Research Article - Journal of Biochemistry and Biotechnology (2023) Volume 6, Issue 3
Research on Combinations of Cuscuta chinensis and Cnidium monnieri Extracts for Osteoporosis Therapy
Ya-Jyun Liang1, Wei-Ting Kuo1, Christina Soong1,2, Hsuan-Yu Chen1,3,4* and Feng-Huei Lin 1,5*
1Institute of Biomedical Engineering, College of Medicine and College of Engineering, National Taiwan University, Taipei
2Department of Physical Medicine and Rehabilitation, National Taiwan University Hospital, Taipei
3Department of Orthopedics, College of Medicine and National Taiwan University Hospital, National Taiwan University, Taipei
4Department of Orthopedics, National Taiwan University Hsin-Chu Hospital, Hsin-Chu, Taiwan
5Division of Biomedical Engineering and Nanomedicine Research, National Health Research Institutes, Taiwan.
- Corresponding Author:
- Hsuan-Yu Chen
Feng-Huei Lin. Institute of Biomedical Engineering
College of Medicine and College of Engineering
National Taiwan University, Taipei
E-mail: hychen83@ntu.edu.tw, double@ntu.edu.tw
Received: 24-May-2023, Manuscript No. AABB-23-93330; Editor assigned: 27-May-2023, PreQC No. AABB-23-93330(PQ); Reviewed: 10-Jun-2023, QC No. AABB-23-93330;
Revised: 15-Jun-2023, Manuscript No. AABB-23-93330(R); Published: 22-Jun-2023, DOI:10.35841/aabb-6.3.146
Citation: Liang YJ, Kuo WT, Soong C, Chen HY, Lin FH. Research on combinations of Cuscuta chinensis and Cnidium monnieri extracts for osteoporosis therapy. J Biochem Biotech. 2023;6(3):146
Abstract
The treatment of osteoporosis using current medication has several drawbacks, including unpleasant side effects, increasing medication restrictions, and limited effectiveness. To address these issues, the aim of present research was to investigate the potential of a novel herbal supplement formulated by combining extracts of Cuscuta chinensis and Cnidium monnieri to promote osteogenic activity. In the investigation, extracts of M (1:0), 3MC (3:1), MC (1:1), M3C (1:3), and C (0:1) were used in a variety of ratios. According to the results of Cell Viability, Cytotoxicity, and DNA content during the 3-day culture period, the 3MC and MC groups showed encouraging biocompatibility and exhibited significant osteogenic proliferation effects in Mg-63 cells. Alkaline phosphatase activity and mineralization greatly increased in MG-63 cell culture under 3MC and MC treatments. Gene and protein expression results in MG-63 cells showed that 3MC and MC treatment improved some indicators for bone mass and bone formation. In addition, 3MC treat-ment greatly increased the mRNA expression of ALP, type I collagen, BMP-2, Osteocalcin (OCN), and Osteoprotegerin (OPG) in the MG- 63 cells in comparison to MC treatment. These results sug-gest that combining 3MC has potential for promoting osteoblastic bone formation, which could lead to the development of a bone-building supplement
Keywords
Osteoporosis, Cuscuta chinensis, Cnidium monnieri, Herbal extracts.
Introduction
Osteoporosis, one of the most prevalent geriatric syndromes increasing of bone fracture risk, is a disease caused by loss of bone mass and decreasing of bone strength [1, 2]. The incidence of osteoporosis is directly proportional to age. Its incidence is directly related to age, especially while women experiencing gradual estrogen decline after menopause, a critical factor in preventing bone resorption. In addition, other aging phenomena such as decline neuromuscular function, cognitive impairment, and sarco-penia will increase the likelihood of stumbling, which could increase the risk of fragility fractures and raise social economic burden. Bisphosphonate is currently recommended as the first-line option for osteoporosis [3-5].
Because it has a high affinity with bone matrix and can
inhibit bone resorption, bringing about a net gain in bone mass. However, it still accompanies with disadvantages on the absorption rate might be low and some adverse events including fever, flu-like symptoms, related jaw osteonecrosis and atypical fracture [6]. Even though some drugs are effective in osteoporosis, they provide only temporary symptom relief, and none have demonstrated the ability to cure or prevent disease progression [3, 7]. Thus, another molecule should be chosen to exert more effects and fewer side effects on bone metabolism. Traditional herb medicines have been proved by many research in recent years that they could have potential effects on osteoporosis [8].
Cnidium monnieri (M) has been used in traditional medicine for thousands of years as a treatment for eczema and warms kidneys [9, 10]. The main ingredient of Cnidium monnieri, are coumarins, flavonoid, osthole, monoterpenoid glucosides and other com-pounds. Some studies have shown that the chemical components of Cnidium monnieri may have some impact on bone metabolism. For example, Cnidium monnieri contains some compounds that with potential antioxidant, anti-inflammatory, and anti-aging ef-fects which may have a protective effect on the function and metabolism of bone cells. The flavonoid, which can effectively increase bone density, and it has been shown to promote reproductive ability [9]. In recent years, many investigators have shown that osthole inhibits bone resorption, promotes bone formation, and increases bone mass. However, more research is needed to determine whether Cnidium monnieri can be used as an effective herbal ingredient for preventing and treating osteoporosis.
