Research Article - Journal of Cardiovascular Medicine and Therapeutics (2017) Volume 1, Issue 3
Ratio E/E' and culprit artery predict ventricular remodeling after STEMI.
Muratalla-González R1, Zaldivar-Fujigaki JL2, Van Der Harst P3, Aceves Chimal JL4, Morales-Portano JD1, García-García JF1, Merino-Rajme JA1*
1Department of Cardiology, National Medical Center, Institute for Social Security and Services for State Workers (ISSSTE), Mexico City, Mexico
2Department of Clinical Research, National Medical Center, Institute for Social Security and Services for State Workers (ISSSTE), Mexico City, Mexico
3Department of Cardiology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands
4Department of Cardiovascular Surgery, National Medical Center, Institute for Social Security and Services for State Workers (ISSSTE), Mexico City, Mexico
- *Corresponding Author:
- Dr. Merino-Rajme JA
General Director Department of Cardiology National Medical Center Institute for Social Security and Services for State Workers (ISSSTE) C.P. 03100, Mexico City Mexico
Tel: 52 55 5200 5003
E-mail: alfredo.merino@issste.gob.mx
Accepted on November 20, 2017
Citation: Muratalla-González R, Zaldivar-Fujigaki JL, Van Der Harst P, et al. Ratio E/E' and culprit artery predict ventricular remodeling after STEMI. J Cardiovasc Med Ther. 2017;1(2):24-28.
Abstract
Background: ST-Elevation Myocardial Infarction (STEMI) is one of the most severe manifestations of atherosclerosis; prognosis is determined by remaining left ventricular function and the importance of systolic function has been well established. The aim of this study was to determine the E/E’ ratio, to predict the adverse ventricular remodeling (VR) in patients presenting with STEMI. Methods: From March 2011 to January 2012 we prospectively enrolled patients presenting with STEMI in the admission unit. Echocardiography was performed at presentation and 6 months after STEMI. VR was defined as the increase ≥ 20% of the left ventricular end diastolic volume. Uni and multivariate linear regression analyses were conducted. Results: Sixty-six STEMI patients (60.8 ± 10.3 years, 81% male) were recruited. In 56% the culprit artery was left anterior descending (LAD), right coronary artery in 39% and circumflex in 5%. VR was predicted by LAD as culprit artery (92%, p<0.001, rho 0.528), Septal E’ Velocity (p<0.001, rho 0.370) and the ratio E/E’ (p<0.001, -0.663). Six months after the STEMI patients with VR had lower left ventricular ejection fraction, and higher end systolic and diastolic volume. ROC analysis suggested an optimal cutoff for a E/E' ratio of ≥ 12 (S:91%, E:77%) at baseline to predict adverse VR. Multivariate analysis showed that the ratio E/E’, septal E´ velocity, culprit artery, and Killip Kimbal classification were predictors of adverse VR after STEMI (rho 0.750). Conclusion: E/E´ ratio ≥ 12 and LAD as the culprit artery are important predictors for adverse ventricular remodeling and should be considered for early medical treatment.
Keywords
Ventricular remodeling, Ratio E/E’, Prognostic parameter, STEMI.
Introduction
ST-elevation myocardial infarction (STEMI) is one the most severe manifestation of coronary artery disease (CAD). CAD causes more than a third of deaths in developed countries annually [1-3]. According to the Mexican National Institute of Geography and Statistics (INEGI), heart disease is the most common disease in Mexico and CAD alone accounted for approximately 82,000 deaths in 2014 [4].
After STEMI and myocyte necrosis, the resulting increase in working load triggers a cascade of biochemical intracellular signaling processes. The modifications in the myocardium include dilatation of the ventricle, hypertrophy, and the formation of a scar [5]. This so called ventricular remodeling may continue for weeks to months after STEMI [6]. Echocardiography has become an essential image modality to follow changes in both systolic and diastolic function after STEMI [7]. Adverse ventricular remodeling (VR) has been defined as an increased ≥ 20% of the Left Ventricular End Diastolic Volume (LVEDV) at 6 months after STEMI [8,9]. Systolic dysfunction has been well established as an important predictor for long-term prognosis. Adverse ventricular remodeling after STEMI has been associated with an increased up to 3 times the risk of overall mortality in 5 years and in the presence of symptoms even up to 10 times [10]. Severe reduction of left ventricular function mandates additional treatment and device therapy to improve prognosis. In addition, adverse changes in diastolic function had been linked to worse outcome. Especially the restrictive left ventricular filling had been linked to early cardiac death after myocardial infarction [11].
