Research Article - Biomedical Research (2017) Volume 28, Issue 15
Resveratrol modulates the proliferation and migration of retinal pigment epithelial cells through TGFβ1-induced EMT signal pathway
Li Junling1,2, Li Xiaorong2, Xiao Bowen1,2 and Wu Jianguo1,2*1Department of Ophthalmology, Tianjin Beichen Hospital, Tianjin, PR China
2Tianjin Medical University Eye Hospital, PR China
Accepted date: April 25, 2017
Abstract
Retinal Pigment Epithelial (RPE) cells play important roles in progression of human ophthalmic diseases. Evidences have showed that Resveratrol (RV) presents benefits for the treatment of ophthalmic diseases. In this study, we investigated the potential molecular mechanism mediated by RV in RPE cells. Transforming Growth Factor beta1 (TGFβ1) and Epithelial-Mesenchymal Transition (EMT) signal pathway was analysed in RPE cells determined by Western blot analysis and RT-PCR. Results demonstrated that RV treatment (10 mg/ml) significantly inhibited proliferation and migration of RPE cells. We showed that RV treatment inhibited TGFβ1 and EMT markers Fibronectin (FIB), alphasmooth muscle actin (α-SMA), and Vimentin (VIM) expression in RPE cells. Inhibition of endogenous TGFβ1 decreased FIB, α-SMA and VIM expression levels in RPE cells as well as inhibited proliferation and migration of RPE cells. TGFβ1 overexpression stimulated EMT markers FSP1, E-cadherin and Snail expression levels in RPE cells. Overexpression of TGFβ1 cancelled RV-down-regulated FIB, α- SMA and VIM expression in RPE cells. Overexpression of TGFβ1 also abolished RV-suppressed proliferation and migration of RPE cells. In conclusion, this study describes the RV-regulated molecular mechanism in RPE cells through TGFβ1-induced EMT signaling and suggests that RV would be a potential therapeutic agent for the prevention or treatment of Proliferative Vitreoretinopathy (PRV).
Keywords
Resveratrol, Retinal pigment epithelial, TGFβ1, Epithelial-mesenchymal transition.
Introduction
Currently, proliferation and migration of Retinal Pigment Epithelium (RPE) cells plays important role in the progression of Proliferative Vitreoretinopathy (PVR) [1,2]. Previous reports have showed that distribution of Transforming Growth Factor- β (TGF-β) and cells derived from RPE cells can be regarded as a major source for TGF-β in Subretinal Strands (SRSs), originating from patients with Proliferative Vitreoretinopathy (PVR) and proliferative diabetic vitreoretinopathy [3,4]. In addition, Study also demonstrated that EMT molecular markers play important role in the many ophthalmic diseases via regulation of proliferation of RPE cells [5]. Therefore, understanding the role of TGF-β and EMT in RPE cells is essential to present and treat human ophthalmic diseases.
Resveratrol (RV) is multifunctional biological polyphenol. RV plays ameliorative effects on higher inflammatory factor concentration, metabolic syndrome and neurological diseases [6,7]. Previous study has showed that RV can protect against ultraviolet A-mediated inhibition of the phagocytic function of human retinal pigment epithelial cells via large-conductance calcium-activated potassium channels [8]. In addition, inhibitory effects of RV on proliferation of human retinal RPE cells have been investigated in vitro, which may aid treatment of proliferative PVR [9]. Furthermore, inhibitory effects of RV on PDGF-BB-induced retinal pigment epithelial cell migration have showed association with PDGFRβ, PI3K/Akt and MAPK pathways [10]. However, the associations between RV and TGFβ1/EMT signal pathway in RPE cells have not been well understood yet.
In this study, we investigated the role of RV in the proliferation and migration of RPE cells. We also explored the potential mechanism mediated by RV in RPE cells. In addition to this, we reported that RV inhibited proliferation and migration of RPE cells through TGFβ1-induced EMT signal pathway.
Materials and Methods
Cell culture and reagents
The ARPE-19 human RPE cell line was were obtained from PromoCell GmbH (Heidelberg, Germany) and cultured in DMEM medium (GIBCO, Invitrogen) with 1% penicillin/ streptomycin sulfate (GIBCO, Invitrogen), 1% L-glutamine (GIBCO, Invitrogen) and 10% fetal bovine serum (GIBCO, Invitrogen) in humidified atmosphere containing 5% of CO2 at 37°C. RV was purchased from Sigma-Aldrich.