Cuscuta chinensis(C), a commonly used traditional medicine, has antioxidant, an-ticancer, neuroprotective, and other obvious pharmacological activities. Besides, it has been used as a functional food to prevent osteoporosis and aging and improve sexual function [11].
Some experiments have also shown that Cuscuta chinensis contains some chemical components that may be beneficial for bone metabolism, such as protease in-hibitors and flavonoids [12]. Furthermore, previous reports demonstrated that Cuscuta chinensis could increase BMP-2 expression and ALP activity, modulate RANKL and OPG signals, and promote osteoblast mineralization [13, 14]. However, more research is needed to confirm the exact role and efficacy of Cuscuta chinensis in osteoporosis.
Even though there are a lot of evidence of therapeutic application of the Cuscuta chinensis and Cnidium monnieri in osteoporosis treatment, the efficacy of the combina-tion of Cuscuta chinensis and Cnidium monnieri in experimental models and the further mechanism are still not clearly clarified. In this study, we aimed to combine these in-gredients extracts to develop a safe, effective, and convenient supplement for the pre-vention and treatment of osteoporosis
Material and Methods
Preparation of MC extracts
Current good manufacturing practices were followed in the production of MC, which is a combination of dried extracts of Cnidium monnieri and Cuscuta chinensis. NuLiv Science USA, Inc. provided the standardized Cnidium monnieri extract (hydroethanolic extract, 10:1 extract ratio) and Cuscuta chinensis extract (hydroethanolic extract, 20:1 extract ratio) (Brea, CA, USA). The experiment group used a varied ratio that included M (1:0), 3MC (3:1), MC (1:1), M3C (1:3), and
C (0:1) extracts.
Cell culture and cell viability assay
The human osteoblastic cell line, MG-63 cell, purchased from the Bioresource Col-lection and Research Center (BCRC, Taiwan), were cultured in Dulbecco’s Modified Ea-gle’s Medium (DMEM; Gibco, MD, USA) containing 10% fetal bovine serum (FBS; Hy-clone, USA) and 1% antibiotics (100 U/mL penicillin and 100 g/mL streptomycin, Gibco) and incubated at 37°C in a humidified atmosphere containing 5%
CO2 (Nozaki, Nikai, Okabe, Nagahama, and Eto, 2016).
Evaluation of cell viability
MG-63 cells will be used to test the cell viability of MC using the water-soluble te-trazolium (WST-1, Takara) assay. Briefly, MG-63 cell were seeded in 96-well plates at a density of 104 cells and cultured for 24 h to full adhesion. The experiment group prepared a mixture of M, 3MC, MC, M3C, and C extracts in a variety of ratios. The solution medium was cultured with MG-63 cell for 24 h. Thereafter, 100 μL WST-1 solutions was added to the wells and incubated for 2 h. The absorbance values of each well were measured at 450 nm using an ELISA plate reader (SpectraMax® iD3 Multi-Mode Micro-plate Reader, Molecular Devices).
Evaluation of cytotoxicity
The cytotoxicity of MC was evaluated using the lactate dehydrogenase (LDH) assay (Sigma-Aldrich, USA). Briefly, MG-63 cells at a concentration of 1×105 cells/well in 96-well plate. Incubate cells for 24 h at 37°C and 5% CO2. Remove the medium and add the following medium 100 μL. The experiment group prepared a mixture of M, 3MC, MC, M3C, and C extracts in a variety of ratios. Incubate cells for 24 h at 37°C and 5% CO2. Remove the medium and add LDH working solution 100 μL, incubate for 30 minutes. The results of the LDH assay were measured at a wavelength of 490 nm by ELISA plate reader (SpectraMax® iD3 Multi-Mode Microplate Reader, Molecular Devices).
Evaluation of Alkaline Phosphatase (ALP) assay
Seed MG-63 cells at a concentration of 105 cells per well in a 96-well plate. Incubate cells for 24 h at 37°C with 5% CO2. Remove the medium and add the following medium: 100 L with cells for 24 h. The control group received DMEM with 10% FBS. The experiment group prepared a mixture of M, 3MC, MC, M3C, and C extracts in a variety of ratios. After 7 days, remove the medium and wash with 1X PBS twice. Add 100 L of pNPP to each well and incubate at room temperature for 30 minutes. Measure the absorbance with an ELISA reader at 405 nm.
Evaluation of Alizarin Red S
105 cells/well MG-63 was cultured on a 24-well plate. Incubate cells for 24 h at 37 °C with 5% CO2. Remove the medium and add the following medium: 100 μL with cells for 24 h. The control group received DMEM with 10% FBS. The experiment group prepared a mixture of M, 3MC, MC, M3C, and C extracts in a variety of ratios. After incubation, remove the medium and wash with 1X PBS twice. Fix for 30 minutes in 250 L of 10% formalin. Remove formalin, rinse twice with 500 μL 1X PBS pH 4, add 250 μL Alizarin Red S stain, remove ARS stain, rinse twice with 500 μL 1X PBS pH 4, and place at room temperature. Air-dry and observe with a dissecting microscope. The removed ARS stain was quantitatively analyzed by measuring absorbance at 562 nm with an ELISA reader.
