- Biomedical Research (2013) Volume 24, Issue 3
Role of total antioxidant capacity and lipid peroxidation in fertile and infertile men.
Anju Mehrotra1*, D.K. Katiyar2, Anju Agarwal3, Vineeta Das4, K.K. Pant51Department of Pharmacology & Therapeutics, Lucknow, 226003, U.P, India.
2Department of Pharmacology & Therapeutics, Lucknow, 226003, U.P, India.
3Department. of Obstetrics/Gynaecology, Lucknow, 226003, U.P, India.
4Department. Of Obstetrics/Gynaecology, Lucknow, 226003, U.P, India.
5Department of Pharmacology & Therapeutics, King George’s Medical University, Lucknow, 226003, U.P, India.
- Corresponding Author:
- Anju Mehrotra
Department of Pharmacology& Therapeutics
King George’s Medical University
Lucknow, 226003, U.P. India
Accepted Date: March 30 2013
Citation: Mehrotra A, Katiyar DK, Agarwal A, Das V, Pant KK. Role of total antioxidant capacity and lipid peroxidation in fertile and infertile men. Biomed Res- India 2013; 24 (3): 347-352.
Abstract
Aim of the present study is to investigate the total antioxidant capacity (TAC) and lipid peroxidation end product, malondialdehyde (MDA) concentration in seminal plasma of fertile and infertile men and its relationship with different spermatozoa parameters. Semen samples were collected from 15 fertile men (normozoospermic as control) and 40 infertile men (15 oligozoospermic, 17 asthenozoospermic, and 8 oligoasthenozoospermic). With the semen sample, investigation was done for volume, total sperm counts, sperm motility grading and abnormal sperm morphology. In all cases TAC and MDA levels were measured spectrophotometrically. Mean antioxidant capacity of control group was found to be higher (2.41 ± 0.07) compared to oligozoospermic (1.73 ± 0.03), asthenozoospermic (1.63 ± 0.08) and oligoasthenozoospermic (1.48 ± 0.04) groups. MDA levels were significantly higher in three infertile groups in comparison to normozoospermic group. MDA level was negatively associated to sperm motility (-0.24) and sperm count (-0.35). There was a positive correlation between lipid peroxidation and the percentage of abnormal morphology. Present study suggested that decreased antioxidant capacity in the infertile group might be due to either increased ROS production or insufficient antioxidant capacity. Sperm motility increased with increased level of ROS production in the infertile groups. It was observed that increased MDA levels in the abnormal groups were associated with lipid peroxidation on sperm function. This study has correlated the degree of lipid peroxidation with sperm viability in infertile patients.
Keywords
Male infertility, TAC, MDA
Introduction
Infertility is a worldwide problem and approximately 8- 10% of couples within reproductive age group are infertile. It is estimated that globally 60-80 million couples suffer from infertility every year, of which probably between 15-20 million are in India alone. In spite of extensive research, no identifiable cause can be found in 25% of infertile males [1].
Oxidative stress has become the focus of interest as a potential cause of male infertility. High concentration of reactive oxygen species (ROS) was detected in the semen of 30-80% infertile men. Normally, equilibrium exists between ROS production and antioxidant scavenging activities in the male reproductive tract. Under physiological conditions, spermatozoa produces small amount of ROS, which are needed for capacitation, acrosome reaction and fertilization. However, excessive amount of ROS produced by leukocytes and immature spermatozoa cause damage to the normal spermatozoa by inducing lipid peroxidation and DNA damage [2]. Thus, reduction of oxidative stress may be effective in the treatment of male infertility.
Seminal plasma contains a number of enzymatic antioxidants as well as a variety of non-enzymatic antioxidants. The antioxidant power of biological fluids can be evaluated either by quantification of individual antioxidant or by assessing their aggregate cumulative action and synergic effect. The later concept is known as the total antioxidant capacity (TAC). The findings on total antioxidant capacity are still controversial [3].
