Research Article - Biomedical Research (2017) Volume 28, Issue 17
Protective effect of new fuzheng chuyi granule on the lung tissue in acute upper respiratory tract infection mice and its mechanisms
Xin Su1, Shuang Jiang1, Guang Yang2 and Xiaoyan Gong1*
1Changchun University of Chinese Medicine, Changchun, Jilin 130117, PR China
2Second Hospital of Jilin University, Changchun, Jilin 130000, PR China
Accepted on August 17, 2017
Abstract
This research studied the protective effect of New Fuzheng Chuyi Granule (NFCG) on the lung tissue of mice with acute upper respiratory tract infection (AURI) and its mechanisms. A mouse model of AURI was established by intranasal instillation of Streptococcus pneumoniae, and high-dose (5.28 g/kg), medium-dose (2.64 g/kg) and low-dose (1.23 g/kg) of NFCG were intragastrically given to the mice, respectively, and on the 1st, 4th and 7th day after the administration, lung wet/dry weight ratio and lung index were calculated, pathological changes of the lung tissue and the spleen lymphocyte proliferation were observed, and TNF-α, IL-6, IL-1, IFN-γ, TLR2, TLR4, MyD88, and NF-κB expressions in the lung tissue were detected. The results showed that NFCG could reduce the lung wet/dry weight ratio and the lung index, alleviate the pathological changes in the lung tissue, and increase the spleen lymphocyte proliferation in mice; in addition, NFCG could reduce the expression of several pro-inflammatory factors, including TNF-α, IL- 6, IL-1 and IFN-γ, and TLR2, TLR4, MyD88 and NF-κB proteins. These results suggest that NFCG can protect the lung tissue of mice with AURI, which may be related to its improving the immunity, anti-inflammation and regulation of TLR2/4-MyD88 signaling pathway.
Keywords
New fuzheng chuyi granule (NFCG), Immunity, Anti-inflammation, TLR2/4-MyD88 signaling pathway.
Introduction
Acute upper respiratory tract infection (AURI) is an acute inflammation caused by bacteria or viruses and one of common diseases in clinic, and will develop into pneumonia and many other respiratory diseases that affect human health if not timely treated [1]. Currently, antibiotics and immunopotentiators are often chosen for the treatment of AURI caused by bacteria or complicated with bacterial infections [2,3]. Although antibiotics have bactericidal and bacteriostatic effects on some bacteria, the bacteria can develop the resistance to them, which may result in a reduced treatment effect of antibiotics, and some antibiotics are expensive [4,5]. Traditional Chinese medicines have shown some advantages in the treatment of AURI, such as the multi-link and multi target effect of multiple components, and the comprehensive regulation of active components in compound traditional Chinese medicines [6,7].
New Fuzheng Chuyi Granule (NFCG) consists of four herbal medicines, including Rhodiola, Polygonum cuspidatum, Folium Isatidis, and Cyrtomium fortunei, with a function of strengthening vital Qi to eliminate pathogenic factors, clearing heat and removing toxin, and a definite therapeutic effect on upper respiratory tract infection and influenza [8]. In this study, a mouse model of AURI was established by a nasal drip of Streptococcus pneumoniae, and lung wet/dry weight ratio, lung index, pathological changes of the lung tissue, spleen lymphocyte proliferation, inflammatory factor expressions and TLR2/4-MyD88 pathway were observed and investigated in mice with AURI, to evaluate the protective effect of NFCG on the lung tissue of the mice and explore the underlying mechanisms.
Materials and Methods
Materials and chemicals NFCG (prepared by the Changchun University of Traditional Chinese Medicine); Amoxicillin (Sichuan Pharmaceutical Co. Ltd, China); SP and DAB kits (Fuzhou Maixin Biotechnology Development Co., Ltd, China); TNF-α, IL-6, IL-1 and IFN-γ detection kits (Bio-Techne China Co. Ltd, China); Rabbit anti-mouse TLR2, TLR4, MyD88, NF-κB and β-actin antibody (Cell Signaling Technology, USA); HRP-labeled goat antirabbit IgG antibody (Beijing Dingguochangsheng Biotechnology Co., Ltd, China); ECL luminescence kit (Pierce Company, USA).
Preparation of Streptococcus pneumonia liquid
Lyophilized Streptococcus pneumoniae CICC 10913 was streak-inoculated in 5% sheep soup solid medium and cultured at 37°C in an incubator overnight. On the second day, the activated single clone was selected to be transferred into another 5% sheep soup solid medium for its inoculation, and the medium was left to stand in a 5% CO2 incubator for the culture; the logarithmic growth phase bacteria were washed with PBS 2 times, and then the bacteria concentration of the liquid was adjusted to 1 × 108 CFU/mL for use.