The E/E’ ratio is a parameter of diastolic function and is based on the end pressure of left ventricle and left atrium. In the first hours of the infarction an increase in E wave of ventricular filling and a decrease of the mitral annulus wave E´ causes an increase of the E/E’ ratio. This ratio appears useful in the evaluation of atrial pressure as it is less dependent of the left ventricular preload and afterload compared with the conventional measures like the pattern of transmitral flow. An E/E' ratio>15 has been proposed as the optimal cut-off to predict outcomes in Caucasian populations [12].
The aim of this study was to determine the value of the E/E’ ratio presenting with STEMI to predict the adverse VR and to identify factors associated with the change in E/E’ ratio based after the STEMI.
Methods
Study design and participants
Prospective, single-center study conducted in the National Medical Center 20 de Noviembre ISSSTE, Mexico City, Mexico. This protocol was approved by the institutional Medical Ethical Committee and was in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients.
Patients aged ≥ 18 years presenting with STEMI at the National Medical Center 20 de Noviembre ISSSTE between March 2011 to January 2012 were recruited. Exclusion criteria included concomitant cardiovascular disease known to influence diastolic function (severe valvulopathy, cardiomyopathy or pericardial disease).
Study procedure
At presentation, cardiovascular risk factors were inventoried, Killip Kimbal classification (KK), Canadian Cardiovascular Society grading for angina pectoris (CCSGAP), and Forrester classification were scored. Echocardiography was performed within the first 7 days after admission. Follow-up reassessment, including echocardiography, was performed at 6 months.
Echocardiography
Two-dimensional and Doppler transthoracic echocardiography was performed with Tissue Doppler Image (TDI) and M mode in the left lateral position, using a Phillips IE33 (Stockholm, Sweden-Royal Phillips electronics) with a 3.5-MHz transducer. All measurements and evaluation were performed according to the guidelines of the American society of echocardiography [13]. In brief, left Ventricle Ejection Fraction (LVEF), Left Ventricular End Diastolic Diameter (LVEDD), Left Ventricular End Systolic Diameter (LVESD), peak transmitral filling velocities in early diastole (E) and late diastole (A) were obtained by pulse wave Doppler at the tip of the mitral leaflets from the apical four chamber view, TDI was performed to obtain systolic wave (S'), early diastolic wave (E') and late diastolic wave (A'), these were measured at the level of the mid-portion of the inter-ventricular septum and at the medial and lateral annuli of the mitral valve and left atrial volume index (LAVI) were determined. The E/E’ ratio was defined as the relation between the rapid filling stage of the left ventricle and the early diastolic velocity, <8 is considered a normal value [14]. Adverse VR was defined as an increase in the 20% of the LVEDV after 6 months of the STEMI.
Statistical analysis
Data are present as percentage and mean with standard deviation. Differences between subjects with and without VR were assessed by T student test. Associations between echocardiographic variables and clinical variables with VR were studied with univariate and multivariable linear regression. In order to assess the optimal cutoff value of the E/E’ ratio a ROC curve was used. The statistical program SPSS 20.0 (SPSS Inc., 2011) was used for all statistical analyses. A difference was considered statistical significance if p<0.05.
Results
Patients characteristics and treatment for STEMI
Sixty-six STEMI patients were recruited (mean age 60.8 ± 10.3 years, 80% male) all had at least 2 cardiovascular risk factors (Table 1). The majority (81%) did not have a history of angina and did not use anti-ischemic treatment before presentation, only 3% had a history of CCS functional class III and 16% of class I or II angina, all of the patients had at least two cardiovascular risk factors (Table 1).