It has been postulated that oxidants interfere with normal sperm function via membrane lipid peroxidation and fragmentation of nucleic acids, which result in sperm dysfunction. Due to membrane polyunsaturated fatty acids (PUFAs) and their capacity to generate ROS, human spermatozoa are highly susceptible to oxidative stress [4]. The more important marker of lipid peroxidation is MDA. This by-product has been used in biochemical assays to monitor the degree of peroxidative damage sustained by spermatozoa. The results of such assay exhibit an excellent correlation with the degree to which sperm function is impaired. High levels of seminal MDA represent increased lipid peroxidation rates, which diminishes fertility [5].
The quantification of antioxidants is a complicated, expensive & time-consuming task. In comparison, single measurement of total antioxidant capacity (TAC) is less costly and also useful in routine practice. The oxidative stress (OS) adversely affects sperm function by altering membrane fluidity, permeability and impairs sperm functional competence. Therefore, the purpose of our present study was to evaluate the TAC & MDA profiles of infertile men and their impact on semen parameters.
Materials and Methods
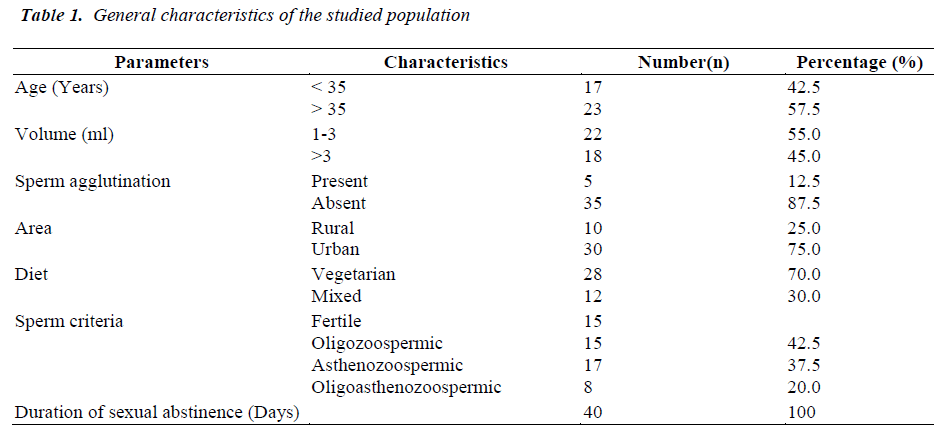
Forty infertile subjects were selected from the couples attending the infertility clinic in the Department of Obstetrics & Gynaecology. Fifteen normal healthy men were taken as control. A total of 55 subjects were divided into four groups
Normozoospermic (15 cases)
Subjects of this group represented healthy fertile men (controls). They were characterized by normal values for sperm motility (> 30%), sperm concentration (> 20 × 106 sperm/ml) and normal sperm morphology (> 30%).
Oligozoospermic (15 cases)
Infertility of this group was defined mainly by sperm concentration (< 20 × 106 sperm/ml), with normal levels for sperm motility (> 30%) and morphologically normal sperm (> 30%).
Asthenozoospermic (17 cases)
This study group was consisted of asthenozoospermic men in which infertility was determined less than normal levels for sperm motility (< 30%), with normal sperm morphology (< 30%) and sperm concentration (> 20 × 106 sperm/ml).
Oligoasthenozoospermic (8 cases)
Subjects of this group presented sperm concentration >20×106 spermatozoa/ml with >30% sperm motility and <30% normal forms of sperms.
Inclusion and exclusion criteria for selection of infertile males
Inclusion criteria: Subjects’ age should be between 25- 40 years. Couples living together with regular unprotected coitus for a reasonable period of time and without contraception. Semen examination showing normal pH, normal viscosity with spermatozoa concentration >20 million /ml, motility >30% and >30% spermatozoa should be normal.
Exclusion criteria: A detailed medical history was performed for all studied cases. Subjects currently on any medication or antioxidant supplementation were not included. In addition, subjects with testicular varicocele, genital infection, leukocytospermia, chronic illness and serious systemic diseases, smokers and alcoholics were excluded from the study because of their well-known high seminal ROS levels and decreased antioxidant activity.
