- Biomedical Research (2015) Volume 26, Issue 4
Quantitative determination of resveratrol in Polygonum cuspidatum and its anti-proliferative effect on melanoma A375 cells
Ying Sun1,2#, Yan. Qi3#, Zhen Mu2, Keyu Wang1*1Department of Dermatology, QiLu Hospital, Shandong University, No.107, Wenhua Xi Road, Jinan, 250012, Shandong Province, P.R.China;
2Department of Dermatology, affiliated Hospital, Taishan Medical College, Tai?an, 271000, Shandong Province, P.R.China;
33Department of Urology, affiliated Hospital, Taishan Medical College, Tai?an, 271000, Shandong Province, P.R.China.
#Equally contributed to this work
- Corresponding Author:
- Wang Keyu
Department of Dermatology
QiLu Hospital, Shandong University, No.107
Wenhua Xi Road, Jinan, 250012
Shandong Province, P.R.China
E-mail: keyuwangsd@126.com
Accepted Date: July 23 2015
Abstract
Objective To establish HPLC method for determination of resveratrol content in Polygonum cuspidatum, and to study its inhibitory effect on human melanoma A375 cells. Methods HPLC with an Agilent column (model Angilent ZORBAX Eclips Plus C18, 4.6 × 250 mm, 5 μm) is used; mobile phase: methanol-water (75:25); flow rate: 1.0 mL/min; UV detection wavelength: 300 nm; injection volume: 5 μL; and column temperature: 25 ?. Anti-melanoma activity of resveratrol is analyzed by observing changes in cell morphology under inverted microscope and by MTT assay. Results Resveratrol shows a good linearity within a 1.248-12.48 μg range, and its recovery is 98. 58%, with a RSD of 1.62% (n = 6). MTT assay results show that the viability of A375 cells decreases with increasing concentration of resveratrol; and A375 cell inhibition rate of resveratrol exhibits rather evident dose-response relationship within the experimental concentration range. At high magnification, cells in the control group have clear nuclear membranes; a large number of mitochondria are visible within cytoplasm; double membranes and ridge structure are all clear. A small number of rough endoplasmic reticula, free ribosomes and lysosomes are seen, and cell structure is intact. Cells treated with resveratrol change rather greatly, with swollen organelles, increased number of intracellular granules, and even cell membrane disintegration, liquefaction degeneration cytoplasm, substantial disappearance of mitochondria and endoplasmic reticulum structure, release of cellular contents and occurrence of apoptosis. Cell morphology is not intact, and number of adherent cells is reduced. Conclusion HPLC method is accurate, simple and reproducible, which is suitable for quantitative analysis of resveratrol in Polygonum cuspidatum. Resveratrol can effectively inhibit the proliferation of human melanoma A375 cells.
Keywords
Polygonum cuspidatum; resveratrol; melanoma A375 cell
Introduction
Polygonum cuspidatum is the dried root and rhizome of Polygonum cuspidatum Sieb. et Zucc. in the genus Polygonum of the family Polygonaceae. As a perennial shrubby herb, it is mainly distributed in China's various regions south of the Yangtze River, as well as Shaanxi, Hubei and Sichuan. Polygonum cuspidatum is used for joint arthralgia, amenorrhea, water and fire burns, bruises, carbuncles, sores, cough, excessive phlegm, etc. [1]
Polygonum cuspidatum mainly contains anthraquinones & anthraquinone glycosides, stilbenes, flavonoids, phenolic compounds, etc [2-4], of which monomer components like polydatin and physcion have been widely used in medicine industry. Polygonum cuspidatum has become an important source of raw material for these monomer components. Modern pharmacological studies have shown that Polygonum cuspidatum has antibacterial, anti-inflammatory, anti-viral [5-6], vascular smooth muscle dilating, microcirculation improving, antiplatelet aggregation, hypolipidemic, anti-tumor, antioxidation, hepatoprotective and choleretic effects [7-9]. Particularly in recent years, American Institute of Natural Medicine has reported that resveratrol in Polygonum cuspidatum has an anti-HIV effect, which has further promoted the R & D of Polygonum cuspidatum. In order to further research and develop Polygonum cuspidatum, this paper studies the method for quantitative determination of resveratrol, a signature component of Polygonum cuspidatum, and explores its anti-melanoma activity, in order to provide evidence for its clinical application.
