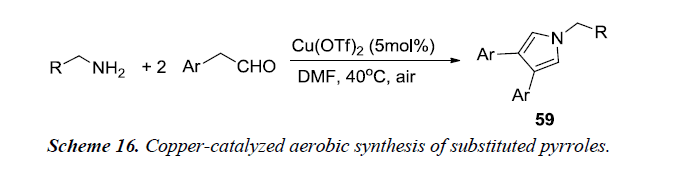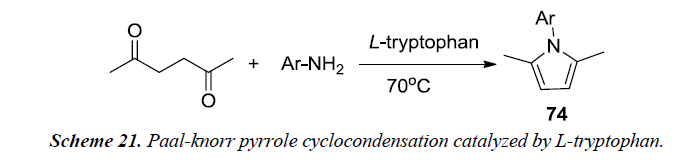Review Article - Journal of Pharmaceutical Chemistry & Chemical Science (2017) Journal of Pharmaceutical Chemistry & Chemical Science 2017
Recent synthetic and medicinal perspectives of pyrroles: An overview.
Ramandeep Kaur1, Vibhuti Rani1, Vikrant Abbot1, Yagyesh Kapoor2, Debabrata Konar3, Kapil Kumar1,2,3*
1Department of Pharmaceutical Chemistry, Indo-Soviet Friendship College of Pharmacy (ISFCP), Moga, India
2School of Pharmaceutical Sciences, Apeejay Stya University, Haryana, India
3National Institute of Pharmaceutical Education and Research, Punjab, India
- *Corresponding Author:
- Dr. Kapil Kumar
Department of Pharmaceutical Chemistry Indo-Soviet Friendship College of Pharmacy (ISFCP) Moga India
Tel: 0163 632 4201
E-mail: kapil.py@gmail.com
Accepted date: December 20, 2017
Citation: Kaur R, Rani V, Abbot V, et al. Recent synthetic and medicinal perspectives of pyrroles: An overview. J Pharm Chem Chem Sci. 2017;1(1):17-32.
Abstract
Pyrrole is a privileged scaffold with assorted nature of biological activities. Many active compounds have been developed by amalgamation of different pharmacophores in a pyrrole ring system. Pyrroles are an active component of complex macrocycles, including the porphyrins of heme, chlorins, bacteriochlorins, chlorophyll, porphyrinogens. Pyrrole and its derivatives are widely used as intermediates in synthesis of pharmaceuticals, agrochemicals, dyes, photographic chemicals, perfumes and other organic compounds. The pyrrole skeleton is an imperative structural framework found in extensive range of biologically active natural products and pharmaceutically active molecules. They are an element of polymers, indigoid dyes and large aromatic rings. Pyrroles are utilized as a catalyst for polymerization process, corrosion inhibitor, preservative, solvent for resins and terpenes. It is functional in various metallurgical process, luminescence chemistry, spectrochemical analysis and transition metal complex catalyst for uniform polymerization. Furthermore, some of the compounds are useful intermediates in the synthesis of biologically important naturally occurring alkaloids and synthetic heterocyclic derivatives. In this review, attempts are made to disclose various tactical approaches to synthesize pyrrole and its derivatives. The structure activity relationship studies have been discussed along with their pharmacological applications.
Keywords
Pyrrole, Combretastatin, Anticancer, Anti-viral, Anti-mycobacterial.
Introduction
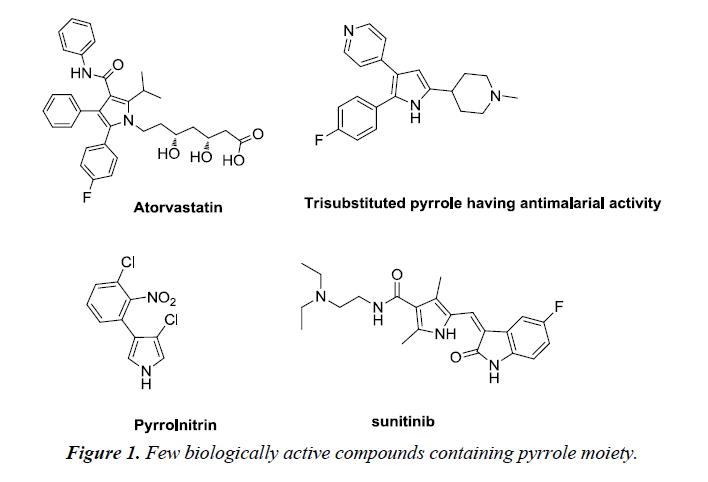
Heterocyclic compounds are cyclic compounds containing at least two different elements as ‘ring member’ atoms. They may be organic or inorganic, with carbon atoms along with one or more atoms of elements such as sulphur, oxygen, nitrogen within the ring structure. Consequently, the non-carbon elements that replace the carbon atoms in a chemical structure are commonly termed as heteroatoms [1]. Biologically, pyrroles tend to construct the key structure of porphyrin rings, which act as an active moiety in chlorophyll, heme, vitamin B12 or bile pigments [2]. It is a colorless volatile liquid that darkens immediately upon exposure to air and polymerizes in light. It has low basicity than amines and other aromatic compounds due to the delocalization of the lone pair of electrons of the nitrogen atom in the aromatic ring. Pyrrole and its derivatives are recognized to inhibit reverse transcriptase (human immunodeficiency virus type 1 (HIV-1)) and cellular DNA polymerases protein kinases. They exhibit wide range of biological activities such as antibacterial, anti-fungal, anti-viral, anti-inflammatory [3], anticancer and antioxidant activity [4] (Figure 1).
Literature Review
Synthetic strategies
Synthesis of pyrrole derivatives and their utilization for the preparation of pyrolo-pyrimidine scaffold includes various synthetic approaches, one of which has been discussed below.
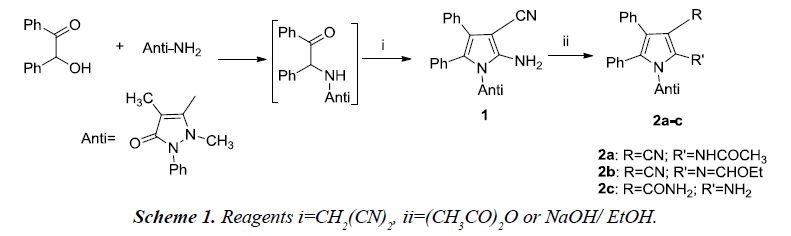
The reaction was initiated using benzoin, antipyrine amine and malononitrile which afforded pyrrole derivative 1 which was further utilized for the preparation of pyrrole derivatives using appropriate reagents and reaction conditions. The pyrrole derivatives 2a-c were further converted to the corresponding pyrrole[2,3-d] pyrimidines [5].
