Research Article - Biomedical Research (2021) Red Cell Immunology and Genotyping
Review on SNARE proteins in cellular membrane trafficking
Kiflay Mulugeta1*, Kbrom Gerezgiher1, Maezu G/slassie2
1Departement of Biomedical science, Collage of Medicine and Health Sciences, Adigrat University, Adigrat, Ethiopia
2Department of Midwifery, Collage of Medicine and Health Sciences, Adigrat University, Adigrat, Ethiopia
- Corresponding Author:
- Dr. Kiflay Mulugeta
Department of Biomedical science
Adigrat University
Adigrat
Ethiopia
Accepted date: 04 October, 2021
Abstract
Membrane trafficking encompasses the wide variety of processes that go into the movement of cargo using membrane bound transport vesicles. Vesicle-mediated membrane traffic includes, vesicle budding, transport, tethering, and fusion. SNARE proteins mediate membrane fusion as fusion process during the fidelity of membrane trafficking together with coats, tethers, and Rabs. Functionally, SNAREs can be classified into v-SNAREs associated with the vesicle (or other forms of transport intermediates) and t-SNARE associated with the target compartment. A v-SNARE on the transport vesicle interacts with the cognate t-SNARE on the target membrane compartment to form trans-SNARE complexes with the help of SM proteins and tethering factors. The interaction between v- and t-SNAREs is thought to bring the membranes close enough together so that they can fuse. After fusion the ATPase NSF unravels the v- and t-SNAREs. The v-SNAREs are recycled to the starting membrane compartment. SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) are now generally accepted to be the major players in the final stage of the docking and the subsequent fusion of diverse vesicle-mediated transport events. The SNARE-mediated process is conserved evolutionally from yeast to human, as well as mechanistically and structurally across different transport events in eukaryotic cells. Mutation of SNAREs or disruption of SNARE complex formation may result in cellular or physiological defects from yeast to human.
Introduction
The organized eukaryotic cell contains numerous compartment with many functionally and structurally different organelles that are separated from the cytosol by lipid bilayers. Such compartmentalization requires precise protein and lipid transport between the different organelles. In the endocytic and exocytic pathways, cargo proteins are transferred between compartments by transport vesicles.
Membrane trafficking of eukaryotic cell is necessary and it is driven by a SNARE protein complex. To accomplish this, transport vesicles bud off from an intracellular donor organelle and then target, dock and fuse with an acceptor organelle. The vesicles form by budding from an organelle’s surface for cargo packaging, mediated by coat proteins (such as COPI, COPII and clathrin) and small GTPase’s. Vesicle translocation is mediated by motor proteins driving vesicle movement along the cytoskeleton. Tethering and Rab proteins regulate vesicle docking to the acceptor compartment. The SNARE hypothesis was first conceived in 1993, which suggested that a v-SNARE located on the vesicular membrane pair with cognate t- SNAREs at the intended target membrane and forms a trans- SNARE complex that brings the two membrane lipid bilayers into close proximity, which induces the fusion of the two close positioned membranes. After fusion, the trans-SNARE complex becomes a cis-SNARE complex on the target membrane. The concerted action of a-SNAP and NSF causes disassembly of SNARE complex by the ATPase of NSF hydrolyses ATP to allow the individual SNAREs for use in the next round of vesicle transport complex is an essential step in the membrane fusion cycle. In vitro experiments on large number of SNARE proteins encoded in the yeast genome demonstrated that specific pairing of cognate SNARE proteins provides the inherent molecular mechanism for vesicle docking and compartmental specificity. SNAREs may have diverse combinations with each other to generate many SNARE complexes, however, only a specific SNARE complex can function to regulate membrane fusion, suggesting that the function of SNARE and the formation SNARE complexes are specifically regulated. SNARE proteins play a central role in the process of intracellular membrane fusion. They bring two membrane lipid bilayers into close proximity, which induces the fusion of the two close positioned membranes.
Materials and Methods
Mutation of SNAREs or disruption of SNARE complex formation may result in cellular or physiological defects from yeast to human. Works from China suggest that SNARE proteins are involved in many diseases such as cancer, neuronal diseases and other physiological disorders. For example, GS28 can stabilize p53 by preventing its ubiquitin-mediated degradation and promote apoptosis. SNARE proteins have been implied in tumorigenesis. Targeting SNARE protein in various types of cancer cell through selective proteolysis of SNAREs may present an opportunity to explore.
Snare Proteins
Definition of snares
"SNAP" (Soluble NSF Attachment Protein) Receptor" are a large protein complex consisting of at least 24 members in yeasts and more than 60 members in mammalian cells. SNARE proteins dock the transport vesicle at the correct membrane location and catalyse membrane fusion, the final step in cargo delivery. SNAREs bring the opposing membranes of the transport vesicle and the target region closely together so that the lipids from the different bilayers can mix, and this eventually results in fusion, and the release of cargo [1].