Study consent: A written consent of each subject was taken after explaining the aims and objectives of the study and its benefits on individual and society. The study was approved by the Ethical Committee of the University.
Semen analysis: Semen samples were collected by masturbation into sterile cups following 3 days of sexual abstinence. After 30 minutes of liquification, standard semen parameters (volume, motility, concentration and morphology) were immediately evaluated according to the World Health Organization guidelines [6].
Motility grading
Sperm motility was classified into four categories: rapid progressive motile (Type a), slow progressive motile (Type b), non-progressive motile and immotile spermatozoa.
Total progressive motility was defined as the combination of ‘Type a’ rapid motility and ‘Type b’ slow progressive (at least 30% of sperm should have normal motility (categories a + b). Sperm concentration was determined with an improved Neubauer Hemacytometer® counting chamber and normal values of concentration were “> 20 × 106 sperm/ml".
Evaluation of spermatozoa morphology: Spermatozoa morphology includes - (i) ejaculated volume/ml (ii) sperm concentration (million /ml) (iii) morphology - head, mid piece and tail.
Staining method for spermatozoa morphology
Safranin/cresyl violet staining assay was performed to analyze spermatozoa morphology. Staining solution contains methanol, 0.1% safranin solution, buffer and 0.25% cresyl violet stain solution.
Preparation of seminal plasma: Semen samples were collected from subjects with 3-4 days of sexual abstinence. These samples were centrifuged at 4000 rpm for 10 minutes. Supernatant seminal plasma was collected and frozen at -80°C until examined.
Total Antioxidant Capacity measurement [7]: TAC was measured spectrophotometrically (UV Spectrophotometer, Shimadzu, Japan) at 532 nm. The reaction mixture contained 0.5 ml of phosphate buffer (100m mol/ l, pH 7.4), 0.5ml of sodium benzoate (10m mol/l), 0.2ml of ethylenediaminetetraacetic acid (Fe- EDTA), 2m mol /l EDTA + 2m mol/l of ferric ammonium sulphate (FeNH4(SO4)2, 0.2 ml of H2O2 (10m mol/l) and 20μl of seminal plasma was incubated for 60 minutes at 37ºc. The reaction was then stopped by addition of 1 ml of 20% acetic acid. Each sample also served as its own control. TAC of the sample was then calculated by the following formula:
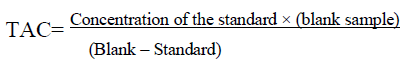
Assessment of oxidative stress by measurement of Lipid Peroxidation [8]: Lipid peroxidation was measured by determining the MDA production using thiobarbituric acid (TBA) method. After liquification, semen samples were centrifuged at 4000 rpm for 10 min to get seminal plasma. Then, 0.1 ml of seminal plasma was added to 0.75 ml of TBA reagent (0.8 g of 2-TBA dissolved in 80 ml of distilled water with 0.5 ml of NaOH). Perchloric acid (7%) was added to this mixture in order to adjust the pH = 7.4). This mixture was heated for 1 hour at 95°C in a warm water bath. After cooling, the tube was centrifuged for 10 min at 4000 rpm and the supernatant's absorbance was read on a spectrophotometer at 535 nm.
Statistical Analysis
Mean ± SD were calculated for each of the quantitative value. The differences were considered to be significant at values of P <0.05. Pearson's correlation was performed to examine the correlation between parameters and semen quality.