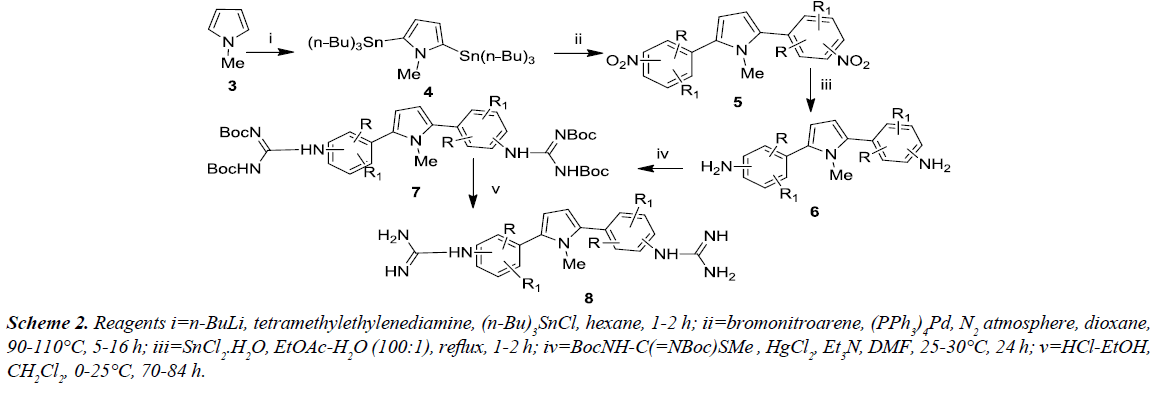
An alternative approach for the synthesis of various 2,5-bis (guanidinoaryl)-1-methyl-1H-pyrroles was proceeded from 1-methyl-1H-pyrrole via multi-step procedure and were evaluated for antifungal property. The 1-methyl-1H-pyrrole [6], was reacted with tri-n-butyltin chloride along with n-butyl lithium and N,N,N’,N’-tetramethylethylenediamine under reflux afforded 2,5-bis(tri-n-butylstannyl)-1-methyl-1Hpyrrole. Further, this compound undergoes ‘stille’ coupling [7] with substituted bromonitroarene in the presence of tetrakis ((triphenylphosphine) palladium) afforded the corresponding nitro intermediates which were then reduced with tin(II) chloride dihydrate to produce the amino compounds 6. Compound 6 was reacted with Boc-protected S-methylthiourea in the presence of mercury (II) chloride in order to yield the Boc-protected diguanidino analogues 7. Compound 7 was deprotected using ethanolic-HCl in dichloromethane at 0oC to afford the corresponding 2,5-bis(guanidinoaryl)-1-methyl-1Hpyrrole derivatives 8 in good to excellent yield as illustrated in Schemes 1 and 2.
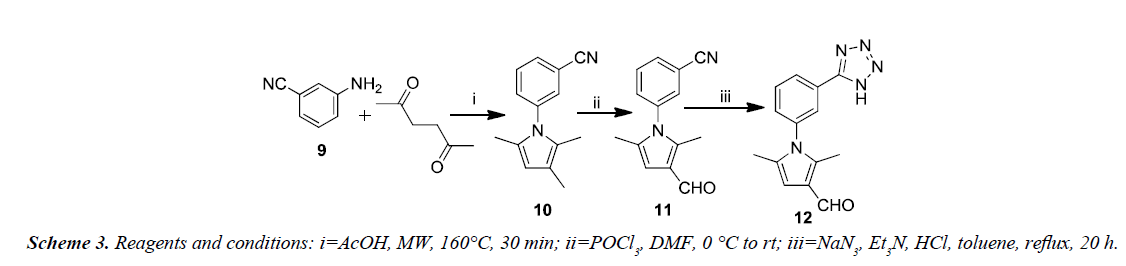
Xiao-Yang et al. synthesized a new series of 2,5-dimethyl-3- (5-(N-phenylrhodaninyl)methylene)-N-(3-(1H-tetrazol-5-yl) phenyl)pyrrole derivatives with improved anti-HIV-1 activity on the basis of structure of binding site HIV-1 gp41 as shown in Scheme 3. The Paal-Knorr reaction was reinvestigated for the synthesis of 3-(2,5-dimethyl-1H-pyrrol-1-yl) benzonitrile 10 by condensing 3-aminobenzonitrile 9 with 2,5-hexanedione. An aldehyde group was introduced at the 3-position of the pyrrole-ring in order to obtain the 3-(3-formyl-2,5-dimethyl- 1H-pyrrol-1-yl) benzonitrile 11. The cyano group present in 11 was converted into tetrazolyl by treating with sodium azide and triethylamine hydrochloride in reflux using toluene as solvent to afford the 2,5-dimethyl-N-(3-(1H-tetrazol-5-yl)phenyl)pyrrole- 3-carbaldehyde 12.
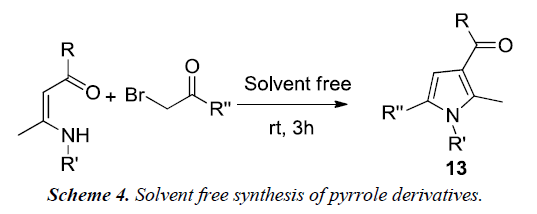
Yavari et al. reported a synthesis of 1,2,3,5-tetrasubstituted pyrroles 13 by simple mixing of enaminones and α-bromoketones at room temperature for 3h without the use of any solvent or catalyst as illustrated in Scheme 4 [8].
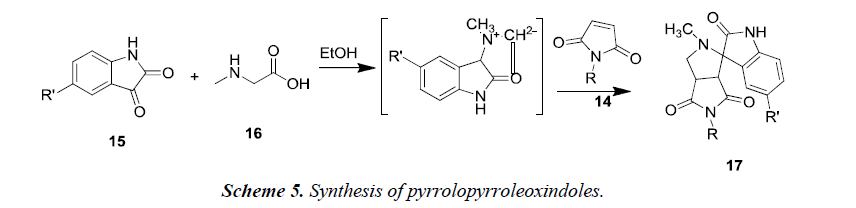
Another reaction of 1-aryl-1H-pyrrole-2,5-diones 14 was proceeded using unstabilized azomethineylides. This was generated in situ via decarboxylative condensation of isatin 15 and sarcosine 16, which finally afforded only a single product i.e. 4’-aryl-5’a,6’-dihydro-1’-methyl-spiro[3H-indole- 3,2’(1’H)-pyrrolo[3,4-c]pyrrole]-2,3’,5’(1H,2’aH,4’H)-triones 17 as described in Scheme 5. The synthesized compounds 17 possessed moderate anti-tumor properties against HCT116 (colon), MCF-7 (breast) and HEPG2 (liver) human tumor cell lines [7].
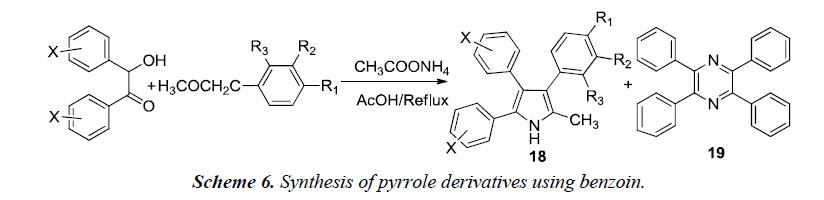
Goel et al. described the synthesis of 2-methyl-3,4,5-triphenyl pyrrole derivatives 18 which was carried out by refluxing a mixture of benzoin, benzyl methyl ketone and ammonium acetate in acetic acid as shown in Scheme 6. A minor byproduct 19 was formed during the course of reaction which was probably formed due to self-condensation of benzoin with ammonium acetate in presence of acetic acid in the presence of air. This byproduct was avoided by synthesizing 3,4,5-triphenyl-1H-pyrroles under nitrogen atmosphere using anhydrous ammonium acetate.
After the synthesis, compounds were evaluated for antihyperglycemic potential. The results suggested that unsubstituted-phenyl ring at positions 4 and 5 of the pyrrole lowers the elevated blood sugar levels while substitution at positions 3 or 4 of phenyl ring leads to either reduction or a complete loss of antihyperglycemic activity. A compound substituted with trifluoromethyl group at the 3rd position of the aryl ring exhibited good antidiabetic activity [9].
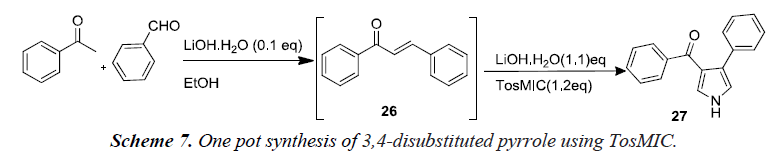
Nair et al. reported that the generation of the TosMIC anion by lithium hydroxide in ethanol followed by the reaction of anion with α,β-unsaturated carbonyl compounds. Their efforts resulted in a simple one-pot protocol both for chalcone 26 formations from aromatic aldehydes and enolisable ketones and the subsequent cyclocondensation to form pyrrole 27 as illustrated in Scheme 7 [10].