DNA content analysis
105 MG-63 cells were cultured in a 24-well plate. The experiment group prepared a mixture of 3MC and MC extracts in a variety of ratios. After 3 days of incubation, remove the supernatant, add 600 μL of cell lysing solution to lyse the cells, add 3μL of RNase solution to remove RNA, add 200 μL of protein precipitation solution, mix and centrifuge to remove protein, add 600 μL of isopropanol into the centrifuge tube, and mix carefully After centrifugation, the DNA pellet was obtained, which was washed with 70% alcohol to remove more than salt. After centrifugation, it was air-dried and stored at 2-8°C by adding DNA Rehydration Solution.
Gene expression analysis
To assess the mineralization effects of CM, we analyzed the levels of Osteogene-sis-related gene expression - ALP, type I collagen, Osteocalcin (OCN), Osteoprotegerin (OPG), Osteopontin (OPN), RANKL and BMP-2- using MG-63 as target cells. Total RNA was extracted using Qiazol reagent (Qiagen, Valencia, CA, USA) following the manu-facturer’s protocol. Random hexamers (Vivantis Inc., California, CA, USA) and reverse transcriptase (Vivantis Cat No: RTPL12) were used for the first-strand cDNA synthesis with the following PCR parameters: 95°C for 3 min (denaturation), 40 cycles of 95°C for 20 s, 60°C for 30 s (annealing), and 72°C for 30 s (elongation). The real-time RT-PCR was performed using the TOOLS 2X SYBR qPCR Mix (Biotools Co., Ltd., Taipei, Taiwan) on a CFX Connect Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The changes in expression of the target genes were calculated using GAPDH as an endogenous control.
Determination of type I collagen content
105 cells/well MG-63 was cultured in 6-well plate. The experiment group prepared a mixture of 3MC and MC extracts in a variety of ratios. After 1 day of culture, adding the different concentrations MC extracts co-cultured with cell, the cells were collected to measure the collagen content. After the cells were lysed with 0.1% Triton X-100, centri-fuged at 10,000 g for 5 minutes, and the supernatant was collected. Using type I collagen as the standard, the protein concentration was analyzed by BCA protein assay kit. Read absorbance at 540 nm wavelength with ELISA reader.
Assaying levels of Osteocalcin and BMP-2
104 cells/well MG-63 was cultured in a 96-well plate. The experiment group prepared a mixture of 3MC and MC extracts in a variety of ratios. After 1 day of culture, adding the different concentrations MC extracts cocultured with cell, the cells were collected to measure the collagen content. Refer to the kit instructions for the experimental procedure, Osteocalcin and BMP-2 were measured using Human Osteocalcin ELISA Kit and Human BMP-2 ELISA Kit, respectively. Add the collected supernatant to the 96 well plate of the Coating primary antibody, and incubate for 2 hours at room temperature. Remove the supernatant and wash 5 times with wash buffer. Add 100 μL of different Conjugate to each well for 2 hours, remove the supernatant, and wash 5 times with wash buffer. Add 100 μL of substrate solution to each well for 30 minutes at room temperature, add 100 μL of stop solution to each well, and measure the absorbance at 450 nm with an ELISA reader to quantify OPG.
Osteoprotegrin (OPG) immunoassay
104 cells/well MG-63 was cultured in a 96-well plate. The experiment group prepared a mixture of 3MC and MC extracts in a variety of ratios. After 1 day of culture, adding the different concentrations MC extracts cocultured with cell, the supernatant was collected after 7 days of extract addition. The test uses Quantikine® MOP00 ELISA kit. Add the collected supernatant to a 96 well plate of Coating OPG antibody, and incubate for 2 hours at room temperature. Remove the supernatant and wash 5 times with wash buffer. Add 100 μL OPG Conjugate to each well for 2 hours, remove the supernatant, and wash 5 times with wash buffer. Add 100 μL of substrate solution to each well for 30 minutes at room temperature, add 100 μL of stop solution to each well, and measure the absorbance at 450 nm with an ELISA reader to quantify OPG.
Statistical analysis
Statistical data are expressed as mean ± standard deviation (SD). Statistical analysis was done using one-way ANOVA. Differences were considered significant at a p-value of less than 0.05. (p < 0.05, *; p < 0.01, **; p < 0.001, ***).