Results
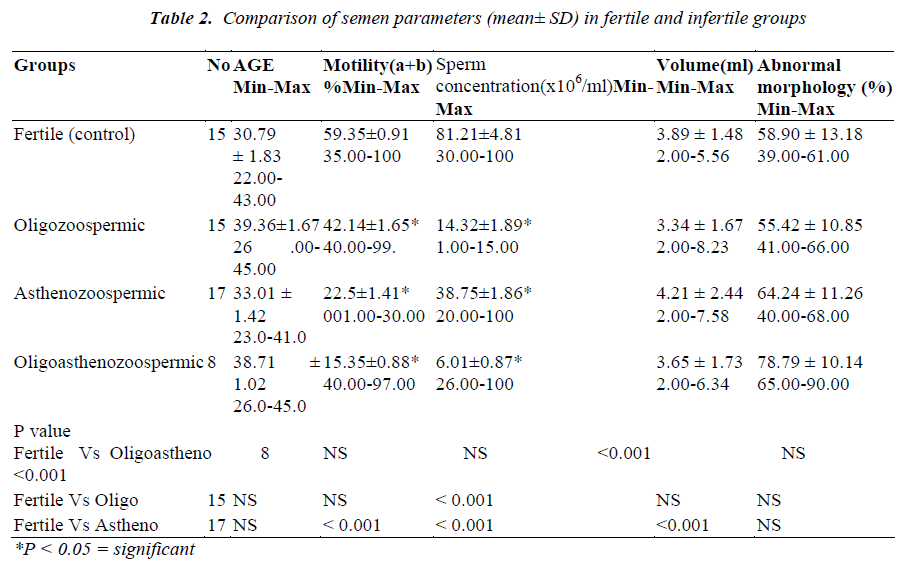
General characteristics of 55 subjects are presented in Table 1. The mean values of the sperm parameters in controls and infertile groups are shown in Table 2. The mean age of the fertile group (30.79 ± 1.83 years) was not significantly different in comparison to asthenozoospermic (33.01 ± 1.42), oligozoospermic (39.36 ± 1.67) and oligoasthenozoospermic (38.01 ± 1.02 ) groups. There was no significant correlation between patient's age and seminal antioxidant status. Sperm count and motility were significantly higher in fertile group than the infertile groups (Table 2).
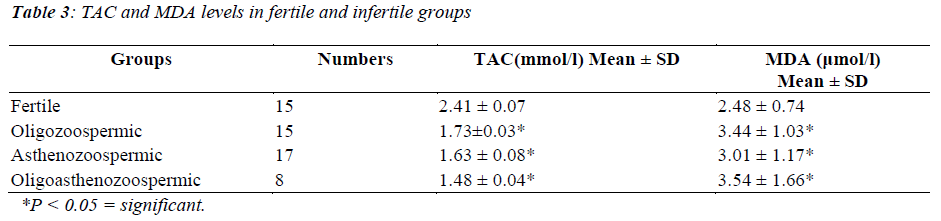
The mean antioxidant capacity of control group was found to be significantly higher (2.41 ± 0.07) compared to oligozoospermic (1.73±0.03, p<0.01), asthenozoospermic (1.63± 0.08, p<0.01) and oligoasthenozoospermic (1.48 ± 0.04, p<0.01) groups, respectively. Lipid peroxidation as expressed by MDA level in the seminal plasma was found to be significantly higher in the three abnormal groups than in the fertile group. Mean MDA levels in abnormal groups were found to be 3.01 ± 1.17 μmol/l in the asthenozoospermic group, 3.44 ± 1.03 μmol/l in the oligozoospermic group and 3.54 ± 1.66 μmol/l in the oligoasthenozoospermic group, respectively (Table 3).
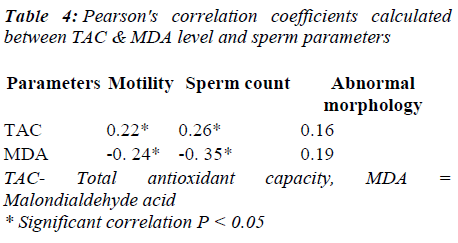
Rates of seminal MDA were negatively associated to sperm motility (r = -0. 24) and sperm concentration ( r = - 0.35). There was a positive correlation between seminal lipid peroxidation and the percentage of abnormal morphology (r = 0.19) (Table 4).