Classification of snares
Functionally, SNAREs can be classified into v-SNAREs associated with the vesicle (or other forms of transport intermediates) and t-SNARE associated with the target compartment. Evidence suggests that t-SNAREs form stable sub complexes which serve as guides for v-SNARE binding to complete the formation of the SNARE complex. Structurally, SNAREs can be categorize into R-SNAREs (those having an Arg/R residue) and Q-SNAREs (those having a Q/Gln residue.
Structure of snares
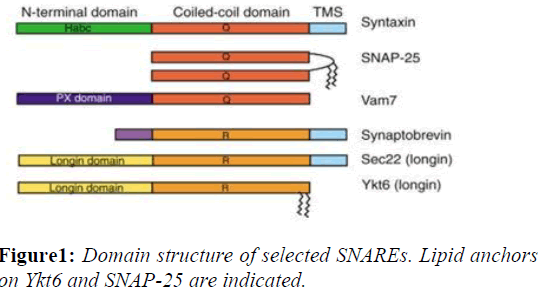
SNAREs are small, abundant, sometimes tail-anchored proteins which are often post-translationally inserted into membranes via a C-terminal transmembrane domain. A few SNAREs lack a TMS. The Q-SNAREs SNAP-25 and SNAP-23 do not have a transmembrane domain and are instead attached to the membrane via lipid modifications such as palmitoylation. These alternative anchors might facilitate regulation of membrane association and the fusion reaction. Tail-anchored proteins can be inserted into the plasma membrane, endoplasmic reticulum, mitochondria and peroxisomes among other membranes, though any particular SNARE is targeted to a unique membrane. The targeting of SNAREs is accomplished by altering either the- composition of the C-terminal flanking amino acid residues or the length of the transmembrane domain (Figure 1).
Mechanism of fusion by snares
Assembly
SNARE proteins must assemble into trans-SNARE complexes to provide the force that is necessary for vesicle fusion. The V- SNARE and T-SNARE have characteristic helical domains, and the V-SNARE interact with T-SNARE forms four –helix bundle. The SM protein Munc18 is thought to play a role in assembly of the SNARE complex. One hypothesis suggests that, during SNARE-complex assembly, the Munc18 clasp releases closed syntax in, remains associated with the N- terminal peptide of syntax in, and then reattaches to the newly formed four-helix SNARE complex [2].
Zippering
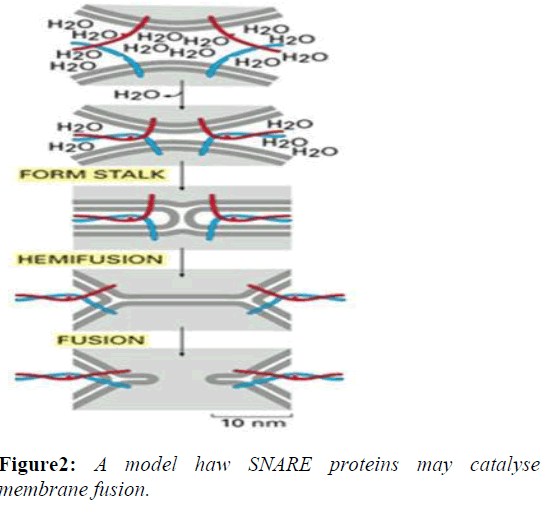
The formation of a SNARE complex acts in a zipper-like fashion, zippering from the distal N-terminal region to the proximal C-terminal region, bringing two opposing membranes closer and eventually completing the fusion. During this "zippering" of the SNARE complex, a fraction of the released energy from binding is thought to be stored as molecular bending stress in the individual SNARE motifs. As the SNARE complex forms, the tightening helix bundle puts torsional force on the Trans membrane domains of SNAREs. This causes the TM domains to tilt within the separate membranes as the proteins coil more tightly. The unstable configuration of the TM domains eventually causes the two membranes to fuse and the SNARE proteins come together within the same membrane, which is referred to as a "cis"-SNARE complex [3].
Disassembly
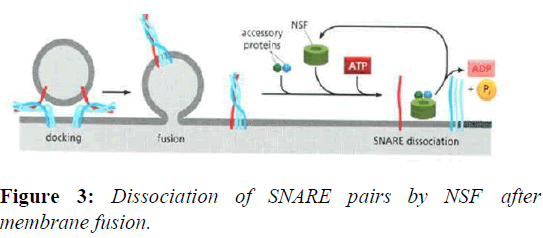
The disassembly of the cis-SNARE complex is an essential step in the membrane fusion cycle because it frees up the individual SNAREs for use in the next round of vesicle transport. The stable, zipped SNAREs can be pried apart by NSF.NSF homohexamers, along with the NSF cofactor α- SNAP, bind and dissociate the SNARE complex by coupling the process with ATP hydrolysis (Figures 2 and 3).