| Age-yr | 60.8 ± 10.3 |
|---|---|
| Gender | |
| Male sex-no. (%) | 53 (80) |
| Female sex-no. (%) | 13 (20) |
| Cardiovascular risk factor-no. (%) | |
| Physical inactivity | 62 (94) |
| Diabetes Mellitus type 2 | 32 (48) |
| Hypertension | 38 (57) |
| Dyslipidemia | 40 (60) |
| Smoking | 32 (48) |
| Chronic renal failure | 4 (6) |
| Chronic angina functional class before presentation-no. (%) | |
| Absent | 53 (81) |
| Class I | 4 (6) |
| Class II | 7 (10) |
| Class III | 2 (3) |
| Treatment for STEMI-no. (%) | |
| Thrombolysis | 28 (42) |
| Effective treatment | 8 (12) |
| Percutaneous coronary intervention | 3 (4.5) |
| Revascularization surgery | 3 (4.5 |
| Infarct medical treatment-no. (%) | |
| Optimal | 45 (68) |
| Incomplete | 21 (32) |
| Glycoprotein IIb/IIIa receptor antagonist | 21 (32) |
| Culprit Aartery-no. (%) | |
| Anterior descendent artery | 37 (56) |
| Circumflex | 3 (5) |
| Right coronary | 26 (39) |
STEMI: ST-Elevation Myocardial Infarction
Table 1: Characteristics of the patients.
At presentation to our center: 28 patients (42%) were in time (first 24 hours of the onset of syntoms 13) to receive thrombolytic treatment, resulting in a resolution of clinical, electrocardiographic and biochemical parameters in 29% (8 patients). All patients underwent coronary angiography. The Left descending artery (LAD) was the most frequent culprit artery, followed by the right coronary and circumflex (Table 1). In 3 patients (4.5%) percutaneous coronary intervention (PCI) was performed and in 3 patients (4.5%) coronary artery bypass grafting (CABG). In addition, 21 patients (32%) received treatment with a glycoprotein IIb/IIIa receptor antagonist (Table 1). 45 patients (68%) received optimal medical treatment of ischemic heart disease according to the AHA guidelines [15].
Ventricular remodeling after STEMI
Echocardiographic parameters at baseline and 6 months after the STEMI were compared in those with and without VR (Table 2). After 6 months follow-up, the average increase of LVEDV was 13.1 ± 24.51 ml (baseline 99.06 ± 29.3 ml, follow up 112.16 ± 30.86 ml); 25 (38%) patients were classified as adverse VR, in this group the LVEDV increased from 90.28 ± 26.15 to 127.68 ± 32.21 ml (range 20.19% to 83.61%) compare with the baseline.
| Variable | WLVR (n=25) | WOLVR (n=41) | p-value |
|---|---|---|---|
| *E wave (cm/s) | 74.6 ± 19.1 | 68.48 ± 20.1 | 0.223 |
| A wave (cm/s) | 73.2 ± 29.2 | 74 ± 23.2 | 0.912 |
| Deceleration time of the E wave (ms) | 185.7 ± 62 | 194.5 ± 52.4 | 0.542 |
| *Septal E' Velocity (cm/s) | 4.9 ± 1.3 | 6.4 ± 1.9 | 0.002 |
| *Ratio E/E´ | 15.9 ± 4.7 | 11 ± 3 | <0.001 |
| Left Atrial Volume Index (ml/m2) | 34.2 ± 6 | 34.6 ± 10.3 | 0.375 |
| LVEF (%) | 47.3 ± 10.5 | 50.7 ± 8.7 | 0.164 |
| LVEDV (ml) | 90.2 ± 26.1 | 104.4 ± 30.2 | 0.057 |
| LVESV (ml) | 46.9 ± 19.6 | 50.6 ± 21.9 | 0.818 |
| At 6 months | At 6 months | At 6 months | At 6 months |
| *LVEF (%) | 41.6 ± 11.3 | 54.1 ± 9.2 | <0.001 |
| *LVEDV (ml) | 127.6 ± 32.2 | 102.7 ± 26.1 | 0.001 |
| *LVESV (ml) | 79 ± 34.5 | 47.5 ± 18 | <0.001 |
WLVR: With Left Ventricular Remodeling; WOLVR: Without Left Ventricular Remodeling; LVEF: Left Ventricle Ejection Fraction; LVEDV: Left Ventricular End Diastolic Volume; LVESV: Left Ventricular End Systolic Volume; *=p<0.05
Table 2: Echocardiographic parameters.
We observed a change in patients with adverse VR after the STEMI for septal Ea velocity (4.9 to 6.4 cm/s, p=0.002), E/E’ ratio (15.9 to 11, p ≤ 0.001), in the follow-up at 6 months in LVEF (41.6 to 54.1%, p ≤ 0.001), LVEDV (127.6 to 102.7 ml, p=0.001), LVESV (79 to 47.5 ml, p ≤ 0.001).