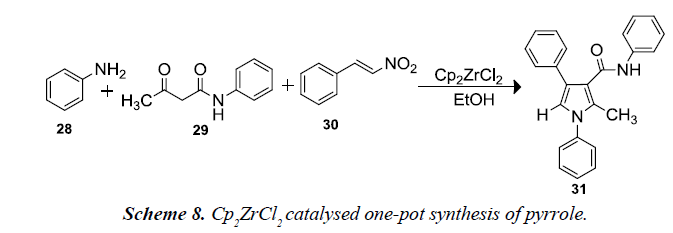
Nair and co-workers also developed a one-pot, three-component assembly between aniline 28, 3-oxo-N-phenylbutanamide 29 and β-nitrostyrene 30 was envisaged to afford a multi-substituted pyrrole. Reacting these substrates, the reactions would proceed through the enamine formation from the amine and β-ketoamide and a subsequent Michael addition of the resulting enamine to afford the pyrroles. To provide an environmentally benign protocol, EtOH was employed as the reaction medium and relatively inexpensive transition metal salts zirconocene dichloride afforded excellent yields [11].
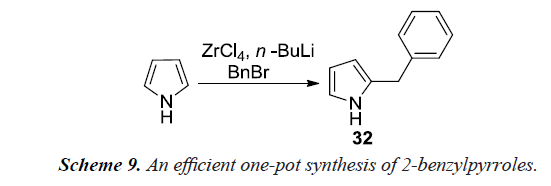
Nair et al. also reported a new methodology for the benzylation of pyrrole using benzyl bromide (Schemes 8 and 9) in a one-pot reaction by in-situ generation of di (1H-pyrrol-1-yl) zirconium (IV) chloride. A solution of pyrrole in dry tetrahydrofuran when treated with n-butyl lithium at -78°C under nitrogen atmosphere provides lithium pyrrole-1-ide. The solution of lithium pyrrole-1-ide on addition to zirconium (IV) chloride in dry tetrahydrofuran maintained at the same temperature would afford di(1H-pyrrol-1-yl)zirconium (IV) chloride. Addition of n-butyllithium to this suspension generates carbanion at C-2 position. The reaction mixture was subsequently allowed to attain room temperature and reacted with benzyl bromide to afford 2-benzyl pyrrole [12].
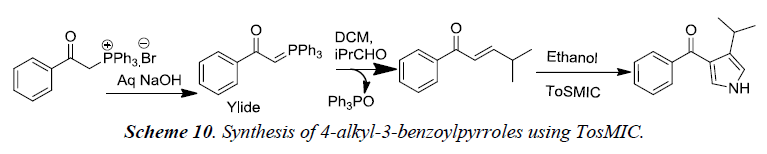
Our group recently developed a synthetic methodology for the synthesis of alkyl substituted pyrroles using tosylmethylisocyanide via Wittig approach. Detailed reaction sequence is illustrated in Scheme 10 [13].
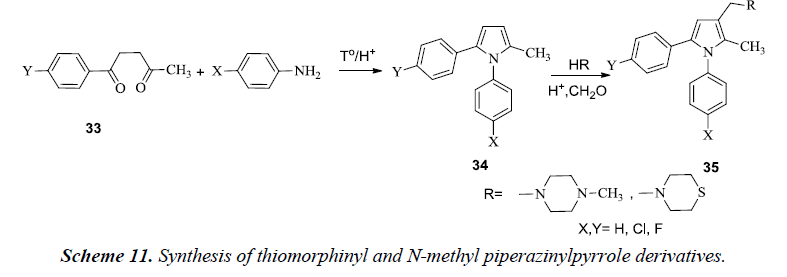
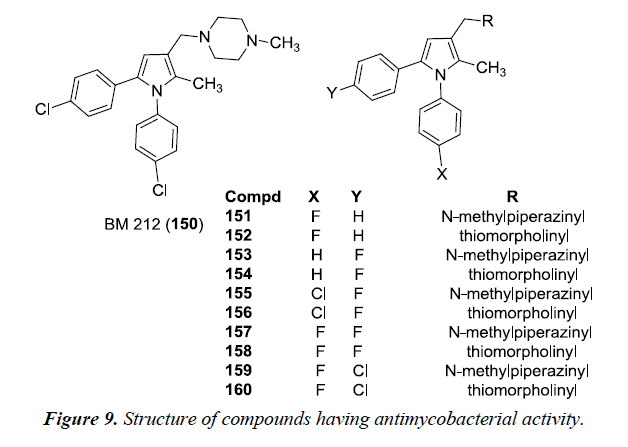
Biava et al. described the synthesis of pyrrole compounds 34 by alternatively introducing thiomorpholine and N-methyl piperazinyl at C-3 of the pyrrole and thereby substituting para position of the phenyl ring in N1 and /or C5 of the pyrrole ring with Cl /F atoms. The essential fundamentals of chemistry were involved in the synthesis of these compounds by allowing 1,4-diketones 33 to react with levulinic acid and chlorobenzene in the presence of AlCl3 as shown in Scheme 11 [14]. The compound 35 also exhibited good in vitro activity against Mycobacterium tuberculosis, various other species of Candida, viruses, gram-negative and gram-positive bacteria and pathogenic plant fungi.
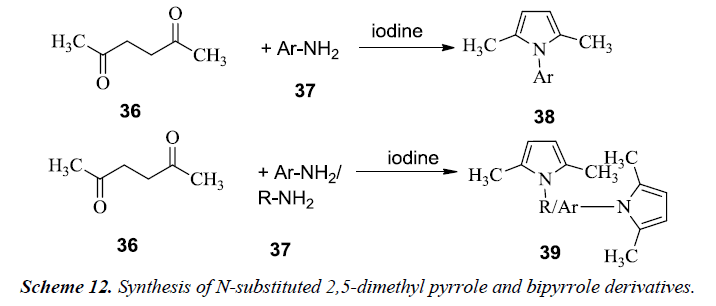
Patil et al. reported the synthesis of N-substituted 2,5-dimethyl pyrrole 38 and bipyrrole 39 by utilizing the most common method for pyrrole synthesis i.e. Paal-Knorr method. The reaction mixture consisted of hexane 2,5-dione 36 and amine solution 37 in THF along with iodine as shown in Scheme 12. The mixture was stirred at room temperature and finally dichloromethane was then added. Similarly, bipyrroles were synthesized in similar fashion [15].
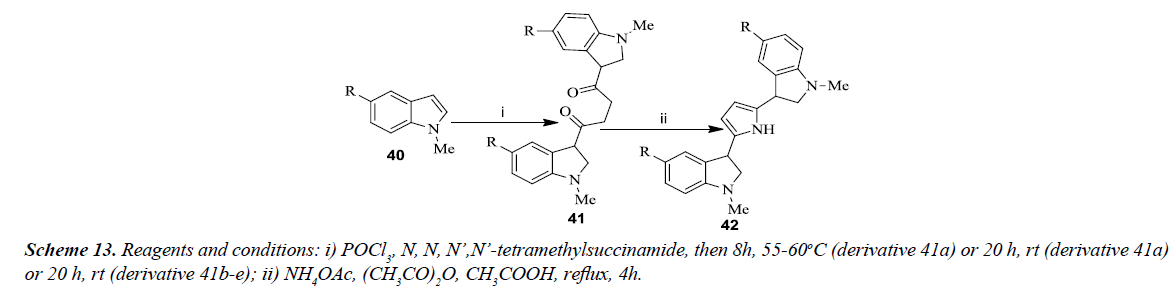
Carbone et al. developed the general synthesis of 2,5-bis(3’indolyl) pyrroles as illustrated in Scheme 13. The reaction involved N-methyl indoles 40 (a-e) which was converted into 1,4-butanediones 41(a-e) in appropriate amount via Vilsmeier-Haack reaction with phosphorus oxychloride and tetramethylsuccinamide. All the symmetrical 1,4-diketones 41 were converted into corresponding 2,5-bis(3’indolyl) pyrroles 42(a-e) using ammonium acetate, acetic anhydride in acetic acid under reflux as shown in Scheme 13. Purification of 1,4-diketones 41 (a-c) was carried out using flash chromatography whereas 1,4-diketones 41 (d-e) were found to be unstable for purification and were used as such for the next step [16].