Discussion
The relationship between total antioxidant capacity and male infertility has been a subject of investigation for a long period of time. Mahafouz et al. [9] observed that fertile subjects showed higher TAC value in comparison to infertile men. They proved that fertile donors showed higher TAC values (median and range: 1700; 1440-2290 microM); compared to infertile patients: (1310; 1040- 1600 microM). The best cut-off to distinguish between fertile (controls) and infertile subjects was 1420 μM. In contrast, our findings confirmed the findings of Koca et al. [10] that seminal plasma TAC level is lower in asthenozoospermic and asthenoteratozoospermic groups in comparison to control group. The decreased level of TAC in seminal plasma may be one of the causes of male infertility. They also reported a positive correlation between seminal plasma TAC and sperm motility. Our findings, moreover support the results of Khorrowbeygi [11] that levels of TAC have a positive correlation with sperm motility (r=0.45, p<0.05) and morphology. Their study showed that decreasing seminal plasma antioxidants status especially TAC might have significant effect on impaired sperm function. Siciliano et al. [12] reported that non-enzymatic antioxidant capacity does not alter in asthenozoospermic males than normozoospermic males.
Keskes-Ammar et al. [13] suggested that higher level of antioxidant status prevents lipid peroxidation in spermatozoa and therefore results in higher sperm motility. Iwaski et al. [14] observed that higher level of ROS was correlated with a decreased number of motile sperm; conversely greater sperm motility was observed in samples with lesser amount of ROS. Our results suggest that TAC level is directly proportional to the sperm motility grade.
Increased formation of ROS has been correlated with reduction of sperm motility [15]. The link between ROS and reduced motility may be explained by a cascade of events that result in a decrease in axonemal protein phosphorylation and sperm immobilization [16]. Another hypothesis is that H2O2 can diffuse across the membranes into the cells and inhibits the activity of enzymes such as G6PDH leading to a decrease in the availability of NADPH and an accumulation of oxidized glutathione and reduced glutathione. These changes can cause a decrease in the antioxidant defences of the spermatozoa, which ultimately leads to the peroxidation of membrane phospholipid [17].
Guzman et al. [18] also observed that there is an inverse significant correlation between seminal plasma TAC and ROS levels. They suggested that the inverse correlation between TAC and ROS might be associated with an increase in the consumption of soluble, non-enzymatic antioxidant in seminal plasma, which results from overproduction of ROS. The findings of the present study suggested that decreased antioxidant capacity in infertile groups may be due to either increased ROS production or insufficient antioxidant capacity
Altered antioxidant status was observed in the seminal plasma of infertile men which is responsible for oxidative damage and makes the sperm highly susceptible to lipid peroxidation. Lipid peroxidative degradation of sperm membrane may be responsible for abnormal sperm motility, concentration and morphology. MDA production reflects the peroxidation of membrane polyunsaturated phospholipids [19, 20]. Our results showed a significant increase in the amount of seminal MDA in abnormal groups compared to normozoospermic. Our results were corroborative with Hesham et al. [21] and Ben Abdallah et al. [22] who reported that MDA content was elevated in oligozoospermic and asthenozoospermic groups. However, there was controversy about seminal MDA activity and sperm quality. We showed that higher seminal MDA levels were negatively associated with sperm motility and sperm count. A positive relationship was observed between increased lipid peroxidation and abnormal sperm morphology, which was similar with the findings of Suleiman et al. [23]. Increased MDA levels in seminal plasma of abnormal groups could represent the pathological impact of lipid peroxidation on the spermatozoa membrane and consequently on sperm motility and viability.
A negative correlation of motility with seminal MDA indicates that sperm membrane lipid peroxidation affects the fluidity and mobility of sperm axoneme. This affects functional competence of the sperm and acts like a biological safeguard. It was also shown that increased MDA levels in the abnormal groups could represent the pathological effects of lipid peroxidation on sperm function. Present findings suggested that routine determination of antioxidant status of semen during infertility investigation is useful. Furthermore, future research should focus on cohort study, with genetic susceptibility and their repercussions on semen quality.
References
- Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update 2003; 9: 331-345.
- Wang X, Sharma RK, Sikka SC, Thomas AJ Jr, Falcone T, Agarwal A. Oxidative stress is associated with increased apoptosis leading to spermatozoa DNA damage in patients with male factor infertility. Fertil Steril 2003; 80 : 531-535.