Results
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn
The effect of different ratio MC preparations on MG-63 cell viability
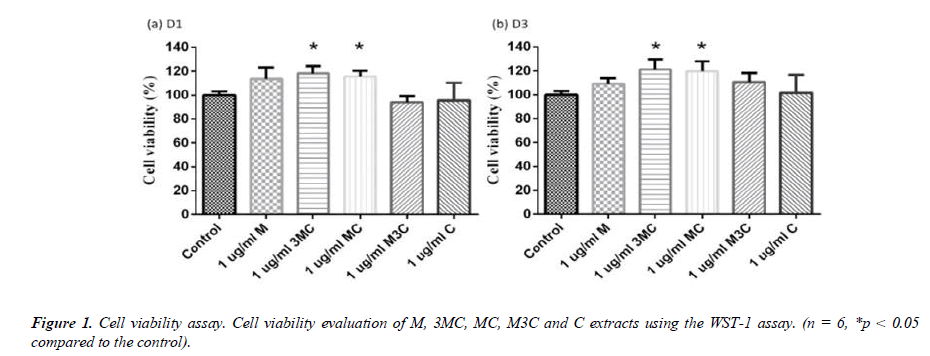
The WST-1 assay was used to determine the effect of M, 3MC, MC, M3C and C ex-tracts on cell viability (\ 1). Each group was normalized to the control group, which had 100% cell viability. After 1 and 3 days, the difference ratio of MC extracts had no negative effects on the survival of MG-63 cells. Furthermore, the 3MC and MC groups exhibit good cell proliferation on days 1 and 3.
Cytotoxicity assays
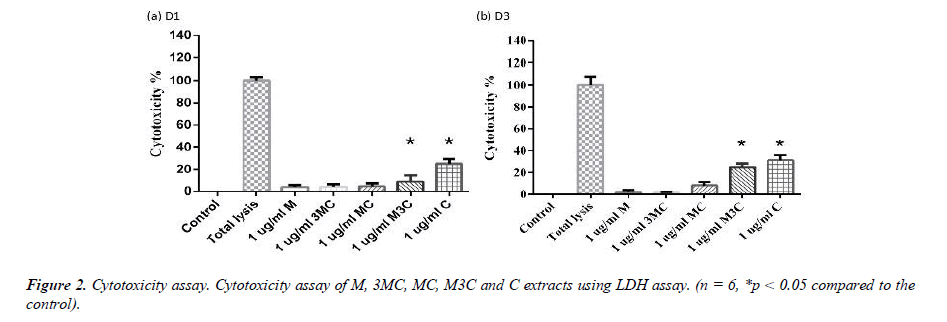
The LDH assay was used to determine the cytotoxicity of M, 3MC, MC, M3C and C groups (Figure 2). Each group was normalized to that of the Total lysis group, which had 100% cytotoxicity. After 1 and 3 days, the M, 3MC and MC groups had no negative effects on the survival of MG-63 cells. Furthermore, compared to the Control group, the M3C and C groups exhibit more cytotoxicity.
ALP assay
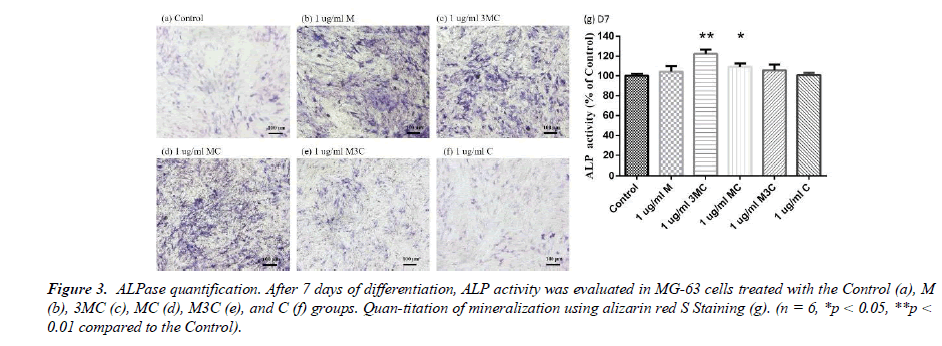
The experiment involved a quantitative and staining ALP assay to evaluate osteo-genesis, as shown in Figure 3. The results indicated that MG-63 cells from the M, 3MC, and MC groups displayed more purple than the Control group, indicating higher levels of alkaline phosphatase activity and thus greater potential for osteogenesis. Furthermore, the quantitative analysis of ALP concentration in the 3MC and MC groups revealed sig-nificantly higher values than the control group, suggesting that these groups are effective in promoting osteoblastic differentiation and osteogenesis.