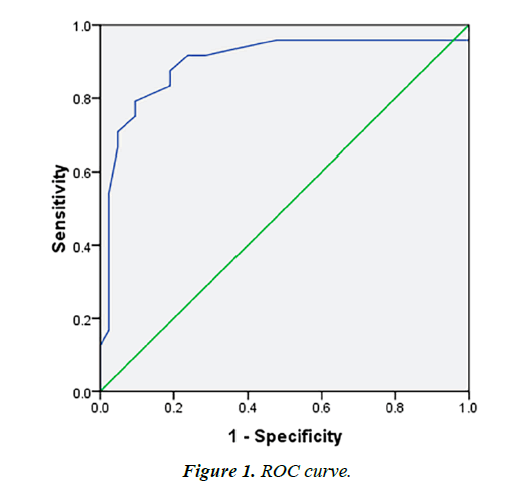
ROC analysis suggested that an index >12 of the E/E' ratio at baseline was best to predict adverse VR at 6 months (sensitivity 91% and specificity of 77%, AUC 0.897) (Figure 1). Univariate linear regression analysis (Table 3) exhibited that the robust predictors for VR were Killip-Kimball Classification (R 0.381), culprit artery (R 0.546), ratio E/E' (R 0.539) and septal E' velocity (R 0.370).). The multivariate linear regression was made incorporated the highest parameters in the univariate regression showed a strongest association for VR (R 0.750, p<0.001), mainly with Ratio E/E', Culprit Artery and Killip- Kimball Classification (R 0.748, p<0.001), even when Septal E' Velocity was incorporated the difference was slightly improved (Table 4).
| Variable | R | β | SE | p |
|---|---|---|---|---|
| Age | 0.041 | 0.002 | 0.006 | 0.743 |
| Gender | 0.199 | -0.250 | 0.154 | 0.110 |
| Sedentarism | 0.067 | 0.137 | 0.254 | 0.591 |
| Diabetes | 0.117 | 0.114 | 0.120 | 0.348 |
| Hypertension | 0.025 | -0.024 | 0.123 | 0.843 |
| Dyslipidemia | 0.182 | 0.181 | 0.122 | 0.143 |
| Smoking | 0.067 | 0.013 | 0.024 | 0.594 |
| Chronic renal failure | 0.198 | -0.403 | 0.249 | 0.110 |
| CCSGAP | 0.190 | -0.072 | 0.047 | 0.127 |
| Killip-Kimball classification | 0.381 | -0.249 | 0.076 | 0.002 |
| Forrester classification | 0.342 | -0.233 | 0.080 | 0.005 |
| Culprit artery | 0.546 | 0.275 | 0.053 | <0.001 |
| Trombolytic treatment | 0.038 | -0.038 | 0.123 | 0.760 |
| Primary PCI | 0.020 | -0.048 | 0.291 | 0.871 |
| E wave (cm/s) | 0.152 | -0.004 | 0.003 | 0.223 |
| A wave (cm/s) | 0.014 | <0.001 | 0.002 | 0.912 |
| Deceleration time of the E wave (ms) | 0.076 | 0.001 | 0.001 | 0.542 |
| Septal E' Velocity (cm/s) | 0.370 | 0.096 | 0.030 | 0.002 |
| Ratio E/E´ | 0.539 | -0.060 | 0.012 | <0.001 |
| Left Atrial Volume Index (ml/m2) | 0.100 | 0.005 | 0.007 | 0.425 |
| LVEDV (ml) | 0.235 | 0.004 | 0.002 | 0.057 |
| LVESV (ml) | 0.086 | 0.002 | 0.003 | 0.493 |
| LVEF (%) | 0.173 | 0.009 | 0.006 | 0.164 |
CCSGAP: Canadian Cardiovascular Society grading for angina pectoris; LVEF: Left Ventricle Ejection Fraction; LVEDV: Left Ventricular End Diastolic Volume; LVESV: Left Ventricular End Systolic Volume
Table 3: Univariable linear regression.