- Agarwal A, Prabhakaran S, Allamaneni SS. Relationship between oxidative Stress, varicocele and infertility: a meta-analysis. Reprod Biomed Online 2006; 12: 630-633.
- Hesham N, Moemen LA, Abu Elela MH. Studying the levels of malondialdehyde and antioxidant parameters in normal and abnormal human seminal plasma. Aust J Basic Appl Sci. 2008; 2: 773-778.
- Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003; 79: 829-843.
- Agarwal A, Gupta S, Sikka S. The role of free radicals and antioxidants in reproduction. Curr Opin Obstet Gynecol 2006; 18: 325-332.
- World Health Organization. WHO laboratory manual for the examination of human semen and semencervical mucus interaction. 4. Cambridge, UK: Published on behalf of the World Health Organization by Cambridge University Press; 1999, 1-86.
- Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol 2001; 54; 93: 356-361.
- Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med. 1976; 5: 212-216.
- Mahfouz R, Sharma R, Sharma D, Sabanegh E, Agarwal A. Diagnostic value of the total antioxidant capacity (TAC) in human seminal plasma. Fertil Steril 2009; 91: 805-811.
- Koca Y, Ozdal OL, Celik M, Unal S, Balaban N. Antioxidant activity of seminal plasma in fertile and infertile men. Arch Androl 2003; 49: 355-359.
- Khosrowbeygi A, Zarghami N. BMC Clin Pathol. 2007; 1: 7-6.
- Siciliano L, Tarantino P, Longobardi F, Rago V, De Stefano C, Carpino A. Impaired seminal antioxidant capacity in human semen with hyperviscosity or oligoasthenozoospermia, J Androl 2001; 22: 798-803.
- Keskes-Ammar L, Feki-Chakroun N, Rebai T, Sahnoun Z, Ghozzi H, Hammami S, Zghal K, Fki H, Damak J, Bahloul A. Sperm oxidative stress and the effect of an oral vitamin e and selenium supplement on semen quality in infertile men. Arch Androl 2003; 49: 83-94.
- Iwasaki A, Gagnon C. Formation of reactive oxygen species in spermatozoa of infertile patients. Fertil Steril 1992; 57: 409-416.
- Agarwal A, Ikemoto I, Loughlin KR. Relationship of sperm parameters to levels of reactive oxygen species in semen specimens. J Urol 1994; 152: 107-110.
- De lamirando E, Gagnon C, Reactive oxygen species and human spermatozoa II. Depletion of adenosine triphosphate (ATP) plays an important role in the inhibition of sperm motility. J Androl 1992; 13: 379- 386.
- Griveau JF, Dumont E, Renard B, et al. Reactive oxygen species, lipid peroxidation and enzymatic defence systems in human spermatozoa. J Reprod Fert 1995; 103: 17-26.
- Gil-Guzman E, Ollero M, Lopez MC, Sharma RK, Alvarez JG, Thomas AJ Jr, Agarwal A. Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Hum Reprod 2001; 16: 1922-3019.
- Storey BT. Biochemistry of the induction and prevention of lipo-peroxidative mechanisms damage in human spermatozoa. Mol Hum Reprod. 1997; 3: 203- 213.
- Agarwal A, Allamaneni SS. Free radicals and male reproduction. J Indian Med Assoc. 2011; 109:184–187.
- Hesham N, Moemen LA, Abu Elela MH. Studying the levels of malondialdehyde and antioxidant parameters in normal and abnormal human seminal plasma. Aust J Basic Appl Sci 2008; 2: 773-778.
- Ben Abdallah F, Dammak I, Attia H, Hentati B, Ammar-Keskes L. Lipid peroxidation and antioxidant enzyme activities in infertile men: correlation with semen parameter. J Clin Lab Anal 2009; 23: 99-104.
- Suleiman SA, Ali ME, Zaki ZM, El Malik EM, Nasr MA. Lipid peroxidation and human sperm motility: protective role of vitamin E. J Androl 1996; 17: 530- 537.