Establishment of animal model, grouping and drug administration
The 180 BALB/c mice (half male and half female) were randomly divided into control group, model group, high-, medium- and low-dose NFCG groups, and positive drug group, 30 mice in each group; mice in each group were randomly divided into 1 d group, 4 d group, and 7 d group, 10 mice in each group. Except those in the control group, the mice in the other groups was anesthetized with ether and then 50 μL of Streptococcus pneumoniae liquid were slowly dropped into the mice’s nasal cavity for waiting for the inhalation of the liquid naturally; mice in the control group were given the same volume of PBS in the same way, and NFCG on the 1st hour after the administration of Streptococcus pneumoniae liquid. Mice in the high-, medium- and low-dose NFCG groups were intragastrically given 5.28 g/kg, 2.64 g/kg and 1.23 g/kg of NFCG, respectively, those in the positive drug group were intragastrically given 0.3g/kg of amoxicillin [9], and those in the control group were intragastrically given normal saline, in which the intragastric administration volume was 0.2 mL, once daily. After the intragastric administration, the general state of mice was observed, and the organ samples were taken on the 1st, 4th and 7th day after the administration.
Measurement of lung wet/dry weight ratio and lung index
The mice’s body weights were exactly weighed, and then their lung tissues were taken after they were sacrificed; after weighed, the wet lung tissues were placed at 60°C in an electric thermostatic oven to be dried for 72 h and the dried lung tissues were weighed, for the calculation of the wet/dry weight ratio; the ratio of lung weight to body weight was considered the lung index.
Spleen lymphocyte proliferation test
The mice were sacrificed by dislocating their necks, then their spleens were removed, and their spleen lymphocytes were collected with the conventional methods; the spleen lymphocytes were cultured in RPMI 1640 medium containing 5% fetal bovine serum to the logarithmic growth phase. The lymphocytes were collected, the cell concentration was adjusted to 2 × 106/mL, and the cells were seeded at a density of 100 μL/well in 96-well culture plates; each sample was repeatedly seeded in 3 wells, Con A at a final concentration of 2 g/mL was added to the wells to be used as the specific stimulation. The plates were placed in a 5% CO2 incubator for 48 h for the culture of cells, then 20 μL of 5 mg/mL MTT solution were added to each well for the further culture of the cells for 4 h, and finally 100 μL DMSO solution was added to each well. OD values of the samples were detected at 570 nm.
Pathological changes of the lung tissue detected by HE staining
The left lungs of mice in each groups were fixed by quickly immersing them in the newly-prepared 4% paraformaldehyde solution, then dehydrated, made transparent, waxed and paraffin-embedded, and the samples were made into 5 μm slices. The dried slices were dewaxed, soaked, stained by HE staining, dehydrated, made transparent and sealed by following the conventional preparation steps of lung tissue pathological specimens. Pathological changes of the mice’s lung tissue were observed under an Olympus optical microscope.
Detection of TNF-α, IL-6, IL-1 and IFN-γ levels in the lung tissue by ELISA
The lung tissue was washed with pre-cooled PBS solution, and the surface of the washed lung tissue was wiped dry to remove the blood; 100 mg of the lung tissue was cut into pieces with ophthalmic scissors and the cut lung tissue pieces were placed in 9 times volume of PBS solution. Then the solution containing the cut lung tissue pieces was transferred into a tissue homogenate tube, in which the lung tissue was ground by rotating the tissue homogenate rod against the tube wall 10 times, for 6~10 min each time, and finally the grinding fluid was centrifuged to obtain the supernatant. TNF-α, IL-6, IL-1 and IFN-γ levels in the supernatant were measured according to the instructions of ELISA detection kits.
Detection of TLR2, TLR4, MyD88 and NF-κB protein expressions in the lung tissues by immunohistochemistry
The lung tissue was fixed by immediately immersing it in a newly-prepared 4% paraformaldehyde solution for 24 h, and paraffin-embedded and made into 4 μm slices; the tissue slices were attached to slides treated with polylysine and the slides were stained with immunohistochemical SP method according to the instructions of the kits. Motic Images Advanced 3.2 image analysis software was applied to evaluate the expression of the different proteins, in which the greater the gray value was, the lower the positive expression was and vice versa.
Statistical analysis
The data were analyzed with SPSS 17.0 statistical software and single factor analysis of variance was used. Any two means were compared with LSD. P<0.05 was considered a statistically significant difference.