Discussion
The goal of this study was to analyze the ratio E/E’ as a predictor of ventricular remodeling after STEMI and other factors that could associate to have a better outcome predicting VR, as far as our concern this is the first study to determine the independent predictors of adverse VR after STEMI in our population. We observed that Killip-Kimball Classification, LAD as a culprit artery, ratio E/E' and septal E' velocity independently had a strong relation for adverse VR (Table 3); nevertheless, in a multivariate model (Table 4), septal velocity was not as important as Killip-Kimball Classification, culprit artery and ratio E/E' although it may be associated with LV remodeling [16] we do not find a strong relation as a predictor.
| Variable | R | β | SE | P |
|---|---|---|---|---|
| Ratio E/E´ | 0.539 | -0.060 | 0.012 | <0.001 |
| + Culprit Artery | 0.705 | 0.233 | 0.046 | <0.001 |
| + Killip-Kimball Classification | 0.748 | -0.168 | 0.057 | <0.001 |
| + Septal E' Velocity (cm/s) | 0.750 | 0.015 | 0.027 | <0.001 |
Table 4: Multivariable linear regression.
In our results we found that the optimal E/E’ ratio in our population was 12, with strong sensitivity and specificity in contrast which is lower compared to the commonly used ratio of 15 in Caucasians [17,18], this highlights the importance to start an early treatment to prevent adverse VR, nevertheless other factors such as the artery involve should be taken in consideration since a medium correlation was exhibited; Killip- Kimball Classification has not been associate as a predictor for ventricular remodeling after STEMI, even though is a clinical scale in our results a moderate relation was showed nevertheless more studies should be done to support this results as a parameter for start an early treatment.
Several risk factors have previously been considered to predict VR after STEMI, especially those related with systolic dysfunction have been highlighted by Sutton and Navqvi [6,19], including deterioration of the ejection fraction, and involvement of the LAD artery. Our analyses confirm the role of LAD involvement and we also identified E/E´ ratio and the E´ wave speed of the mitral annulus as independent predictors suggesting echocardiography could be helpful for the clinician to decide for a chronic treatment to prevent VR.
There is no doubt that when the myocardial infarction involves greater ventricular mass the possibility of adverse VR increases; therefore, when the LAD is affected could be related directly to adverse VR since this artery supplies around 45% to 55% of the irrigation to the heart, which correlates with our results, contrary when the vessel involve are the right coronary or the circumflex it depends mostly on the dominancy of the vessel.
In our study, only 21% of the patients received an effective reperfusion treatment (8 with effective thrombolytic therapy, 3 PCI and 3 CABG), which may explain the high prevalence of VR after STEMI. Ineffective reperfusion was caused by patient delay resulting in presentation to our hospital beyond the time in which thrombolysis is indicated; unfortunately in Latin America still exist a considerable delay for the patient to attend on time for proper treatment due to incorrect information, difficulty access to the hospital and self-medication, together with delay in medical attention in the emergency typically retard the medical valuation, all of these culminate in an inopportune treatment.
To prevent adverse VR, international guidelines recommend, in addition to optimal reperfusion therapy, treatment with antiplatelet agents, beta-blockers [20-26], and inhibitors of the renin-angiotensin-aldosterone system [27-29]. Especially aldosterone inhibitors post-myocardial infarctions are indicated in patients with systolic dysfunction [29,30]. These studies did not consider diastolic dysfunction after myocardial infarction although these individuals are at increased risk to develop systolic dysfunction in the future. In our study we found that after 6 months there were an increase in the LVEF, LVEDV and LVESV which in turn is expected, based on these we considered that the use of antagonist of aldosterone in the treatment of patients suffering from myocardial infarction should be studied further and evaluated as a new indication in patients even if LVEF>40%.
Conclusion
In conclusion, an early echocardiography evaluation of the E/ E´ ratio and determination of the culprit artery are important to identify STEMI patients that are at increased risk of adverse VR. We observed a E/E’ ratio>12 and the affection of the LAD artery could represent an early parameter to identify patients who might benefit from more aggressive treatment aimed to prevent adverse VR. Future prospective studies are required to assess the feasibility and effectiveness of such as a strategy.
Acknowledgements
The authors thank the echocardiograph and coronary care unit of our facilities.
References
- Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. The Lancet. 2016.
- Nichols M, Townsend N, Scarborough P, et al. Cardiovascular disease in Europe: Epidemiological update. Eur Heart J. 2014;35:2950-959.
- Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155-165.
- http://www3.inegi.org.mx/sistemas/sisept/Default.aspx?t=mdemo107&s=est&c=23587
- Flachskampf FA. Raised diastolic pressure as an early predictor of left ventricular remodeling after infarction: Should echocardiography or natriuretic peptides be used for assessment? Rev Esp Cardiol. 2010;63(9):1009-012.
- Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101(25):2981-988.
- Oh JK, Ding ZP, Gersh BJ, et al. Restrictive left ventricular diastolic filling identifies patients with heart failure after acute myocardial infarction. J Am Soc Echocardiogr. 1992;5(5):497-03.
- Bolognese L, Cerisano G, Buonamici P, et al. Influence of infarct-zone viability on left ventricular remodeling after acute myocardial infarction. Circulation. 1997;96(10):3353-359.
- Cerisano G, Bolognese L, Carrabba N, et al. Doppler-derived mitral deceleration time: An early strong predictor of left ventricular remodeling after reperfused anterior acute myocardial infarction. Circulation. 1999;99(2):230-36.
- Naqvi TZ, Padmanabhan S, Rafii F, et al. Comparison of usefulness of left ventricular diastolic versus systolic function as a predictor of outcome following primary percutaneous coronary angioplasty for acute myocardial infarction. Am J Cardiol. 2006;97:160-66.
- Nijland F, Kamp O, Karreman AJ, et al. Prognostic implications of restrictive left ventricular filling in acute myocardial infarction: A serial Doppler echocardiographic study. J Am Coll Cardiol. 1997;30(7):1618-624.
- Hillis GS, Møller JE, Pellikka PA, et al. Noninvasive estimation of left ventricular filling pressure by E/e' is a powerful predictor of survival after acute myocardial infarction. J Am Coll Cardiol. 2004;43(3):360-67.
- Lang RM, Badano LP, Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39.
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22(2):107-33.
- Yancy CW, Jessup M, Bozkurt B, et al. American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-39.
- Dokainish H, Zoghbi WA, Ambriz E, et al. Comparative cost-effectiveness of B-type natriuretic peptide and echocardiography for predicting outcome in patients with congestive heart failure. Am J Cardiol. 2006;97:400-03.
- Shacham Y, Khoury S, Flint N, et al. Serial echocardiographic assessment of left ventricular filling pressure and remodeling among ST-segment elevation myocardial infarction patients treated by primary percutaneous intervention. J Am Soc Echocardiogr. 2016;29(8):745-49.
- Kruszewski K, Scott AE, Barclay JL, et al. Noninvasive assessment of left ventricular filling pressure after acute myocardial infarction: a prospective study of the relative prognostic utility of clinical assessment, echocardiography, and B-type natriuretic peptide. Am Heart J. 2010;159(1):47-54.
- Yusuf S, Peto R, Lewis J, et al. Beta blockade during and after myocardial infarction: An overview of the randomized trials. Prog Cardiovasc Dis. 1985;27:335.
- Norwegian Multicenter Study Group. Timolol-induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N Engl J Med. 1981;304:801.
- Hjalmarson A, Herlitz J, Holmberg S, et al. The Göteborg metoprolol trial. Effects on mortality and morbidity in acute myocardial infarction. Circulation. 1983;67:I26.
- Chadda K, Goldstein S, Byington R, et al. Effect of propranolol after acute myocardial infarction in patients with congestive heart failure. Circulation. 1986;73:503.
- Metoprolol in acute myocardial infarction (MIAMI). A randomized placebo-controlled international trial. The MIAMI Trial Research Group. Eur Heart J. 1985;6:199.
- First International Study of Infarct Survival Collaborative Group. Randomized trial of intravenous atenolol among 16027 cases of suspected acute myocardial infarction: ISIS-1. Lancet. 1986;2:57.
- Chen ZM, Pan HC, Chen YP, et al. COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: Randomized placebo-controlled trial. Lancet. 2005;366:1622.
- ISIS-4: A randomized factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. Lancet. 1995; 345:669.
- GISSI-3. Effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarction. Gruppo Italiano per lo Studio della Sopravvivenza nell'infarto Miocardico. Lancet. 1994;343:1115.
- Swedberg K, Held P, Kjekshus J, et al. Effects of the early administration of enalapril on mortality in patients with acute myocardial infarction. Results of the Cooperative New Scandinavian Enalapril Survival Study II (CONSENSUS II). N Engl J Med. 1992;327:678.
- The Randomized AADActone Evaluation Study (RALES)). Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure. Am J Cardiol. 1996;78:902.
- Pitt B, Remme W, Zannad F, et al. Eplerenone post-acute myocardial infarction heart failure efficacy and survival study investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003; 348:1309.