Materials
Instruments and reagents
Angilent 1100 HPLC system; Angilent ZORBAX Eclips Plus C18 column. Reagents used for preparation of standard and test solutions were all of analytical grade, and those used for liquid chromatography were HPLC grade reagents; water used for liquid chromatography was ultrapure water. DMSO and MTT were purchased from Sigma, USA; FBS was purchased from Gibco; and RPMI1640 from Invitrogen.
Drugs and cells
Polygonum cuspidatum medicinal herb was purchased from the medicine market in Anguo, which was identified as Polygonum cuspidatum. Resveratrol reference substance was self-prepared, whose purity was determined to be 99.5% by area normalization. Melanoma A375 cells were purchased from Shanghai ELISA Kit Assay Center.
Methods
Chromatographic conditions
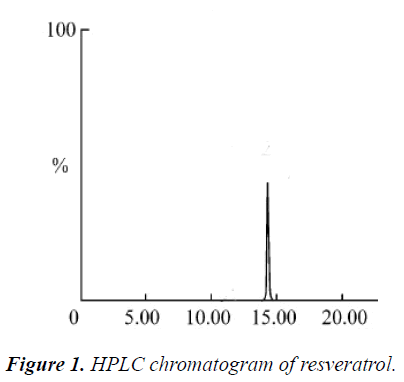
Agilent column (model Angilent ZORBAX Eclips Plus C18, 4.6 × 250 mm, 5 μm); mobile phase: methanol-water (75:25 by volume); flow rate: 1.0 mL/min; UV detection wavelength: 300 nm; injection volume: 5 μL; and column temperature: 25 ℃. Under such chromatographic conditions, resveratrol was eluted, and basically achieved baseline separation; retention time was 13.104 min.
Linearity range
1 mL, 2 mL, 4 mL, 6 mL, 8 mL and 10 mL of 12.48 μg/mL resveratrol reference solutions were precisely drawn, respectively, placed into 10 mL volumetric flasks, diluted to the mark with methanol, and injected at 10 μL separately to measure the peak area of resveratrol under the above chromatographic conditions. Standard curve was plotted with mass concentration (X) versus peak area (Y), regression equation was Y = -18567.73X + 15622.52, r = 0.9997, and linear range: 1.248-12.48 μg/mL.
Stability test
Each 10 μL of the same test solution was precisely drawn, and injected at 0, 2, 4, 6, 8, 12 and 24 h, respectively, for determination according to the above chromatographic conditions, and RSD was found to be 0.85%. The results showed that the test solution was stable within 24 h.
Precision test
Each 10 μL of the same test solution was precisely drawn, and injected repeatedly 5 times for determination according to the above chromatographic conditions. RSD was found to be 1.2% (n = 5).
Recovery test
Six aliquots of 0.5 g of Polygonum cuspidatum coarse powders of known contents were accurately weighed, added separately with 1 ml of 3.18 μg/mL resveratrol reference solution, prepared as per the above preparation method of test solution, and determined according to the above chromatographic conditions. The results showed that the average recovery of resveratrol was 98. 58%, with a RSD of 1.62% (n = 6).
Reproducibility test
Five aliquots of samples of the same batch were accurately weighed, and prepared as per the preparation method of test solution. Then, 10 μL of the above solution was precisely drawn, and injected for determination under the same chromatographic conditions as described above. The results showed that RSD was 1.5% (n = 5).
Sample determination
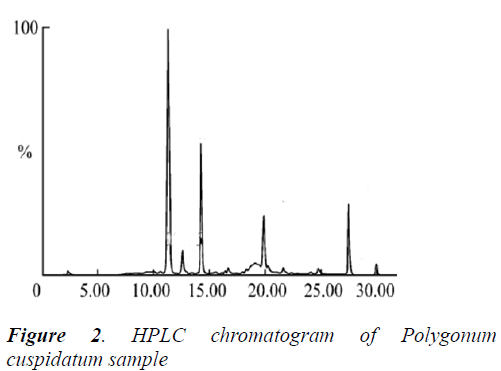
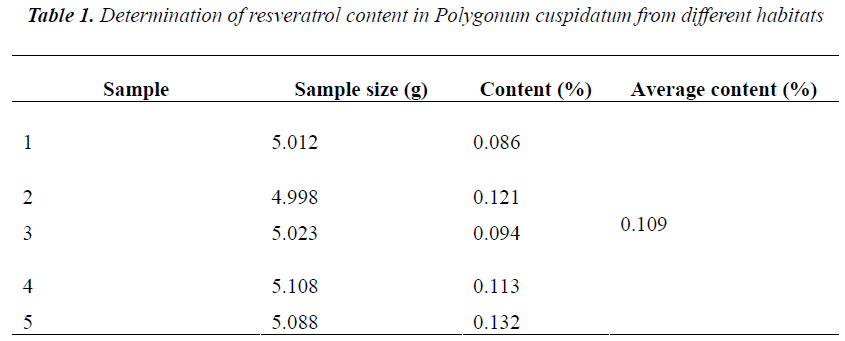
0.5 g of five different batches of Polygonum cuspidatum coarse powders were accurately weighed, and ultrasonically extracted for 1 h with 20 mL of 80% aqueous methanol, then diluted to the mark, and filtered. 1 mL of subsequent filtrates were placed into 10 mL volumetric flasks, diluted to the mark with mobile phase, and ultra-filtered. Afterwards, 10 μL of subsequent filtrates were injected for determination of resveratrol content according to the above method and chromatographic conditions. The results showed rather big difference in resveratrol content of five different batches of samples, with an average of 0.109%. See Table 1 below.