Alizarin-Red S staining
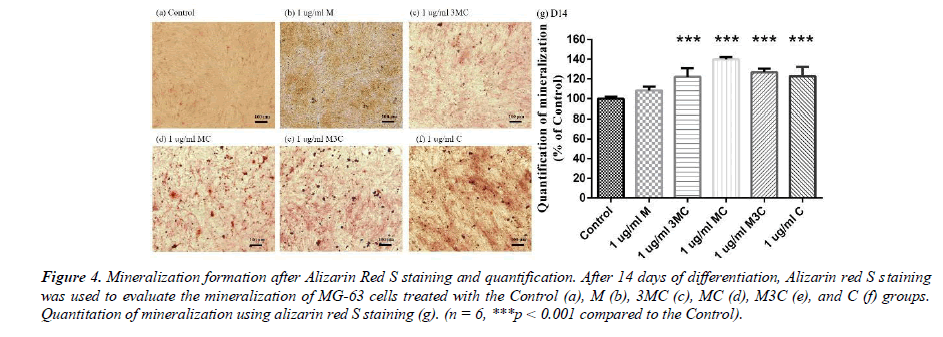
Alizarin-red S staining is the usual method for detecting calcium deposition in the MG-63 cells after 14 days. The results of Alizarin-red S staining are shown in Figure 4. The observations show that the 3MC, MC, M3C, and C groups in MG-63 have more red than the Control group. The amount of mineralization significantly increased in the 3MC, MC, M3C, and C groups. The above experimental results indicate that the 3MC and MC groups have good viability and no cytotoxicity toward MG63 osteoblasts and have higher mineralization activity. Therefore, 3MC and MC were selected for subsequent experiments.
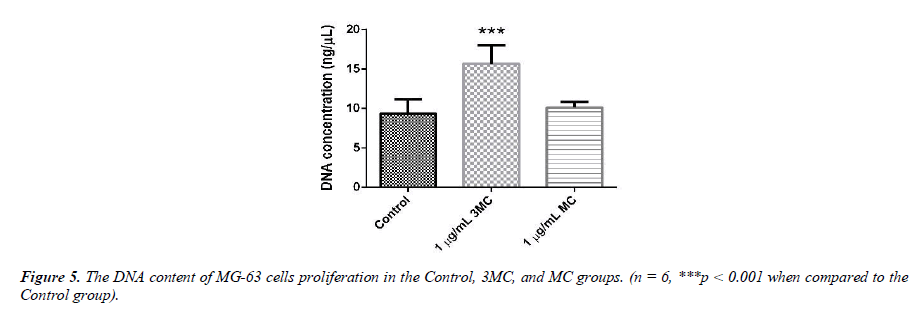
Determination of cellular deoxyribonucleic acid content (total DNA)
The DNA content of MG-63 cells growing in the Control, 3MC, and MC groups is used to evaluate cell proliferation, as shown in Figure 5. The DNA content of the Control group was about 9.8 ± 1.9 ng/μl, that of the 3MC group was about 15.6 ± 2.9ng/μl, and that of the MC group was about 10.1 ± 0.7 ng/μl. DNA levels in the 3MC group were significantly higher than in the Control group. The MC group, however, had no impact on the cells as there was no obvious difference between the Control group and the MC group.
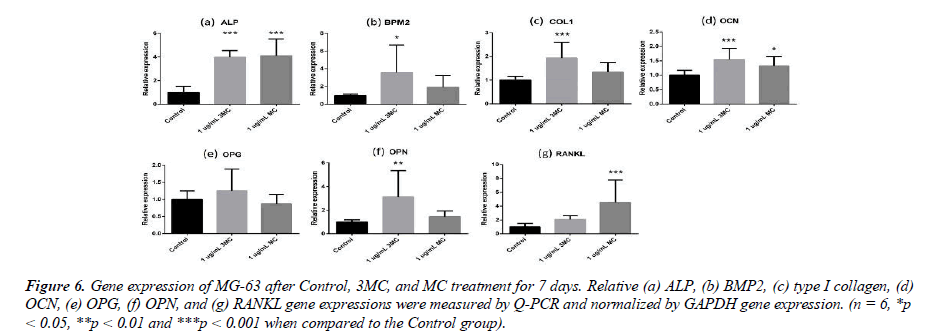
Bone remodeling-related gene expression
To explore the effect of the Control, 3MC, and MC on the MG63 property, several genes, such as ALP, BMP2, type I collagen, Osteocalcin (OCN), Osteoprotegerin (OPG), Osteopontin (OPN), and RANKL, were measured by Q-PCR analysis. The results of gene expression are shown in Figure 6. In comparison to the Control group, the gene expression of the 3MC group significantly differed in the expression of type I collagen (0.9-fold increase), OCN (0.5-fold increase), OPN (2.1-fold increase), ALP (2.9-fold increase), and BMP2 (2.5-fold increase), while the MC group significantly differed in the expression of OCN (0.3-fold increase), ALP (3.1- fold increase), and RANKL (3.5-fold increase). The results demonstrated that osteogenesis-related gene expression was influenced by the 3MC and MC groups.