Known snare complexes (snare pins) in mammalian cells
Several SNARE complexes have been defined to function in various transport events in the secretory and exocytosis (Table 1).
| SNARE complexes | Function | Reference |
|---|---|---|
| Syn5, GS27, Bet1, and Sec22b | Mediating homotypic fusion of ER-derived COPII vesicles into larger transport intermediates referred to as ERGIC | (Zhang et al., 1997; Zhang et al., 1999) |
| Syn5 ,GS28 , Bet1 , and Ykt6 | Transport from the ER to the Golgi- mediate the fusion of matured EGTCs with the cis-face of the Golgi apparatus | (Zhang and Hong, 2001) |
| Syn18 ,Sec20 ,Slt1, and Sec22b | Required for retrograde traffic to the ER | (Nakajima et al., 2004; Dilcher et al., 2003) |
| Syn5 , GS28, GS15 , and Ykt6 | Functions in intra-Golgi traffic and also mediates traffic from the endosomal compartments to the Golgi apparatus | (Tai et al., 2004; Xu et al., 2002; Parlati et al., 2002; Shorter et al., 2002) |
| Syn16 , Vti1a , Syn6 , and VAMP4 | Functioning in the retrograde traffic from early/recycling endosomes to the TGN | (Mallard et al., 2002) |
| Syn1, SNAP-25,and VAMP2 | Fusion of synaptic vesicles with the presynaptic membrane | (Sutton et al., 1998; Söllner et al., 1993b) |
| Syn4 ,SNAP-23, VAMP2 | Mediating fusion with the plasma membrane in response to insulin VAMP2 acts as a v-SNARE of storage vesicles containing GLUT4 and interacts with t-SNARE assembled from | (Bryant et al., 2002) |
| Syn4,SNAP-23 and VAMP7 | Mediate the fusion of secretory lysosomes with the plasma membrane | (Rao et al., 2004) |
Table1: Known mammalian SNARE complexes and their site(s) of action in the exocytic and/or endocytic pathways. The potential v-SNARE are indicated in Bold.
Regulatory effect on exocytosis
Regulatory proteins
Regulatory proteins such as Munc18-1(SM), synaptotagmin and complexin enable the exocytosis to be precisely regulated in living cells in space and time upon signal arrival, especiallyforCa2+-triggered neurotransmitter release. Munc18-1 was found to bind directly to syntaxin in closed- configuration, thus regulating the availability of syntaxin for SNARE complex assembly. The N-terminal domain of syntaxin-1 is regulated by Munc18-1 in its two different conformational states, which impose spatially distinct regulatory mechanisms, either compensating or inhibiting the active state in syntaxin trafficking .It is intriguing that Munc18-1 is also intimately related to the transport of syntaxin to target compartments [4].
Complexin interact with the SNARE complex and acts as a force clamp that controls the transfer of the force generated by SNARE complex assembly to the fusing membranes. The fusion-inhibition effect of complexin was assigned to the positioning of the complexin accessory helix between the vesicle and plasma membranes. Moreover, complex in has to undergo a conformational change from an open to a close conformation so that another player, synaptotagmin, cantriggerfusionupon Ca2+ stimuli [5].
Exocytosis of synaptic vesicles in the synapse is strictly regulated by Ca2+ concentrations and Synaptotagmin I is probably the Ca2+ sensor that couples this ion flux to the exocytosis of synaptic vesicles that occurs on account of an action potential. Synaptotagmin I binds directly to Syn1 and SNAP-25 of the t-SNARE. The binding of synaptotagmin to the SNARE complex is thought to release the force clamp by displacing complexin, thus facilitating the force transfer to the opposing membranes in a Ca2+ dependent manner.
Regulation via snap-25 palmitoylation
SNAP-25 is composed of two a-helix domains connected by a random coil linker. The α-helical domains combine with those of both syntaxin and synaptobrevin to form the 4-α-helix coiled-coil SNARE complex critical to efficient exocytosis.SNAP-25 palmitoylation relies on the of cysteine residues found in its random coil region for docking to the target membrane.
The covalent bond of fatty acid chains to SNAP-25 via thioester linkages with one or more cysteine residues therefore, provides for regulation of docking and ultimately SNARE mediated exocytosis.
Snap-25 regulation of voltage-gated Ca2+ channels in neuronal axon terminals
As an action potential reaches the axon terminal, depolarization events stimulate the opening of voltage gated calcium channel allowing the rapid influx of calcium down its electro chemical gradient. Calcium is going to stimulate exocytosis via binding with synaptotagmin1. SNAP-25 however, has been shown to negatively regulate voltage gated calcium channel function in glutamatergic neuronal cells [1].