Results
Effects of NFCG on the lung wet/dry weight ratio and lung index
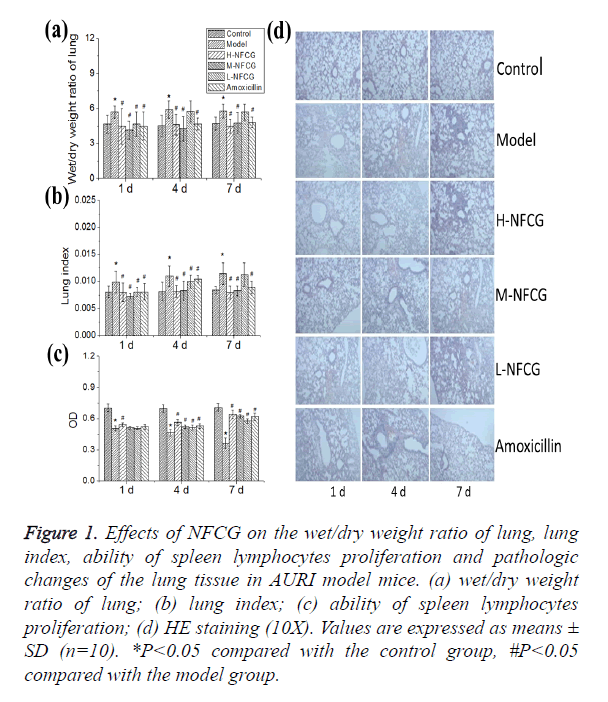
After the infection with Streptococcus pneumoniae, the lung wet/dry weight ratio of mice in the model group was significantly higher than that in the control group (P<0.05); compared with those in the model group, lung wet/dry weight ratios of mice in all NFCG-treated groups were significantly reduced on the 1st day after the infection with Streptococcus pneumonia, and those in high- and medium-dose NFCG groups were significantly decreased on the 4th and 7th day after the infection (P<0.05, Figure 1a). After the infection with Streptococcus pneumoniae, the lung index of mice in the model group was significantly increased with the time of infection, compared with that in the control group (P<0.05); compared with those in the model group, lung indexes of mice in groups treated with the different doses of NFCG were decreased significantly on the 1st and 4th day after the infection with Streptococcus pneumonia, and those in high- and medium-dose NFCG groups were significantly decreased on the 7th day after the infection (P<0.05, Figure 1b).
Figure 1. Effects of NFCG on the wet/dry weight ratio of lung, lung index, ability of spleen lymphocytes proliferation and pathologic changes of the lung tissue in AURI model mice. (a) wet/dry weight ratio of lung; (b) lung index; (c) ability of spleen lymphocytes proliferation; (d) HE staining (10X). Values are expressed as means ± SD (n=10). *P<0.05 compared with the control group, #P<0.05 compared with the model group.
Effect of NFCG on the proliferation of spleen lymphocytes
Compared with that in the control group, the proliferation of mice’s spleen lymphocytes in the model group was significantly decreased (P<0.05), showing an gradual increasing trend with the time of infection; after the administration of NFCG, the proliferation of mice’s spleen lymphocytes was increased to some extent, in which that on the 1st day in the high-dose group and that on the 4th and 7th day in the three NFCG-treated groups were significantly increased compared with that in the model group (P<0.05, Figure 1c).
Effects of NFCG on pathological changes of the lung tissue
The lung tissue HE staining showed that pathological changes in the lung tissue of mice in NFCG-treated groups were less serious than those in the model group, presenting a structural integrity of bronchial walls, a small amount of visible infiltration of inflammatory cells in bronchial walls, no exudation in bronchial cavities, a mild vasodilation and vascular hyperemia in parts of the alveolar walls, and an occasional infiltration of inflammatory cells in a few of alveolar cavities (Figure 1d).
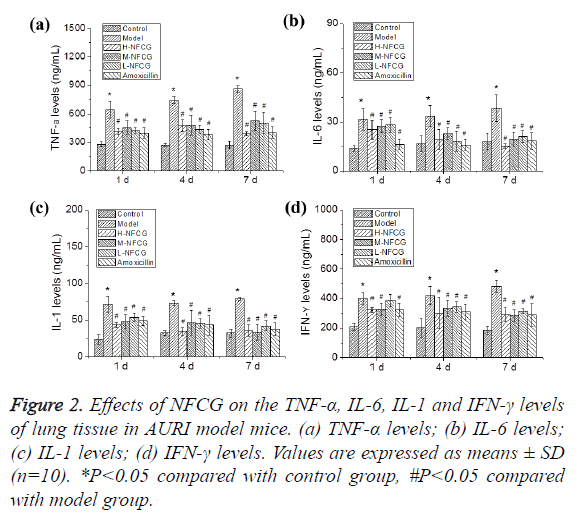
Effects of NFCG on TNF-α, IL-6, IL-1 and IFN-γ levels in the lung tissue
After the infection with Streptococcus pneumoniae, TNF-α, IL-6, IL-1 and IFN-γ levels in the lung tissues of mice in the different groups were detected by ELISA. As shown in Figure 2, TNF-α, IL-6, IL-1 and IFN-γ levels in the lung tissues of mice at the different time points in the model group were significantly elevated compared with those in the control group (P<0.05); compared with those in the model group, TNF-α, IL-6, IL-1 and IFN-γ levels in the lung tissues of mice at the different time points in NFCG-treated groups were significantly reduced (P<0.05) except that the IFN-γ level in the low-dose NFCG group showed no significant change on the 1st day (P>0.05).