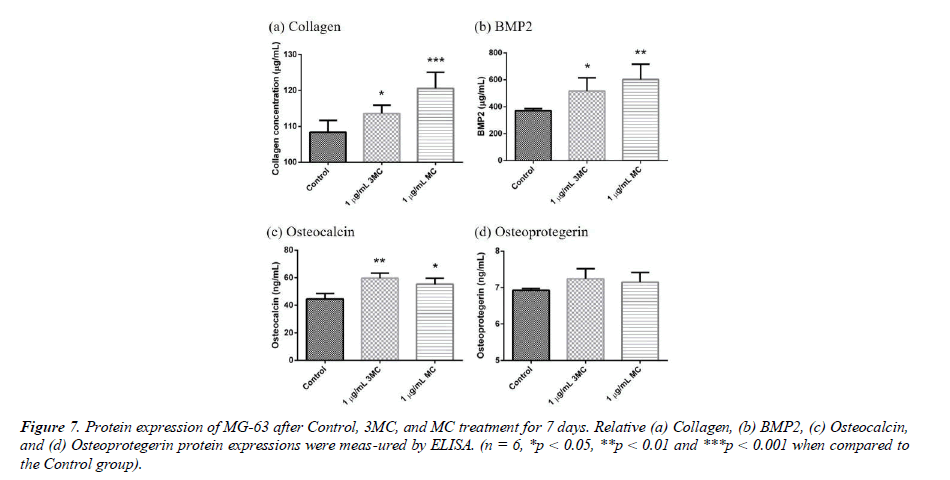
Osteogenesis-related protein expression
To explore the effect of the Control, 3MC, and MC on the MG63 property, several proteins, such as Collagen, BMP2, osteocalcin (OCN), and osteoprotegerin (OPG) were measured by Elisa analysis. The results of protein expression are shown in Figure 7. Collagen expression was 108.3 ± 3.30 μg/ml in the control group, 113.6 ± 2.32 μg/ml in the 3MC group, and 120.5 ± 4.49 μg/ml in the MC group. BMP2 expression was 371.33 ± 15.28 μg/ml in the control group, 515.90 ± 99.09 μg/ml in the 3MC group, and 602.09± 114.38 μg/ml in the MC group, which showed significant difference in the amount of BMP2 expressed in the 3MC and MC groups compared to the control group. The results demonstrated a significant difference in BMP2 levels between the 3MC and MC groups in comparison to the control group. Osteoprotegrin expression was 6.92 ± .05 ng/ml in the control group, 7.24 ± 0.27 ng/ml in the 3MC group, and 7.14 ± 0.26 ng/ml in the MC group, which showed a slight increase in the amount of osteoprotegrin expressed in the 3MC and MC groups compared to the control group. Osteocalcin expression was 44.57 ± 3.99 ng/ml in the control group, 59.66 ± 3.72 ng/ml in the 3MC group, and 55.30± 4.32 ng/ml in the MC group. This difference in osteocalcin expression levels between the 3MC and MC groups and the control group was significant. The results indicated that osteogenic protein production could be enhanced in both the 3MC and MC groups.
Discussion
The condition known as osteoporosis causes bones to become porous, resulting in weakened bones that are more susceptible to fragility fracture and it is associated with loss of independence, morbidity, and mortality. Because the symptoms of osteoporosis are not apparent in the early stages of the disease, most patients are not diagnosed until fragility fracture has occurred. To improve the diagnosis and care of high-risk patients, various efforts have been made, such as the implementation of fracture liaison services, deep-learning algorithm assisted fracture detection, and fracture diagnosis with better modality [15, 16]. Medication commonly used to prevent and treat postmenopausal os-teoporosis by reducing bone loss to boosting bone mass, are bisphosphonates in clinical settings [17]. It is also important to find effective products that are less burdensome to the body to avoid osteoporosis [5]. Herbs are commonly used in traditional medicine to treat orthopedics and osteoporosis, and they are also clinically effective in doing so. As a nat-ural active ingredient in the treatment of osteoporosis, coumarins found in Cnidium monnieri and flavonoids found in Cuscuta chinensis both increase bone density and are thought to be fertility- promoting in medicine. Coumarins also have substantial phar- macological activity and support osteoblast maturation [18, 19].
In this study, MG-63 cells are used as a useful model of osteoblast-like properties to evaluate the biological function and mineralization of various materials. The results in-dicate that M, 3MC, MC extracts have good biocompatibility and no cytotoxicity to os-teoblastic cells, which suggests the possibility of reducing side effects (Figure 1&2). Os-teoblasts will proliferate to a certain number and produce a lot of alkaline phosphatase at the beginning of their differentiation into osteocytes. In the later stage of differentiation, osteoblasts secrete a significant quantity of calcium ions, which precipitate as calcium phosphate around the cells. Calcium ions and alizarin red S can combine to create a complex, which can then be seen or measured as the level of calcium precipitation. The main constituents of Cuscuta chinensis have been shown to be flavonoids, saccharides, and resin glycosides, which are suggested as being responsible for the pharmacologic activities [9, 20]. The increased ALP activity and mineralization demonstrated that 3MC and MC treatments can regulate a significant portion of the closely correlated control between maturation and differentiation in MG-63 cells through increased growth factor synthesis and secretion, which eventually stimulates mineralization (Figure 3&4). According to the above experiment results, the 3MC and MC groups exhibit excellent viability, no cytotoxicity toward MG- 63 osteoblasts, and greater mineralization activity. So, for the following tests, 3MC and MC were chosen.
Figure 3: ALPase quantification. After 7 days of differentiation, ALP activity was evaluated in MG-63 cells treated with the Control (a), M (b), 3MC (c), MC (d), M3C (e), and C (f) groups. Quan-titation of mineralization using alizarin red S Staining (g). (n = 6, *p < 0.05, **p < 0.01 compared to the Control).