Transfection of small interference RNA (Si-RNA)
All si-RNAs were synthesized by Invitrogen (Shanghai, China) including Si-RNA-TGFβ1 (Si-TGFβ1) or Si-RNA-vector. PRE cells (1 × 106) were transfected with 100 pmol of Si-TGFβ1 targeting TGFβ1 (Applied Biosystems) with Si-RNA-vector as control (Applied Biosystems) by using a Cell Line Nucleofector kit L (Lonza).
Construction of lentivirus for TGFβ overexpression
The TGFβ1 was cloned into Lentivirus plasmid using Lentivirus vector system (System Biosciences, Inc.) with vector as control and named pvector (Control) and pTGFβ, respectively. All DNA sequences were synthesized by Invitrogen. All of the plasmids were confirmed by DNA sequencing. Plasmid of pvector or pTGFβ was transfected into RPE cells using lipofectamine 2000. The cells transfected with pvector or pTGFβ were used for further analysis.
Quantitative real-time PCR
The human RPE cells were cultured and transfected with pTGFβ1 or knockdown of TGFβ1 using Si-RNA (Si-TGFβ1). When the concentration of cells grown to 85%, the cells was randomly divided into three groups: Control group, TGF-β1 added to RPE cells for 24 h. Total RNA was isolated from RPE cells using TRIzol reagent (Invitrogen) and transcribed into cDNA using Super Script VILO cDNA Synthesis Kit (Life Technologies). All the forward and reverse primers were synthesized by Invitrogen. Relative mRNA expression changes were calculated by 2-ΔΔCt. The results are expressed as the nfold way compared to control.
Wound healing assay
Wound healing assay was performed by following the protocol provided in the literature [11]. Si-vector, Si-TGFβ1, pvector or pSi-TGFβ1-treated PRE cells were cultured in a 12-well plate for 24 h. After washing with culture medium to remove cell debris, the cells were allowed to migrate for 48 h, followed by observation under a microscope.
Cell proliferation assay
Non-treated, Si-TGFβ, pvector or pSi-TGFβ1-treated PRE cells PRE cells proliferation was detected using CCK-8 kit according to the manufacturer’s instructions. Briefly, RPE cells were cultured in 48-well plates at the density of 1 × 104 cells/ well and then cultured for 24 h. Finally, 10 μl of CCK-8 solution was added to each well and incubated for 2 h. The results were measured using a microplate reader at 570 nm.
Western blot
Human PRE cells were collected and lysed in RIPA buffer (MPER reagent for the cells and T-PER reagent for the tissues, Thermo Scientific) followed homogenized at 4°C for 10 min. A total of 20 μg protein extracts was electrophoresed on 12.5% polyacrylamide gradient gels and then transferred to nitrocellulose membranes. The membranes were incubated in blocking buffer (5% milk) prior to incubation with primary antibodies at 4°C overnight. The primary rabbit anti-human antibodies used in the immunoblotting assays were: TGFβ1 (1:200, ab92486, Abcam), FIB (1: 500, ab6328, Abcam), α- SMA (1: 500, ab7817, Abcam), VIM (1: 500, ab92547, Abcam) and β-actin (1: 500, ab8226, Abcam). Horseradish peroxidase-conjugated anti-rabbit IgG (Bio-Rad, Hercules, CA, USA) were used at a 1:5000 dilution and detected using a Western Blotting Luminol Reagent.
Statistical analysis
The experiments data were expressed as mean ± standard (SD) deviation. The significant difference (p < 0.05) of data of different groups were calculated using Duncan's multiple range test using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
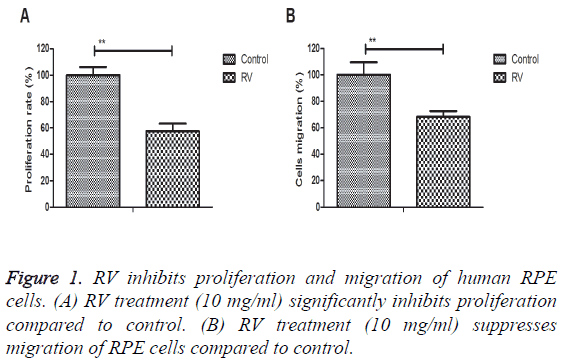
RV inhibits proliferation and migration of human RPE cells
The inhibitory effects of RV were investigated in RPE cells in vitro. Results demonstrated that RV treatment (10 mg/ml) significantly inhibited proliferation compared to control (Figure 1A). We also showed that RV treatment (10 mg/ml) suppressed migration of RPE cells compared to control (Figure 1B). These results suggest that RV treatment can significantly inhibit proliferation and migration of human RPE cells.