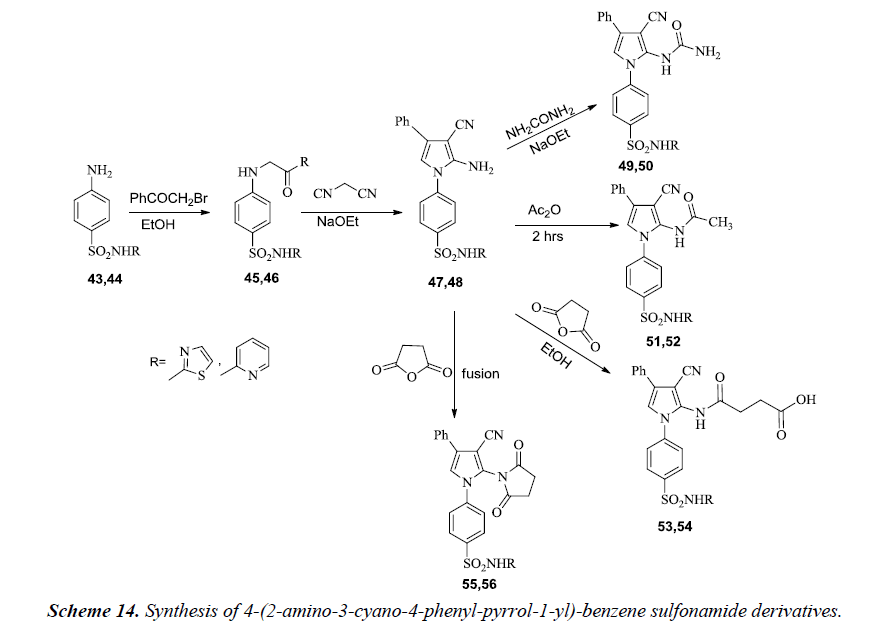
Synthesis of 4-(2-amino-3-cyano-4-phenyl-pyrrol-1-yl)- benzene sulfonamide derivatives 47 and 48 have been described by Ghorab et al. In the reaction sequence, sulfathiazole 43 and sulfapyridine 44 react with phenacyl bromide yields 4-(2-oxo- 2-phenyl-ethylamino)-benzene sulfonamide derivatives 45 and 46 which on further reaction with malonitrile in sodium ethoxide gives pyrrole derivatives 47 and 48. However, further interaction of 47 or 48 with urea in refluxing ethanol containing sodium ethoxide furnished the corresponding pyrrole derivatives 49 or 50 respectively. Acetylation of 47 and 48 using acetic anhydride gives respective monoacetylpyrrole derivatives 51 or 52. A separate reaction of 47 or 48 with succinic anhydride in ethanol gives the corresponding pyrrole derivatives 53 or 54 respectively. Fusion with succinic anhydride yielded the respective pyrrole derivatives 55 or 56 as illustrated in Scheme 14 [17].
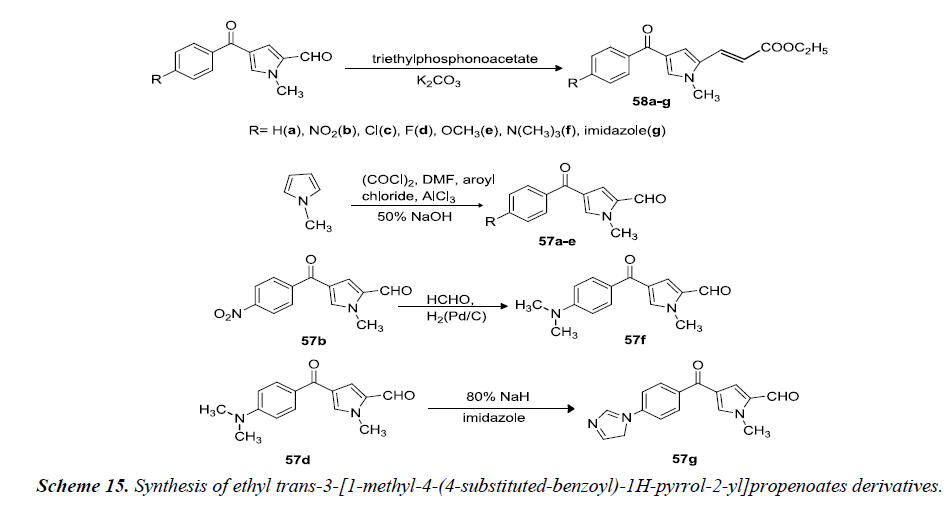
Massa et al. reported that ethyl trans-3-[1-methyl-4-(4- substituted-benzoyl)-1H-pyrrol-2-yl]propenoates 58(ag) were prepared by reacting 1-methyl-4-(4-substitutedbenzoyl)- 1H-pyrrole-2-carboxaldehydes 57(a-g) with triethylphosphonoacetate in absolute ethanol containing anhydrous potassium carbonate. Aldehydes 57(a-e) were obtained via one-pot Vilsmeier-Haack and Friedel-Crafts sequential reactions starting from 1-methylpyrrole in DMF with addition of oxalylchloride, aluminum trichloride and the appropriate aroylchloride as shown in Scheme 15. Reductive methylation of 57b gave aldehyde 57f. 57d was reacted with imidazole in the presence of sodium hydride afforded the resulting pyrrole derivative 57g as shown in Scheme 15 [18].
Huang et al. and co-workers made an attempt to synthesize substituted pyrroles 59 from aryl acetaldehydes and alkyl amines using copper-catalyzed aerobic conditions as described in Scheme 16. This method provides an alternative approach for mild activation of molecular oxygen under copper catalysis. The optimal conditions for the cyclization process utilizes amine, aldehyde and Cu(OTf)2 in DMF at 40°C under air for 24 h [19].
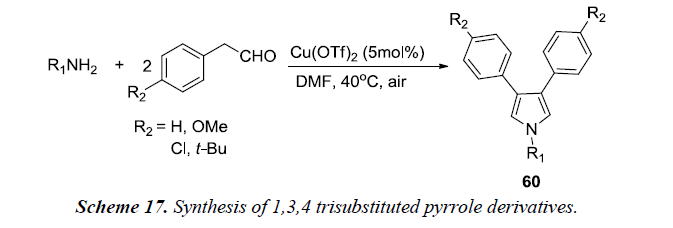
1,3,4 trisubstituted pyrroles 60 (Scheme 17) were also synthesized by reacting aliphatic amines with phenylacetaldehyde with conditions being the same as described in Scheme 16. Aromatic ethylamines afforded good yields of pyrrole derivatives.’
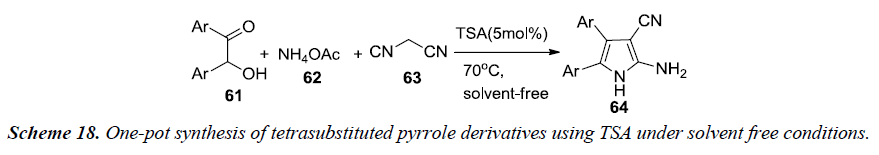
Farahi et al. and co-workers have reported one-pot synthesis of tetrasubstituted pyrrole 64 which involves a three-component reaction between α-hydroxyketones 61, malononitrile 63 and ammonium acetate 62 using tungstate sulfuric acid (TSA) under solvent free conditions at 70°C in 75%-90% yield as illustrated in Scheme 18. TSA is a heterogenous catalyst alternative to sulfuric acid, synthesized by a reaction of anhydrous sodium tungstate with chlorosulfonic acid [20].