Figure 4: Mineralization formation after Alizarin Red S staining and quantification. After 14 days of differentiation, Alizarin red S staining was used to evaluate the mineralization of MG-63 cells treated with the Control (a), M (b), 3MC (c), MC (d), M3C (e), and C (f) groups. Quantitation of mineralization using alizarin red S staining (g). (n = 6, ***p < 0.001 compared to the Control).
The DNA quantification research indicates that the 3MC group can stimulate cell proliferation (Figure 5). Osteoblasts produce and secrete significant amounts of type I collagen, an essential extracellular macromolecule and part of the bone matrix. Osteo-protegrin can stop osteoeclasts from differentiating, surviving, and carrying out their usual functions, thereby halting bone loss [21]. Bone morphogenetic protein 2 (BMP-2) and osteocalcin are significant proteins that support the formation of bone [22]. RANKL is a crucial protein that promotes bone development [23]. Osteopontin can be used to assess how osteoblasts function. The 3MC group in this research is able to increase the gene expression of collagen type I, OCN, OPN, ALP, and BMP2, whereas the MC group is able to increase the gene expression of OCN, ALP, and RANKL. The results indicate that bone formation- related gene expression is influenced by both the 3MC and MC groups (Figure 6). According to the experiment results, both the 3MC and MC groups can increase collagen content and osteocalcin and BMP2 protein expression, suggesting that they can improve the expression of bone formation proteins (Figure 7). This study demonstrates that 3MC and MC treatments increased growth factor synthesis and secretion, which in turn promotes mineralization, can control a sizable part of the tightly correlated control between maturation and differentiation in MG-63 cells.
Figure 6: Gene expression of MG-63 after Control, 3MC, and MC treatment for 7 days. Relative (a) ALP, (b) BMP2, (c) type I collagen, (d) OCN, (e) OPG, (f) OPN, and (g) RANKL gene expressions were measured by Q-PCR and normalized by GAPDH gene expression. (n = 6, *p < 0.05, **p < 0.01 and ***p < 0.001 when compared to the Control group.
The previous studies suggested that Cnidium monnieri and Cuscuta chinensis con-tain abundant polyphenols and flavonoids, which have significant antioxidant effects, reducing the generation of free radicals and slowing the impact of oxidative damage on bone tissue [19, 24]. Meanwhile, studies have also found that the compounds in Cnidium monnieri and Cuscuta chinensis can promote the proliferation and differentiation of bone cells, thereby enhancing the formation and repair of bone tissue while reducing bone resorption and protecting bone tissue health [25-27]. As a result of the present studies, a mixture of Cnidium monnieri and Cuscuta chinensis in a 3:1 or 1:1 ratio has shown sig-nificant potential in the treatment of osteoporosis.
Conclusion
In this study, the results indicate that M, 3MC, and MC extracts have good bio-compatibility and no cytotoxicity to osteoblastic cells. At 3MC and MC treatments, alka-line phosphatase activity and mineralization greatly increased in MG-63 cell culture. DNA content during the 3-day culture period, the 3MC group showed osteogenic pro-liferation effects in Mg-63 cells. According to gene expression results, the 3MC group improved the expression of collagen type I, OCN, OPN, ALP, and BMP2, whereas the MC group improved the expression of OCN, ALP, and RANKL. Further, based on the protein production results, the 3MC and MC groups produced more osteocalcin, collagen, and BMP2 proteins, which in turn increased osteogenesis. These results suggest a possible role for 3MC and MC combinations in osteoblastic bone formation and the possibility of developing a bone-building supplement.
Author Contributions
Conceptualization, Y.J.L; methodology, Y.J.L and W.T.K; validation, Y.J.L, W.T.K and C.S.; formal analysis, Y.J.L; investigation, Y.J.L; resources, F.H.L and H.Y.C; data cura-tion, Y.J.L; writing—original draft preparation, Y.J.L; writing—review and editing, F.H.L and H.Y.C; visualization, F.H.L; supervision, F.H.L.;
Funding
This study was supported by the National Health Research Institutes (BN-112-GP-07 and BN-112-GP-09), Ministry of Science and Technology, Taiwan (MOST 111-2221-E- 002 -037 -MY3), and subsidized by Ministry of Science and Technology and National Taiwan University (NTU), Taiwan.
Acknowledgments
The authors would like to thank the Institute of Biomedical Engineering, Na-tional Taiwan University, the Ministry of Science and Technology, and the National Taiwan Uni-versity Hospital for their financial support. We are deeply grateful for the generous assistance provided by NuLiv Science USA, Inc. in this research.
Conflict of Interest
All authors do not have any possible conflicts of interest.
References
- Salari N, Ghasemi H, Mohammadi L, et al. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J Orthop Surg Res. 2021;16(1):1-20.
- Ji MX, Yu Q. Primary osteoporosis in postmenopausal women. Chronic Dis Transl Med. 2015;1(01):9-13.