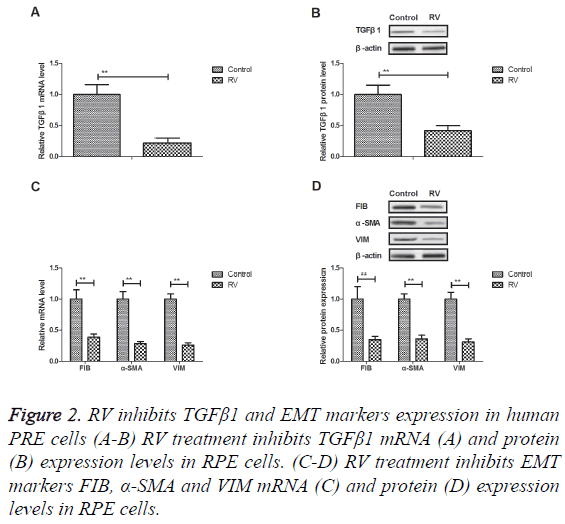
RV inhibits TGFβ1 and EMT markers expression in human PRE cells
Previous study showed that TGFβ1 and EMT signal pathway is associated with proliferation and migration of human RPE cells. We showed that RV treatment inhibited TGFβ1 mRNA and protein expression levels in RPE cells (Figures 2A and 2B). Results revealed that EMT markers FIB, α-SMA and VIM expression levels were down-regulated by RV treatment in RPE cells (Figures 2C and 2D). RV treatment can inhibit TGFβ1 and EMT markers expression in human PRE cells.
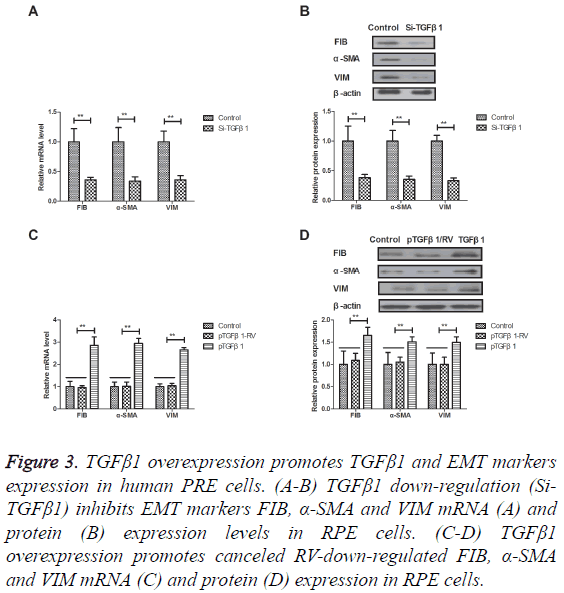
TGFβ1 overexpression promotes TGFβ1 and EMT markers expression in human PRE cells
The efficacy of TGFβ1 in the EMT signal pathway was studied in human PRE cells. As shown in Figures 3A and 3B, TGFβ1 down-regulation (Si-TGFβ1) inhibited EMT markers FIB, α- SMA and VIM mRNA and protein expression levels in RPE cells. Notably, TGFβ1 overexpression promoted and canceled RV-down-regulated FIB, α-SMA and VIM expression in RPE cells (Figures 3C and 3D). These results suggest that TGFβ1 overexpression can canceled RV-inhibited EMT markers expression in human PRE cells.
Figure 3: TGFβ1 overexpression promotes TGFβ1 and EMT markers expression in human PRE cells. (A-B) TGFβ1 down-regulation (Si- TGFβ1) inhibits EMT markers FIB, α-SMA and VIM mRNA (A) and protein (B) expression levels in RPE cells. (C-D) TGFβ1 overexpression promotes canceled RV-down-regulated FIB, α-SMA and VIM mRNA (C) and protein (D) expression in RPE cells.