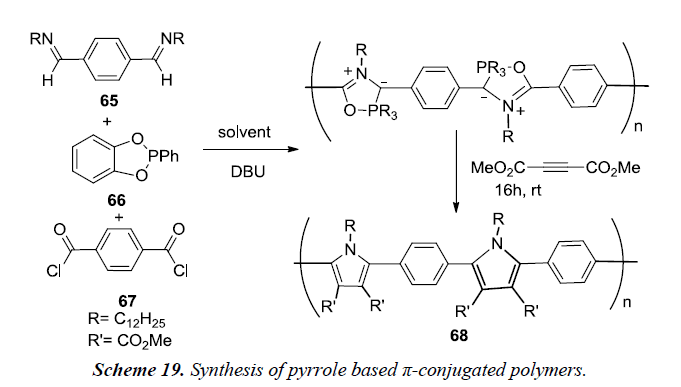
Kayser et al. and co-workers have described an efficient metal free, multi-component reaction assembly for synthesis of pyrrole based π-conjugated polymers. This reaction involves the coupling of imines 65, (catechyl)PPh 66 and acid chlorides 67 to produce phospha-Munchone containing polymers which can be further converted to polypyrrole via cycloaddition as described in Scheme 19. The coupling of imines, acid chloride, (catechyl) PPh and DBU as a base followed by addition of alkyne using dichloromethane as reaction medium at temperature 45-55oC is reported to afford pyrroles 68 in good yields but is not sufficient to generate high molecular weight polymers [21].
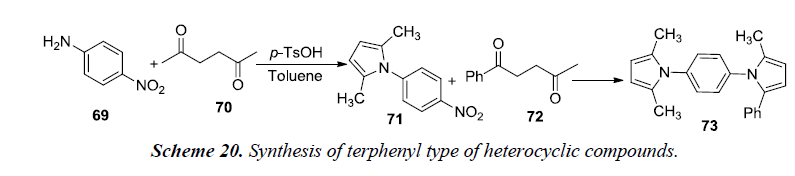
A recent synthetic strategy has been developed by Kim et al. for the terphenyl type of heterocyclic compounds using Paal-Knorr condensation. Reaction utilizes reduction of indium which tends to trigger intermolecular heterocyclization. The reaction was initiated by using nitroaniline with 1,4-dicarbonyls that resulted in the formation of two distinct pyrrole moiety-containing arenes 73. Firstly, Paal-knorr reaction was carried out using nitroaniline 69 with 2,5-hexanedione 70 in the presence of catalytic amount of p-TsOH.H2O using toluene as a solvent for 1.5 hrs under reflux conditions. Secondly, the isolated intermediate, 2,5-dimethyl-1-(3-nitrophenyl)-1H-pyrrole 71 was further reacted with 1-phenyl-1,4-pentanedione 72 in the presence of indium and acetic acid in toluene under reflux for 4 hrs. The desired product, 2,5-dimethyl-1-(4-(2-methyl-5- phenyl-1H-pyrrol-1-yl)phenyl)-1H-pyrrole 73 was afforded in 92% yield as shown in Scheme 20 [22].
Aghapoor et al. developed a proficient solvent-free method by utilizing Paal-knorr pyrrole cyclocondensation synthesis which was catalyzed by L-tryptophan. In reaction sequence, primary aromatic amine, hexane-2,5-dione and L-tryptophan were stirred for 1 hr at 70oC. The catalyst was recovered using ethanol by subsequent filteration and was further reused for the next reaction without the loss of activity. The reaction afforded excellent yield by using L-tryptophan in catalytic amounts [23].
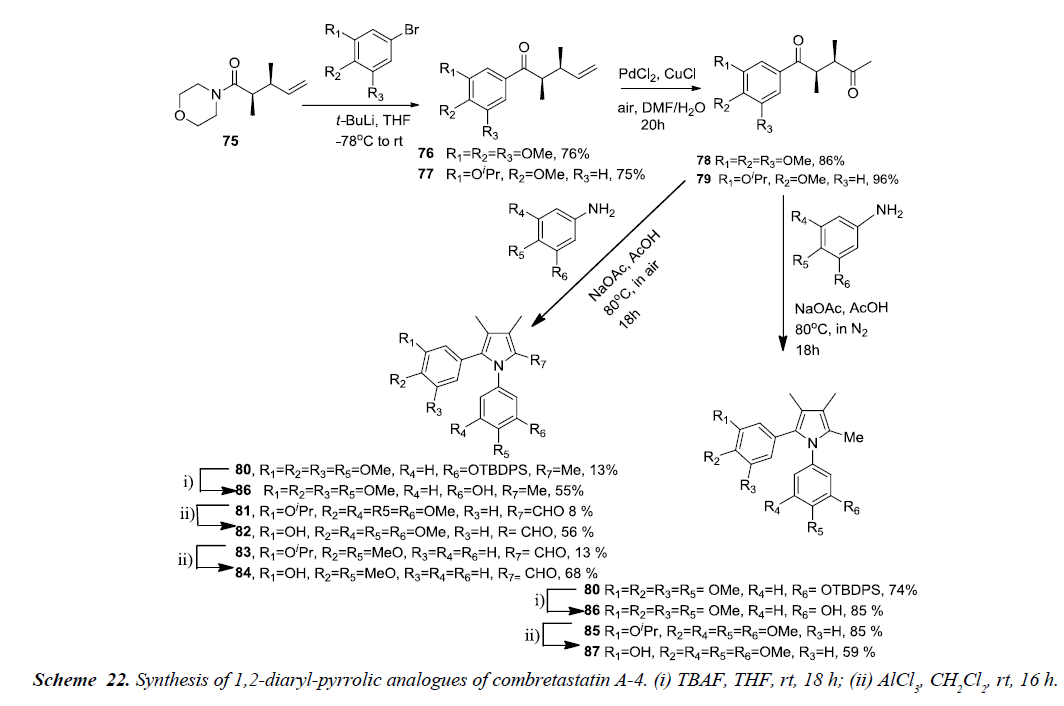
Pyrrole analogues of Combretastatins A-4 i.e. 1,2-diaryl and 2,3-diaryl-1H-pyrrole were synthesized and evaluated for antiproliferative activity. The desired analogues were prepared using Paal-Knorr pyrrole synthesis along with an acyl-claisen rearrangement approach. The acyl-claisen rearrangement of acid chlorides with (E)-allylicmorpholines is highly distereoselective which results in 2,3-syn-disubstituted amides. 2,3-syn arrangement of substituents in 1,4-dicarbonyl compound is the preferred rearrangement for heterocycle formation in the Paal- Knorr synthesis.
The 2,3-syn-dimethyl morpholine amide 75 was substituted using lithiated arenes and afforded ketones substituted with 3,4,5-trimethoxy 76 or 3-isopropyl-4-methoxy phenyl groups 77 respectively. Wacker-Tsuji oxidation of 76 and 77 was carried out using PdCl2 and CuCl, acting as catalyst, in DMF-water to give diketones 78 and 79 in excellent yields. The diketones 78 and 79 was further reacted with 3-TBDPSO-4-methoxyaniline and 3,4,5-trimethoxyaniline under nitrogen atmosphere to produce fully substituted methyl pyrroles 80 and 85 in 74% and 85%, of yield respectively. This reveals that the removal of oxygen minimizes various side products and contributes to an increase in pyrrole formation. Finally, TBDPS group in 80 was removed using TBAF while removal of isopropyl group in 85 was done by using AlCl3 thereby, affording the pyrrole derivatives of CA-4, 86 and 87 in 85% and 59%, respectively as shown in Schemes 21 and 22 [24].