Cell culturing
A375 cells were cultured routinely in a 37℃, 50 mL/L CO2 incubator with RPMI 1640 medium containing 100 mL/L FBS, 100 ku/L penicillin and 100 mg/L treptomycin. Medium was replaced and cells were passaged every 2-3 days.
A375 cell viability assay by MTT
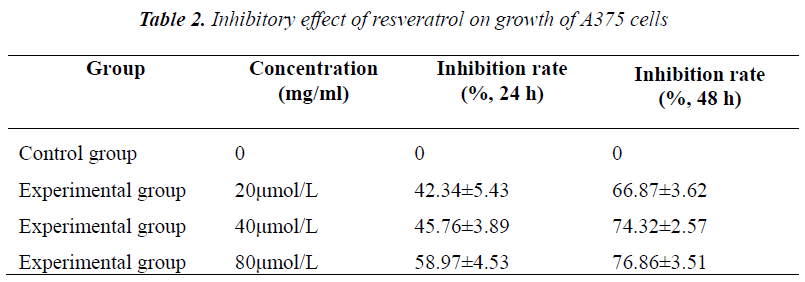
2×106/ml A375 cells were seeded in 96-well flatbottomed plates, divided into five groups, and added with 20, 40 and 80 μmol/L resveratrol for treatment. Six replicate wells were set up for each group. Cells were cultured routinely, and assayed at 48 h and 72 h, respectively. Each well was added with 20 μl of MTT (5 mg/ml), and incubated for 4 h. Before assay, supernatant was discarded, and the crystal was dissolved in 100 μl of DMSO, shaken on a micro oscillator for 5 min, and measured for absorbance at 570 nm on a microplate reader, followed by calculation of cell growth inhibition rate.
Calculation formula for cell inhibition rate: inhibition rate (%) = (OD value of control group - OD value of experimental group) / OD value of control group × 100%.
Experimental results showed that after culturing for 48 h and 72 h, A375 cell growth was all inhibited to varying degrees, exhibiting evident concentration- and timedependence. Compared with the control group, different concentrations of resveratrol all had significantly different inhibition rates on A375 cell growth.
TEM observation of cell morphology
The above concentrations of resveratrol solutions were added into cell culture flasks. After treating for 48 h, cells were collected, and washed. Then, the cells were fixed in 1% osmic acid, wash with 0.1% PBS, dehydrated routinely, impregnated, embedded in epoxy resin, and cut into ultra-thin sections for observation of cell morphology and photography under TEM.
Under electron microscope, cells in the control group had regular nuclear morphology, which were generally elliptical or oval. Nuclear membranes were clear, and a large number of mitochondria were visible within cytoplasm. Double membranes and ridge structure were all clear. After treating with resveratrol for 48 h, part of A375 cells were swollen, and bulges were formed in cell membranes. Swelling and chromatin margination were found in mitochondria, endoplasmic reticula and nuclei. A very small number of cells presented typical early apoptotic changes. In high concentration group, complete disruption of nuclear membranes, structure collapse, karyolysis and cell content overflow, i.e. oncosis-like changes, were observed.
Discussion
Melanoma was first proposed by Rene Laenneic (Jay V.2000) in 1804 [10]. There is only one type of malignant cells that constitute all malignant melanomas, which are malignant pigment-producing cells called melanocytes. Yet they have some small differences in terms of morphology, such as spindle ones and cylindrical ones.