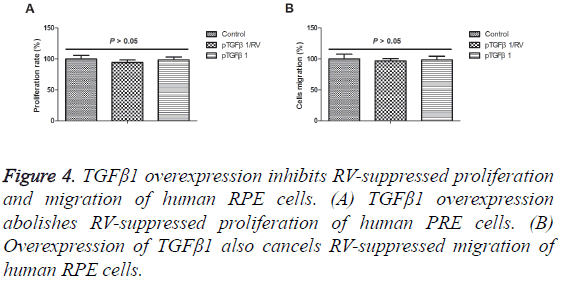
TGFβ1 overexpression inhibits RV-suppressed proliferation and migration of human RPE cells
The association between RV and TGFβ1 was analysed in human PRE cells. As shown in Figure 4A, we showed that TGFβ1 overexpression abolished RV-suppressed proliferation of human PRE cells. We observed that overexpression of TGFβ1 also abolished RV-suppressed migration of human RPE cells (Figure 4B). These results suggest that TGFβ1 overexpression can lead to abolishment of RV-suppressed proliferation and migration of human RPE cells.
Discussion
RPE cells play essential role in maintaining the normal function of the retina, especially the photoreceptor in the optic nerve system [12,13]. The dysfunctions of RPE cells can lead to retina and choroid damage, which further result in the occurrence of retinal degeneration diseases and even impaired vision [14,15]. Importantly, research has showed that RV can suppress expression of VEGF in human RPE cells that indicate RV may be useful as nutraceutical in controlling pathological choroidal neovascularization processes in age-related macular degeneration [16]. In this study, we reported that RV treatment significantly suppressed proliferation and migration of human RPE cells through TGFβ1-induced EMT signal pathway. Findings have suggested that TGFβ1 overexpression can canceled RV-inhibited EMT markers expression in human RPE cells.
Previous study has RV can inhibit EMT of RPE cells and is beneficial for treatment of proliferative VRP [17]. In addition, evidences have indicated that viability of RPE cells is associated with many ophthalmic diseases through regulation of EMT molecular markers [18]. Our results showed that RV treatment present significantly inhibitory effects for proliferation and migration of human RPE cells. Sheu et al. have indicated that RV could protect human RPE cells in acrolein-induced damage [19]. In this study, we indicated that RV down-regulated TGFβ1 expression levels and decreased EMT markers FIB, α-SMA, and VIM expression in RPE cells.
Previous study has showed the regulation of EMT in RPE cells by drugs-regulated signaling may be a valuable therapeutic approach for the prevention or treatment of proliferative PVR [20]. In addition, effect of magnolol on TGFβ1 and FIB expression in human retinal pigment epithelial cells has been analysed under diabetic conditions [21]. Furthermore, TGFβ1 can induceα-SMA expression and fibronectin synthesis in cultured human retinal pigment epithelial cells [22]. We showed that TGFβ1 overexpression stimulated EMT signal pathway and abolished RV-inhibited proliferation and migration of human RPE cells.
In conclusion, the current study identified the role of RV in the proliferation of human RPE cells. Findings have indicated that RV treatment inhibits proliferation and migration of RPE cells through down-regulating TGFβ1-meidated EMT signal pathway in RPE cells. Therefore, RV is a potential agent by modulating the proliferation and migration of RPE cells through regulation of TGFβ1-meidated EMT signal pathway. However, more reports should be investigated in future.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Yan F, He J, Tang L. Transforming growth factor-beta2 increases the capacity of retinal pigment epithelial cells to induce the generation of regulatory T cells. Mol Med Rep 2016; 13: 1367-1372.
- Heimes B, Farecki ML, Bartels S. Retinal pigment epithelial tear and anti-vascular endothelial growth factor therapy in exudative age-related macular degeneration: clinical course and long-term prognosis. Retina 2016; 36: 868-874.
- Mitsuhiro MR, Eguchi S, Yamashita H. Regulation mechanisms of retinal pigment epithelial cell migration by the TGF-beta superfamily. Acta ophthalmologica Scandinavica 2003; 81: 630-638.
- Enzmann V, Hollborn M, Wiedemann P, Kohen L. Minor influence of the immunosuppressive cytokines IL-10 and TGF-beta on the proliferation and apoptosis of human retinal pigment epithelial (RPE) cells in vitro. Ocular Immunol Inflamm 2001; 9: 259-266.