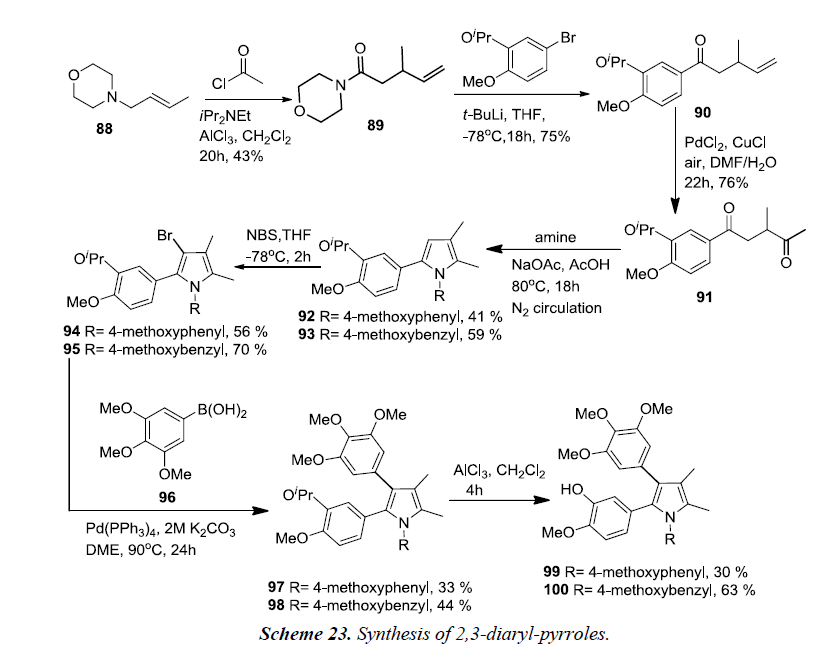
The synthesis of 2,3-diaryl-pyrroles started from acyl-Claisenrearrangement of crotylmorpholine 88 and acetyl chloride, giving morpholine amide 89 in 43% yield. Amide 89 was reacted with the lithiate of 3-isopropyl-4-methoxy bromobenzene which produced ketone 90 in good yield. Alkene oxidation of 90 using the Wacker-Tsuji oxidation resulted diketone 91 in 75% yield. Condensation of diketone 91 with 4-methoxyaniline and 4-methoxybenzylamine afforded pyrroles 92 and 93 in 41% and 59% yields, respectively. Pyrrole formation was again performed under a nitrogen atmosphere in order to prevent oxidation at the 5-methyl group. Bromination of pyrroles 92 and 93 using NBS in THF at -78°C afforded 3-bromo pyrroles 94 and 95, and no poly-brominated products were observed. Suzuki coupling reaction was initiated between 3-bromopyrroles 94 and 95 and 3,4,5-trimethoxyphenylboronic acid 96 thereby affording the desired arylated pyrroles 97 and 98. At the end, isopropyl protecting groups of 97 and 98 were removed using AlCl3 which produced the desired pyrrolic derivatives of CA-4 99 and 100 respectively.
After the synthesis of pyrrole analogues of combretastatin A-4, these were investigated for their anti-proliferative activity against two cancer cell lines, K562 and MDA-MB-231. The results revealed that 2,3-diaryl pyrroles were more active than 1,2-diaryl pyrroles. The most active compound 100 was found to possess IC50 values of 0.21 μM and 0.07 μM against K-562 and MDA-MB-231 cell lines respectively. All these compounds were less active than combretastatin itself. Combretastatin was found to be twice as active as compound 100 against the MDAMB- 231 cell line.
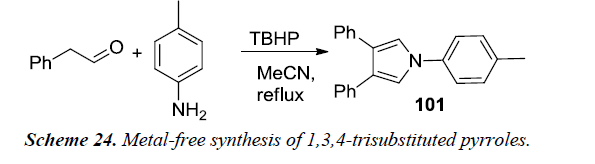
Gao et al. reported first metal-free synthesis of 1,3,4-trisubstituted pyrroles 101 via cascade reaction between acetaldehydes and primary amines. 1,3,4-trisubstituted pyrrole was obtained in 78% yield when phenylacetaldehyde and primary substituted amine were reacted using TBHP as oxidant and MeCN as reaction medium under reflux as illustrated in Schemes 23 and 24 [25].
Biological Importance of Pyrrole and Pyrrole Containing Analogs
Pyrrole is an important scaffold possessing diverse number of biological activities. This skeleton is found in wide range of pharmacologically active compounds, being incorporated either as a substituent or with various substitutions on the ring itself. Some of the drugs containing pyrrole moiety are already available in market while rest are under clinical trials (Figure 2).
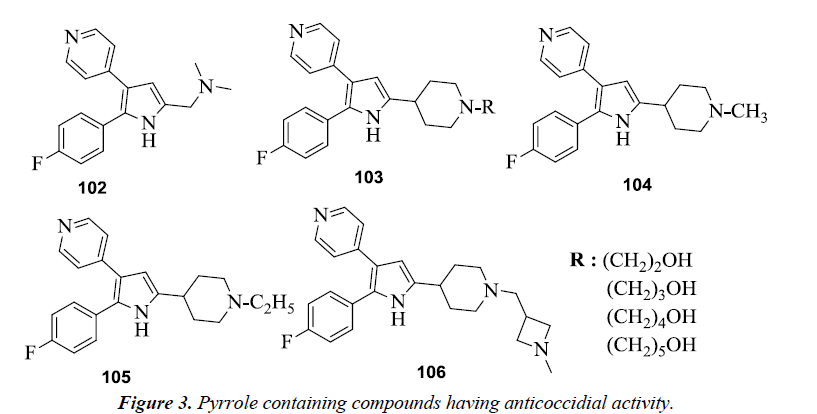
Various diarylpyrrole derivatives were evaluated for the anticoccidial activity by both in vitro and in vivo assays and was observed that dimethyl amine substituted derivative 102 was found to be most potent inhibitor of Eimeria tenella (Et) PKG (cGMP-dependent protein kinase) [26]. On conducting further studies on diarylpyrrole derivatives it was seen that the ω-hydroxylated derivatives 103 were more potent inhibitors as compared to their alkyl analogs [27]. N-substituted derivatives of 2,3-diarylpyrrole were also evaluated against commercially important strains of Eimeria in chickens, among which 5-(N-methyl, N-ethyl and N-methyl azetidine methyl) piperidyl derivatives 104-106 were found to be most potent with a broad spectrum of activity (Figure 3).
Anti-inflammatory activity
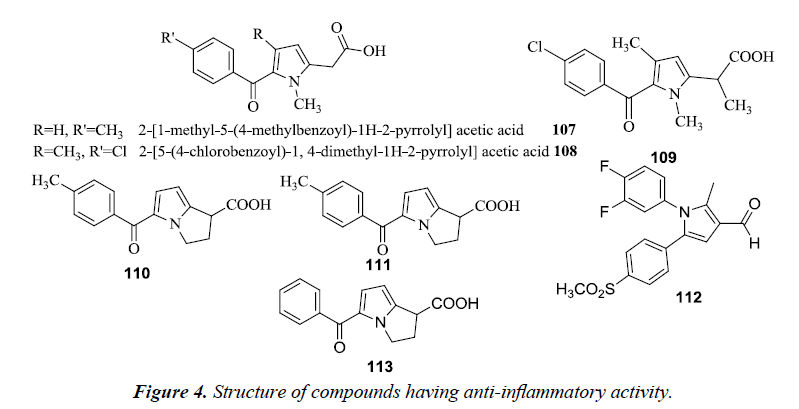
Tolmetin 107 and Zomepirac 108 are two well-known pyrrole acetic acid derivatives utilized for the treatment of rheumatoid arthritis and pain. Carson and Wong reported that methylation of acetic acid chain 109 in the Zomiperac markedly increases the anti-inflammatory potency which was measured by performing the rat paw kaolin edema assay. Benzoylpyrrolopyrrole carboxylic acid series of compounds also possessed high anti-inflammatory and analgesic activity. It was observed that p-methoxy derivative of 5-benzoyl-1,2-dihydro-3H-pyrrolo- [1,2-α] pyrrole-1-carboxylic acid and 4-vinylbenzoyl derivatives were found to be most potent (110 and 111) [28]. Another novel class of pyrrole derivatives were synthesized which consisted of a small appendage fragment (carbaldehyde, oxime, nitrile) on the central core. The compound 112 was found most effective in vivo and exhibited a significant profile when compared to the already marketed reference compounds. This compound was more efficient and potent in inducing a percentage of writhes reduction in comparison to the marketed drug, celecoxib. Another compound 113 RS-37619, (±) 5-benzoyl-1,2-dihydro- 3H-pyrrolo[1,2-a] pyrrole-1-carboxylic acid has been also reported to possess potent analgesic and anti-inflammatory activity. This agent increased the pain threshold to 30 times in yeast inflamed rat paws when compared to naproxen. However, it did not alter the pain threshold in the normal non-inflamed state, similar to NSAID’s. It does not have morphine-like activity and any addiction potential. It revealed mild CNS and cardiovascular activity at doses more than that required for the analgesic and anti-inflammatory activity [29] (Figure 4).