- Sözen T, Öz???k L, Ba?aran NÇ. An overview and management of osteoporosis. Eur J Rheumatol. 2017;4(1):46.
- Walker J. Osteoporosis and fragility fractures: Risk assessment, management and prevention. Nurs Older People. 2022;34(6).
- Hemmati E, Mirghafourvand M, Mobasseri M, et al. Prevalence of primary osteoporosis and low bone mass in postmenopausal women and related risk factors. J Educ Health Promot. 2021;10.
- Hwang JS, Chan DC, Chen JF, et al. Clinical Practice Guidelines For The Prevention And Treatment Of Osteoporosis In Taiwan: Summary. J Bone Miner Metab. 2014;32:10-6.
- Gilbert ZA, Muller A, Leibowitz JA, et al. Osteoporosis Prevention and Treatment: The Risk of Comorbid Cardiovascular Events in Postmenopausal Women. Cureus. 2022;14(4).
- He J, Li X, Wang Z, et al. Therapeutic anabolic and anticatabolic benefits of natural Chinese medicines for the treatment of osteoporosis. Front Pharmacol. 2019;10:1344.
- Liao JM, Zhu QA, Lu HJ, et al. Effects of total coumarins of Cnidium monnieri on bone density and biomechanics of glucocorticoids-induced osteoporosis in rats. Zhongguo Yao Li Xue Bao. 1997;18(6):519-21.
- Zhang Q, Qin L, He W, et al. Coumarins from Cnidium monnieri and their antiosteoporotic activity. Planta Med. 2007;73(01):13-9.
- Tao Y, Chen L, Pan M, et al. Tracing anti?osteoporosis components from raw and salt?processed semen of Cuscuta chinensis by employing a biochemometrics strategy that integrates ultrasonic?assisted extraction, quantitation, efficacy assessment in zebrafish, and grey relationship analysis. J Sep Sci. 2021;44(17):3229-36.
- Yang L, Chen Q, Wang F, et al. Antiosteoporotic compounds from seeds of Cuscuta chinensis. J Ethnopharmacol. 2011;135(2):553-60.
- Yang HM, Shin HK, Kang YH, et al. Cuscuta chinensis extract promotes osteoblast differentiation and mineralization in human osteoblast-like MG-63 cells. J Med Food. 2009;12(1):85-92.
- Mo H, Zhang N, Li H, et al. Beneficial effects of Cuscuta chinensis extract on glucocorticoid-induced osteoporosis through modulation of RANKL/OPG signals. Braz J Med Biol Res. 2019;52.
- Chen HY, Hsu BW, Yin YK, et al. Application of deep learning algorithm to detect and visualize vertebral fractures on plain frontal radiographs. PLoS One. 2021;16(1):e0245992.
- Chen HY, Wu T, Tseng SP, et al. Application of tomosynthesis for vertebral compression fracture diagnosis and bone healing assessment in fracture liaison services. Front Med. 2022;9.
- Tu KN, Lie JD, Wan CK, et al. Osteoporosis: A review of treatment options. Pharmacol Ther. 2018;43(2):92.
- Liu H, Zhang H, Fan H, et al. The preventive effect of Cuscutae Semen polysaccharide on bone loss in the ovariectomized rat model. Biomed Pharmacother. 2020;130:110613.
- Yen FL, Wu TH, Lin LT, et al. Concordance between antioxidant activities and flavonol contents in different extracts and fractions of Cuscuta chinensis. Food Chem. 2008;108(2):455-62.
- Yen FL, Wu TH, Lin LT, et al. Hepatoprotective and antioxidant effects of Cuscuta chinensis against acetaminophen-induced hepatotoxicity in rats. J Ethnopharmacol. 2007;111(1):123-8.
- Blair HC, Larrouture QC, Li Y, et al. Osteoblast Differentiation And Bone Matrix Formation In vivo And In vitro. Tissue Eng Part B Rev. 2017;23(3):268-80.
- Halloran D, Durbano HW, Nohe A. Bone morphogenetic protein-2 in development and bone homeostasis. J Dev Biol. 2020;8(3):19.
- Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 2008;473(2):139-46.
- Zafar S, Sarfraz I, Rasul A, et al. Osthole: A multifunctional natural compound with potential anticancer, antioxidant and anti-inflammatory Activities. Mini Rev Med Chem. 2021;21(18):2747-63.
- Munir N, Mehmood Z, Shahid M, et al. Phytochemical Constituents and In vitro Pharmacological Response of Cnidium monnieri; A Natural Ancient Medicinal Herb. Dose-Response. 2022;20(3):15593258221115543.
- Amin G, Kondori BM, Vazirian M, et al. Evaluation of chemical composition and antioxidant activity of total extract of Cuscuta chinensis Lam. Used in traditional medicine. Planta Med. 2010;76(12):P147.
- Koca U, Küpeli-Akkol E, Sekeroglu N. Evaluation of In vivo and In vitro biological activities of different extracts of Cuscuta arvensis. Nat Prod Commun. 2011;6(10):1934578X1100601005.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.