Role of snare protiens in autophagy
Auto phagosomes aid in degradation of cellular components through fusion with Lysosomes. Auto phagosome biogenesis requires the initiation and growth of phagophores. (Moreau et al., 2011)SNAREs play important roles in mediating vesicle fusion during phagophore initiation and expansion as well as auto phagosome-lysosome fusion in the later stages of autophagy. Though the mechanism of phagophore initiation in mammals is unknown, SNAREs have been implicated in phagophore formation through homotypic fusion of small, chlathrin-coated, single-membrane vesicles containing Atg16L, the v-SNARE VAMP7, and its partner t-SNAREs: Syntaxin7, Syntaxin8 and VTI1B.
Role of snare protiens in neurotransmitter
Neurotransmitter is stored in readily releasable pools of vesicles confined within the pre synaptic terminal. During neurosecretion SNAREs play a crucial role in vesicle docking, priming, fusion, and synchronization of neurotransmitter release into the synaptic cleft.
Regulation of neurotransmitter release by presynaptic Ca2+ influx is likely to be mediated by synaptotagmin in the vesicular, which is physically coupled to the SNAREs. Synaptotagmin is anchored to membranes by a transmembrane domain and has two Ca2+ binding domains. Synaptotagmin binds to SNAREs and lipids in a Ca2+ dependent Manner, which might trigger fusion [5].
Snares and human disorder
Synaptic communication occurs via release of neurotransmitters, and impairment in any of the release steps may lead to neurodegenerative diseases, as well as neurodevelopmental and psychiatric disorders. Proteins at the synapse engage in highly dynamic interactions which, when disturbed, can cause hypo- or hyper-activity in neurotransmitter release, leading to dysfunction.
SNARE proteins and other proteins related to exocytosis may exert a direct impact on mental well-being. In fact, many clinical cases have been reported that connect SNARE genes to neural disorders. Numerous investigations have been prompted by the analysis of humans with genetic disorders. Genetic knockout of -suspected genes in animal models, such as mouse, has also proven to be very useful in understanding the effect of these genes on behaviour, in addition to revealing cellular and molecular mechanisms.
Deficiency in SNAP-25 mRNA has been observed in the hippocampus of certain schizophrenic patients. Further, a single-nucleotide polymorphism in SNAP-25 was reported to be related to hyperactivity in autism-spectrum disorders. Overexpression of the SNAP-25B isoform, due to a variant promoter, leads to early onset of bipolar disorder. It has been suggested that formaldehyde inhalation in mice, producing a decrease in hippocampal expression of SNAP-25 and synaptobrevin 2, leads to learning and memory impairments.
A Mutation in SNAP-29, Coding for a SNARE Protein Involved in Intracellular Trafficking, Causes a Novel Neurocutaneous Syndrome Characterized by Cerebral Dysgenesis, Neuropathy, Ichthyosis, and Palmoplantar Keratoderma. SNAP29 expression was decreased in the skin of the patients, resulting in abnormal maturation of lamellar granules and, as a consequence, in mislocation of epidermal lipids and proteases. Decreased expression of SNAP-29 resulting in abnormal vesicle maturation and secretion [2].
Conclusion
Although, numerous studies were done on SNARE proteins that mediate membrane fusion during the fidelity of membrane trafficking together with coats , tethers, and Rabs, much more work is required for a precise understanding of their various physiological roles, molecular mechanisms of action, and regulation.
SNARE proteins must assemble into trans-SNARE complexes with the help of SM proteins and tethering factors to provide the force that is necessary for vesicle fusion. The V-SNARE and T-SNARE have characteristic helical domains, and the V- SNARE interact with T-SNARE forms four –helix bundle. Formation of the trans-SNARE complex is presumably the core event underlying diverse fusion events and serves as the converging point for various regulatory processes. More regulators of SNAREs are expected to be identified and understanding their precise roles and mechanisms of action are essential to link up vesicle fusion with the rest of the cellular regulatory networks.
Analysis of the mechanisms by which SNARE proteins at the organism level, explored through gene ablation approach with comprehensive assessment of diverse developmental and physiological processes, will rewarding in the future.
References
- Bai J, Chapman ER. The C2 domains of synaptotagmin–partners in exocytosis. Trends Biochem Sci. 2004; 29:143-151.
- Barr JR, Moura H, Boyer AE, et al. Botulinum neurotoxin detection and differentiation by mass spectrometry. Emerging Infect Dis. 2005;11:1578.
- Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153-166.
- Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. 2002;3:267-277.
- Burkhardt P, Hattendorf DA, Weis WI, et al. Munc18a controls SNARE assembly through its interaction with the syntaxin N‐peptide. EMBO J. 2008;27:923-933.