- Chung EJ, Chun JN, Jung SA, Cho JW, Lee JH. TGF-beta-stimulated aberrant expression of class III beta-tubulin via the ERK signaling pathway in cultured retinal pigment epithelial cells. Biochem Biophys Res Commun 2011; 415: 367-372.
- Sheu SJ, Bee YS, Chen CH. Resveratrol and large-conductance calcium-activated potassium channels in the protection of human retinal pigment epithelial cells. Off J Assoc Ocular Pharmacol Therap 2008; 24: 551-555.
- King RE, Kent KD, Bomser JA. Resveratrol reduces oxidation and proliferation of human retinal pigment epithelial cells via extracellular signal-regulated kinase inhibition. Chem Biol Interact 2005; 151: 143-149.
- Sheu SJ, Wu TT. Resveratrol protects against ultraviolet A-mediated inhibition of the phagocytic function of human retinal pigment epithelial cells via large-conductance calcium-activated potassium channels. Kaohsiung J Med Sci 2009; 25: 381-388.
- Alex AF, Spitznas M, Tittel AP, Kurts C, Eter N. Inhibitory effect of epigallocatechin gallate (EGCG), resveratrol, and curcumin on proliferation of human retinal pigment epithelial cells in vitro. Curr Eye Res 2010; 35: 1021-1033.
- Chan CM, Chang HH, Wang VC, Huang CL, Hung CF. Inhibitory effects of resveratrol on PDGF-BB-induced retinal pigment epithelial cell migration via PDGFRbeta, PI3K/Akt and MAPK pathways. PloS One 2013; 8: 56819.
- Stamp ME, Brugger MS, Wixforth A, Westerhausen C. Acoustotaxis-in vitro stimulation in a wound healing assay employing surface acoustic waves. Biomater Sci 2016; 4: 1092-1099.
- Yang P, Baciu P, Kerrigan BC. Retinal pigment epithelial cell death by the alternative complement cascade: role of membrane regulatory proteins, calcium, PKC, and oxidative stress. Investig Ophthalmol Vis Sci 2014; 55: 3012-3021.
- Umazume K, Tsukahara R, Liu L. Role of retinal pigment epithelial cell beta-catenin signaling in experimental proliferative vitreoretinopathy. Am J Pathol 2014; 184: 1419-1428.
- King-Smith C, Vagnozzi RJ, Fischer NE, Gannon P, Gunnam S. Orientation of actin filaments in teleost retinal pigment epithelial cells, and the effect of the lectin, Concanavalin A, on melanosome motility. Vis Neurosci 2014; 31: 1-10.
- Wade JS, Desai TA. Planar microdevices enhance transport of large molecular weight molecules across retinal pigment epithelial cells. Biomed Microdev 2014; 16: 629-638.
- Nagineni CN, Raju R, Nagineni KK. Resveratrol suppresses expression of VEGF by human retinal pigment epithelial cells: potential nutraceutical for age-related macular degeneration. Aging Dis 2014; 5: 88-100.
- Ishikawa K, He S, Terasaki H. Resveratrol inhibits epithelial-mesenchymal transition of retinal pigment epithelium and development of proliferative vitreoretinopathy. Sci Rep 2015; 5: 16386.
- Chen M, Rajapakse D, Fraczek M, Luo C, Forrester JV, Xu H. Retinal pigment epithelial cell multinucleation in the aging eye-a mechanism to repair damage and maintain homoeostasis. Aging Cell 2016; 15: 436-445.
- Sheu SJ, Liu NC, Chen JL. Resveratrol protects human retinal pigment epithelial cells from acrolein-induced damage. J Ocular Pharmacol Ther 2010; 26: 231-236.
- Jun JH, Joo CK. MicroRNA-124 controls transforming growth factor beta1-induced epithelial-mesenchymal transition in the retinal pigment epithelium by targeting RHOG. Investig Ophthalmol Vis Sci 2016; 57: 12-22.
- Kim YS, Jung DH, Kim NH, Lee YM, Kim JS. Effect of magnolol on TGF-beta1 and fibronectin expression in human retinal pigment epithelial cells under diabetic conditions. Eur J Pharmacol 2007; 562: 12-19.
- Stocks SZ, Taylor SM, Shiels IA. Transforming growth factor-beta1 induces alpha-smooth muscle actin expression and fibronectin synthesis in cultured human retinal pigment epithelial cells. Clin Exp Ophthalmol 2001; 29: 33-37.