advantage in RF ablation of perivascular tumors as well. In current reports on perivascular RFA treatment, applications of Internally Cooled Electrodes are more likely to have satisfactory outcomes [15-17].
If a RFA electrode with an expandable active tip was accurately placed within 5mm of a blood vessel, usually an equivalent fine therapeutic effect could be obtained. Half-deployed method of expandable electrode of the same power as in the fully deployed method was reported to concentrate more energy and rapidly raise tissue temperature near large vessels. This RFA technique was now considered as a valuable approach to completely ablate liver tumor proximal to large vessels [26-27].
The majority of research articles deem that the size of the tumor is an important decisive factor for the complete ablation of a liver tumor. Usually larger tumors have a high probability of lying close to large blood vessels, as observed in some related research data[13-15]. In our research, we have noted that the average size of perivascular nodules is larger than the nonperivascular nodules. Taking this into consideration, we thought that the analytic result would be more objective if the nodules were compared by subcategories of stratified sizes. Thus, the subcategories of nodules ≤ 3mm and> 3cm were respectively compared, the closer rates of local residual and recurrence were found between two groups.
The evaluation of RFA effect with respect to different types and diameters of vessels have rarely been reported or discussed. An experimental study using a porcine liver model revealed that the perivascular tissue around veins greater than of 5 mm diameter were more difficult to ablate completely than those around veins at 3-5 mm diameter [24]. Similarly high proportion of residual tumor was observed in our present study.
Nevertheless, no significant differences were found between perivascular tumors around veins greater than 5 mm and those round the veins of 3-5 mm. We made an assumption that the hemodynamics of the hepatic vein were different from those of the portal vein, so the effects of hepatic vein and portal vein blood flow on the thermal coagulation of RFA may differ. However, no significant differences in rates of the incomplete ablation and local recurrence were found between the two different types of veins in our present study.
In summary, this study shows that radiofrequency ablation of liver cancer adjacent to large hepatic vessels is safe and effective. A straight Internally Cooled RFA Electrode, which is easily employed, clearly displays and provides relatively reliable volume of coagulation in a session of RFA. This may have certain advantages over other methods of radiofrequency ablation for perivascular hepatic tumors.
Conflict of Interest
No conflict of interest exits as all authors have approved the submission of this manuscript to this journal. We declare that the manuscript is original and has not been published elsewhere.
Acknowledgements
The authors would like to thank all the colleagues of the Department of Ultrasonography, Zhejiang Cancer Hospital. Sincere thanks are due to Professor Liyu Chen for his keen interest and encouragement for this study.
References
- Feng K, Yan J, Li X, Xia F, Ma K, Wang S, Bie Pand Dong J. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012; 57: 794-802.
- Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology 2003; 228: 235-240.
- Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, XuY, ZengY. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg2010 252: 903-912.
- Tanis E, Nordlinger B, Mauer M, et al. Local recurrence rates after radiofrequency ablation or resection of colorectal liver metastases. Analysis of the European Organisation for Research and Treatment of Cancer #40004 and #40983.Eur J Cancer 2014;50 :912-919.
- Lencioni R, Crocetti L, Cioni D, Della Pina C and Bartolozzi C. Percutaneous radiofrequency ablation of hepatic colorectal metastases: technique, indications, results, and new promises. Invest Radiol2004;39:689-697.
- Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? CurrProblDiagnRadiol 2009; 38:135–143.
- Mann CD, Metcalfe MS, Lloyd DM, MaddernGJand Dennison AR. The safety and efficacy of ablative techniques adjacent to the hepatic vasculature and biliary system. ANZ J Surg 2010; 80: 41-49.
- Al-Alem I, PillaiK, Akhter J, Chua TC and Morris DL. Heat Sink Phenomenon of Bipolar and Monopolar Radiofrequency Ablation Observed Using Polypropylene Tubes for Vessel Simulation. SurgInnov2013; 21: 269 276.
- Metcalfe MS, Mullin EJ, Texler M, Berry DP, Dennison AR and Maddern GJ. The safety and efficacy of radiofrequency and electrolytic ablation created adjacent to large hepatic veins in aporcinemodel. Eur J SurgOncol 2007; 33: 662-667.
- Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, MarchalG, De Wever I and Michel L. Complications of radiofrequency coagulation of liver tumours. Br J Surg 2002; 89: 1206-1222.
- Huang HW. Influence of blood vessel on the thermal lesion formation during radiofrequency ablation for liver tumors. Med Phys 2013;40:073303.
- Ng KK, Lam CM, Poon RT, Shek TW, Fan ST and Wong J. Delayed portal vein thrombosis after experimental radiofrequency ablation near the main portal vein. Br J Surg 2004;91:632-639.
- Lu DS, Yu NC, Raman SS, et al. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver.Radiology 2005;234:954-960.
- Lu DS, Raman SS, Limanond P, Aziz D, EconomouJ, Busuttil R and Sayre J. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J VascIntervRadiol2003;14:1267-1274.
- Ng KK, Poon RT, Lam CM, Yuen J, Tso WK and Fan ST. Efficacy and safety of radiofrequency ablation for perivascular hepatocellular carcinoma without hepatic inflow occlusion. Br J Surg 2006;93:440- 447.
- Lam VW, Ng KK, Chok KS, et al. Incomplete ablation after radiofrequency ablation of hepatocellular carcinoma: analysis of risk factors and prognostic factors. Ann SurgOncol2008;15:782-790.
- Kei SK, Rhim H, Choi D, Lee WJ, Lim HK and Kim YS. Local tumor progression after radiofrequency ablation of liver tumors: analysis of morphologicpattern and site of recurrence. AJR Am J Roentgenol2008; 190:1544-1551.
- Livraghi T, Mäkisalo H and Line PD. Treatment options in hepatocellular carcinoma today. Scand J Surg 2011;100:22-29.
- McGlynn KA and London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis 2011;15:223-243.
- Padma S, MartinieJB and Iannitti DA. Liver tumor ablation: percutaneous and open approaches. J SurgOncol 2009;100:619-634.
- Lai EC, Fan ST, Lo CM, Chu KM, Liu CL and Wong J. Hepatic resection for hepatocellular carcinoma. An audit of 343 patients. Ann Surg 1995;221:291-298.
- Miyazaki M, Kato A, Ito H, et al. Combined vascular resection in operative resection for hilarcholangiocarcinoma: does it work or not? Surgery 2007;141:581-588.
- Ng KK, Lam CM, Poon RT and Fan ST. Portal vein thrombosis after radiofrequency ablation for recurrent hepatocellular carcinoma. Asian J Surg2003;26:50-53.
- Lu DS, Raman SS, Vodopich DJ, Wang M, Sayre J and Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the "heat sink" effect. AJR Am J Roentgenol2002;178:47-51.
- Wright AS, Sampson LA, Warner TF, Mahvi DM and Lee FT Jr. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 2005; 236: 132-139.
- Horigome H, Nomura T, Nakao H and Itoh M. Halfdeployedmethod: percutaneous radiofrequency ablation therapy using clustered electrodes for malignant liver tumors proximal to large vessels. AJR Am J Roentgenol 2001; 177: 948.
- Widmann G, Bodner G and Bale R. Tumour ablation: technical aspects. Cancer Imaging 2009;9: S63-S67.