Melanoma is a highly malignant tumor, which originates from the melanocytes in pigmentation areas of skin, mucosa, eyes and central nervous system. Its clinical symptoms include bleeding, itching, tenderness, ulcers, etc. In general, symptoms of melanoma are age-related; younger patients generally manifest itching, and increased colorific change and area of skin lesions, while elderly patients generally manifest ulcers of skin lesions, which usually indicates poor prognosis [11,12].
Skin lesions of cutaneous malignant melanoma are associated with anatomical location and growth pattern of tumors, that is, associated with histological type. Histological type varies greatly due to age, gender and race differences. Different types of melanomas have different causes and genetic backgrounds. At present, Clark's level is used for clinical histological classification of melanomas, which include four types: lentigo maligna melanoma (LMM); superficial spreading melanoma (SSM); acral lentiginous melanoma/mucosal melanoma; and nodular melanoma (NM). Among malignant melanomas suffered by Caucasians, about 70% are SSM.
When malignant melanomas spread, they usually do not produce melanin, so they are called non-pigmented melanomas, but non-pigmented and pigmented malignant melanomas have almost the same malignant degree. Malignant melanomas occur most commonly in the skin, but 10% occur in the eye. Most common types of cutaneous malignant melanomas are called superficial spreading malignant melanoma and small nodular malignant melanoma.
Pathogenesis of melanoma is generally believed to be the malignant transformation of melanocytic nevus caused by repeated friction, grab and injury; improper excavation or drug corrosion may convert benign melanocytic nevus into malignant melanoma. This study shows that different concentrations of resveratrol can inhibit the growth of A375 cells to varying degrees in a concentration- and time-dependent manner. Under electron microscope, cells in the control group have regular nuclear morphology, which are generally elliptical or oval. Nuclear membranes are clear, and a large number of mitochondria are visible within cytoplasm. Double membranes and ridge structure are all clear. After treating with resveratrol for 48 h, part of A375 cells are swollen, and bulges are formed in cell membranes. Swelling and chromatin margination are found in mitochondria, endoplasmic reticula and nuclei. A very small number of cells present typical early apoptotic changes. In high concentration group, complete disruption of nuclear membranes, structure collapse, karyolysis and cell content overflow, i.e. oncosis-like changes, are observed. The specific mechanisms of action of resveratrol need further confirmation.
References
- Aziz MH, Reagan-Shaw S, Wu JQ, Longley BJ, AhmadN. Chemoprevention of skin cancer by grapeconstituent resveratrol: relevance to human disease. The FASEB journal 2005; 19: 1193-1195.
- Kang H, Xiang L, Fan G Q. Studies on chemicalconstituents in radix and rhizome of R heumnanum. Chin Tradit Herb Drugs 2002; 35: 394-396.
- Lee KR, Hong SW, Kwak JH, Pyo S, Jee OP. Phenolic constituents from the aerial parts of A rtemisiastolonif era. Arch Pharmacol Res 1996; 19: 231-234.
- Jin XM, Jin GZ. Chemical constituents in root and rhizome of Polygonumcuspidatum. Chinese Traditional and Herbal Drugs 2007; 38: 1446-1448.
- Fan XR. Susceptibility testing of pathogenic strains to Polygonumcuspidatum.Lishizhen Medicine and MateriaMedica Research 2000; 11: 108-112.
- OuyangLuming. Experimental study on antimycoticeffect of Chinese herbal medicine on Candida albicans.Chinese Journal of Information on Traditional Chinese Medicine 2000; 7: 26-27.
- Jin CH, Zhao KS, Liu J, Huang ZL. Regulatory effect of polydatin on intra-myocardial cell calcium. Chinese Journal of Pathophysiology 2001; 17: 128-131.
- John DM, Reed PW, Kevin M. Effect of imexontreatment on Friend vires complex infection using genetically defined, nice as a model for HIV-1infection. Antiviral Research 1991; 15: 51-53.
- Bhat KP, Pezzuto JM. Cancer chemopreventive activity of resveratrol. Ann NY AcadSci 2002; 957: 210-229.
- Jay V. The legacy of Laënnec.Archives of pathology &laboratory medicine 2000; 124: 1420-1423.
- Miller AJ, Mihm JMC. Melanoma. New England Journal of Medicine 2006; 355: 51-65.
- Dewing D, Emmett M, Jones RP. The Roles of Angiogenesis in Malignant Melanoma: Trends in Basic Science Research over the Last 100 Years. ISRN oncology 2012; 2012: 546927-546931.