Antipsychotic and anticonvulsant activity
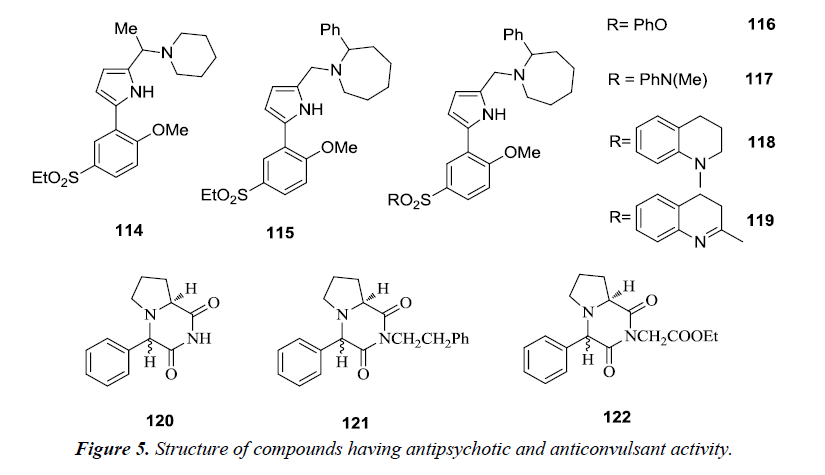
2,5-disubstituted-1H-pyrrole derivatives were synthesized by modifiying basic side chain with the introduction of piperidine 114 and 2-phenylazacycloheptane 115. The synthesized compounds were found to exhibit D3 antagonist activity with 30-fold selectivity for the D3 receptor over the D2 receptor [30]. However, on performing further modifications of the ethyl sulfone substituent to either phenyl sulfonate 116 or sulfonamides 117, 118 and 119 high affinities and selectivity for the dopamine D3 receptor was observed over the D2 receptor. Therefore, these compounds are considered as valuable pharmacological tools for describing the role of dopamine D3 receptor in the central nervous system [31].
A number of novel pyrrole [1,2-a] pyrazine compounds revealed promising seizure protection in the maximal electroshock seizure (MES), subcutaneous metrazol seizure (scMET) and pilocarpine induced status prevention (PISP) tests in epileptic models comparable to the reference anticonvulsant drugs. Among the synthesized pyrrole [1,2-a] pyrazine derivatives it was observed that the 4S,8aS diastereomer 120, its ethoxycarbonylmethyl 121 and 2-phenylethyl 122 derivatives were found to possess potent anticonvulsant activity (Figure 5).
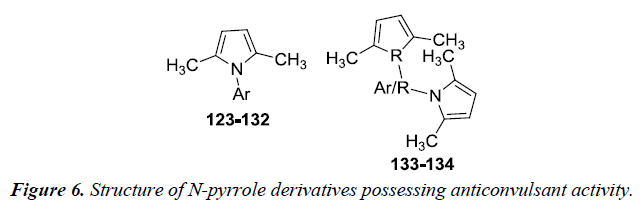
Patil et al. revealed the synthesis of N-pyrrole derivatives 123- 134 with aromatic amines in fairly good yields as compared to aliphatic amines and aliphatic-aromatic amines. All these compounds were investigated for anticonvulsant activity at NIH after intra peritoneal administration to mice by maximal electroshock, subcutaneous metrazol (scMET) induced seizure method and neurotoxicity test at 30, 100, 30 mg/kg dose levels. Compounds 129 and 132 were found to potent against anti-convulsant activity and therefore can be used to develop lead anti-epileptic compounds. Further substitution on pyrrole nucleus can lead to more potent compounds [15] (Figure 6) (Table 1).
| Com. No. | R\Ar | Anticonvulsant activity (mg/kg) MES | Anticonvulsant activity (mg/kg) scMET | Toxicity screening (mg/kg) 0.5 | Toxicity screening (mg/kg) 4.0 |
|---|---|---|---|---|---|
| 123. | Phenyl | NA | NA | 100(12.5%), 300(50%) | - |
| 124. | 4-Nitrophenyl | NA | NA | 100(12.5%) | - |
| 125. | 4-Bromophenyl | NA | NA | 100(12.5%), 300(50%) | - |
| 126. | 4- Flurophenyl | NA | NA | 300(75%) | 300 (50%) |
| 127. | 4-Methoxyphenyl | NA | NA | - | - |
| 128. | 4-Methylphenyl | NA | NA | 300(50%) | - |
| 129. | 4-Iodophenyl | NA | NA | 30(25%), 100(25%) | - |
| 130. | 1-Naphthalenyl | NA | NA | 100(12.5%) | - |
| 131. | 4-Chlorophenyl | NA | NA | - | - |
| 132. | 2-Hydroxyphenyl | NA | NA | - | - |
| 133. | Ethylene | NA | NA | - | - |
| 134. | o-phenylene | NA | NA | - | - |
Table 1: The primary of MES, scMET and toxicity tests were performed by i.p. injection in mice at doses 30,100, 300 mg/kg. NA indicates absence of activity at the maximum dose administered (300 mg/kg).
Antifungal and antibacterial activity
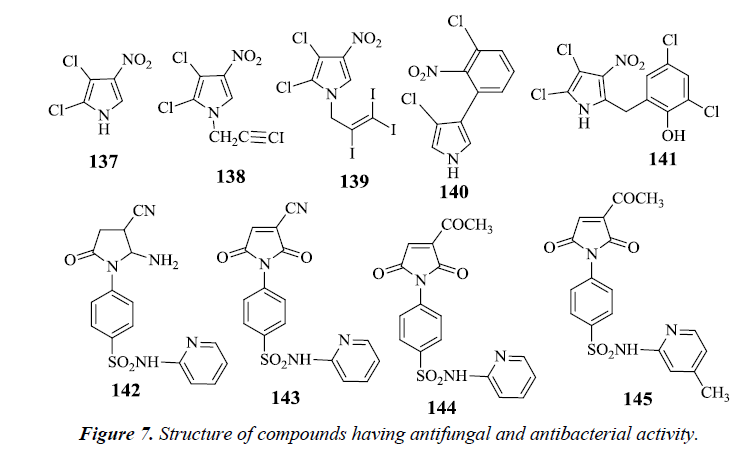
Pyrrolomycins are antibiotics obtained naturally, contains nitropyrrole nucleus which is chemically stable and reactive. On carrying N-alkylation of pyrrolomycin A 137 antimicrobial activity against Candida albicans and Trichophyton mentagophytes strains was found to be reduced, but N-iodoalkylation resulted in an augmentation of the potency. However, it was seen that N-(iodopropargyl) 138 and N-(triiodoallyl) pyrrolomycin A 139 derivatives had better signs of antifungal activity than pyrrolomycin A and clotrinazole [32].
Pyrrolnitrin 140 and Pyrrolomycin B 141 are synthetically obtained pyrroles possessing anti-fungal activity. These pyrroles had bulky substituents on the pyrrole ring which were responsible for the decrease in activity whereas nitro group was found to have a potentiating effect. It was finally stated that the antifungal activity of nitropyrrole was mainly due to electronegative nature of nitro group. Sulfonamides also have power over a variety of biological activities. Antifungal activity tends to increase by the introduction of a heterocyclic sulfonamide in the pyrrole ring. However, absence of substituent on sulfonamide results in partial or complete reduction in antifungal activity. It was observed that compounds 142, 143, 144 and 145 exhibited a remarkable antifungal activity compared with the standard fungicide mycostatine [33] (Figure 7).
Antiviral activity
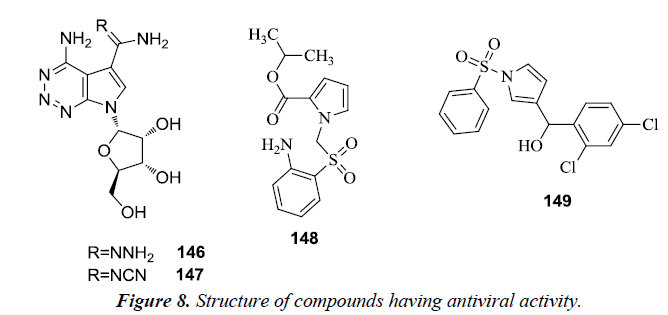
Michael et al. reported the synthesis of numerous heterocyclic analogues of antibiotic toyocamycin and tricyclic nucleoside triciribine. It was revealed that 4-amino-1-(β-D-ribofuranosyl) pyrrole [2,3-d] [1,2,3] triazine-5-carboxamidrazone 146 and 4-amino-1-(β-D-ribofuranosyl) pyrrole [2,3-d] [1,2,3] triazine- 5-carboxamidoxime 147 were active against Human Cytomegalo Virus (HCMV) and Herpes Simplex Virus type I (HSV-I) but their activity was poor and different from cytotoxicity [34]. However, a novel class of non-nucleoside HIV-1 reverse transcriptase inhibitors was recognized as 1-arylsulfonyl-1Hpyrroles because of the presence of a specific diarylcarbinol moiety which tend to correlate well with anti HIV-1 activity. From the series of synthesized derivatives, pyrrolyl aryl sulfones (PAS) 148 and 1-benzenesulfonyl-3-(α-hydroxy-2,4- dichlorobenzyl) pyrrole 149 possessed the highest activity on evaluating in MT-4 cells infected with HIV-1 [35] (Figure 8).
Antimycobacterial activity
Tuberculosis is the most common disease and caused by strains of Mycobacterium tuberculosis. However, it has been observed that in immunocompromised patients, tubercular pathology is always accompanied with mycotic infections caused by Candida albicans, Candida species, Cryptococcus neoformans. A novel pyrrole derivative BM212 150 was found to have good in vitro activity against mycobacteria and candidae. It was suggested that on introduction of N-methyl piperazine or thiomorpholine moiety at C3 of pyrrole and a phenyl ring at N1 and C5 of the pyrrole ring substituted or unsubstituted with a chlorine atom were essential for antimycotic activity. In this study, compound 152, 154, 156, 158 and 160 exhibited remarkable in vitro activity against Mycobacterium tuberculosis but were found to be slightly toxic. Compound 152 and 155 was found to be most active and compound 150 had a good Protection Index (PI). MIC value of 150 was found to be comparable with that of rifampin but is higher than that of BM212 [14] (Figure 9).
Anticancer activity
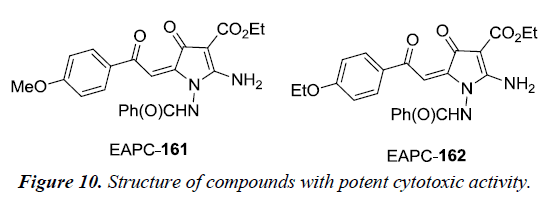
Boichuk et al. has reported that ethyl-2-amino-pyrrole-3- carboxylate (EPAC) 161 is a potent cytotoxic against multiple soft tissue cancer cell lines in vitro. The cytotoxic activity of EPAC was evaluated on different cancer cell lines using microtubule cell proliferation assay. The different cell lines were leiomyosarcoma SK-LMS-1, rhabdomyosarcoma RD, gastrointestinal stromal tumor GIST-T1, A-673 Ewing’s sarcoma and U-2 OS osteosarcoma. It was observed that compounds EAPC 161 and 162 inhibited the cancer cell proliferation in vitro by the inhibition of tubulin polymerization and induction of G2/M cell-cycle arrest which thereby results in accumulation of tumor cells in M phase [36] (Figure 10).
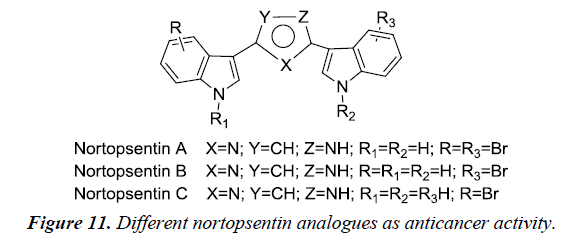
Bis-indole alkaloids are recognized for possesses potent biological activity such as anti-inflammatory, antimicrobial, antiviral and antitumor. Chemically, consists of two indole unit bound to a spacer through their 3rd position. Nortopsentins is an bis-indolyl alkaloid with a potent cytotoxicity against P388 cancer cell lines (IC50- 4.5-20.7μM) whereas their N-methylated derivatives resulted in significant increase in cytotoxicity against P388 (IC50-0.8-2.1μM) when compared to the parent compound [16] (Figure 11).
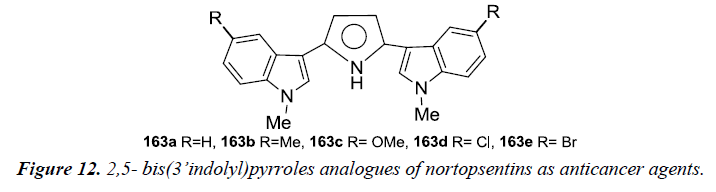
Nortopsentins- 2,5- bis (3’indolyl) pyrroles analogues were synthesized and evaluated for an in vitro cytotoxicity assay using human tumor cell lines and ex-vivo clonogenic assay using human tumor xenograft. Compound 163a and 163b were found to be potent as they affected the concentration-dependent inhibition of tumor cell growth with mean IC50 values of 1.54 μM and 0.67 μM. Selective activity testing was further carried out for these two compounds in which 163a was found to be active against two out of three cell lines of bladder cancer (BXF 1218L, BXF 1352L), LXFL 1121L for lung cancer, PAXF PANC-1 of pancreatic cancer, SXF SAOS-2 (sarcoma), UXF 1138L and two out of three melanoma cell lines (MEXF 1341L; MEXF 276L). However, less sensitivity was observed towards cell lines as colon (HCT-116, HT-29), lung (LXFA 289L), ovarian (OVXF 899L), prostate (DU145), renal cancer (RXF 393NL, RXF 486L). Whereas compound 163b was found to be selectively active towards cell lines derived from bladder cancer (BXF 1218L), melanoma (MEXF 1341L, MEXF 276L), prostate cancer (PRXF PC3M) and sarcoma (SXF SAOS-2). These were evaluated for an ex-vivo clonogenic assay which revealed concentration-dependent activity with mean IC50 values of 5.69 μM and 7.25 μM respectively (Figure 12).
A novel series of 2-substituted-3-cyano-4-phenylpyrrole derivatives bearing either sulfathiazole or sulfapyridine ring were synthesized. These compounds were evaluated for their in vitro cytotoxicity against liver and breast cancer cell line (HEPG2 and MCF7) and were compared with the reference drug doxorubicin. However they were found to act by mechanism known as tyrosine kinase inhibitors. Moreover, some of the compounds were evaluated as radio sensitizing agents in order to prove their ability to enhance the cell killing effect of γ-radiation. Pyrrole-derivatives 164 and 165 had significant activity (IC50=3.49 and 4.6 μM)) and much more potent than doxorubicin. Compound 166 (IC50=3.49 μM), 167 (IC50=3.75 μM) and 168 (IC50=3.49 μM) were the most active compounds amongst the synthesized pyrrole series. The cytotoxic activity on both the cell lines bearing either sulfathiazole or sulfapyridine moiety was not significantly affected. Thus, sulfonamides might be equipotent on both the cell lines. All the chemotherapeutic agents have the ability to sensitize cancer cells to the lethal effects of ionizing radiation. The ability of some of the most potent compounds as a result from in vitro cytotoxic activity 166, 167, 168 to enhance the cell killing effect of γ-irradiation was studied. It was observed that the effect of radiation alone was not significant when compared to control group which contains the cell lines without radiation or drug. On the other hand, the surviving fraction of the tested compounds decreased significantly from the control after being subjected to radiation which indicates the synergism due to combining drugs with radiation [17