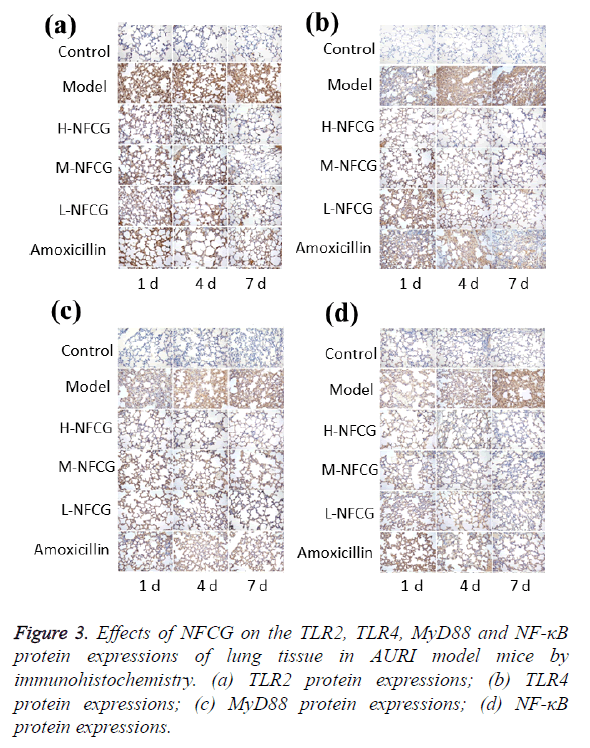
Effects of NFCG on the expression of TLR2, TLR4, MyD88 and NF-κB proteins in the lung tissue of mice
TLR2, TLR4, MyD88 and NF-κB protein expressions in the mice’s lung tissue were detected by immunohistochemistry, and the results are shown in Figure 3. After the infection of Streptococcus pneumoniae, TLR2, TLR4, MyD88 and NF-κB protein expression levels in the lung tissue of mice in the model group were significantly increased compared with those in the control group (P<0.05), showing a gradual increasing trend with the time of infection; compared with those in the model group, TLR2, TLR4, MyD88 and NF-κB protein expression levels at the different time points in NFCG-treated groups were significantly reduced (P<0.05), except that the TLR2 protein expression levels in the low-dose NFCG group showed no significant change on the 1st day (P>0.05).
Discussion
Streptococcus pneumoniae is the main pathogen to cause pneumonia, meningitis, otitis media and sinusitis, and about 1.2 million people die of the infection of Streptococcus pneumoniae around the world each year [10]. The emergence of multiple drug resistant Streptococcus pneumoniae strains have greatly reduced the therapeutic effect of antibiotics on the infection of streptococcus pneumonia, but it is not easy for bacteria to develop a resistance to traditional Chinese medicines with anti-infection activity and their compounds, and the related research has received much concern [11]. In this study, an AURI model was established by intranasal drop of Streptococcus pneumoniae in mice, to observe the effects of NCFG on the lung tissue of mice with AURI and explore the underlying mechanisms. The results of this study showed that NFCG could reduce the lung wet/dry weight ratio and the lung index, and alleviate the pathological changes in the mice’s lung tissue, indicating that NFCG can protect the lung tissue damage of mice with AURI caused by Streptococcus pneumoniae. Immune dysfunction is an important reason for the occurrence of pneumococcal infections and to improve immunity is one of important means for the treatment of Streptococcus pneumoniae infections [12]. Due to this reason, this study further explored the effect of NFCG on the immune function of mice with AURI. The results showed that NFCG could increase the mice’s spleen lymphocyte proliferation, further suggesting that the protective effect of NFCG on the lung tissue of mice with AURI may be associated with its improving the immunity.
Inflammation plays an important role in the process of bacteria infectious diseases, and inflammatory cytokines are important substances in the regulating process of inflammation [13]. In the inflammatory process of infectious diseases, the proinflammatory factors, such as TNF-α, IL-6, IL-1 and IFN-γ, were particularly important. TNF-α is one of cytokines derived from macrophages and the earliest proinflammatory factor generated in the process of inflammation, initiating an inflammatory reaction by transmitting signals through specific receptors on the cell surface [14]. IL-6 is generated in the acute stage of inflammatory responses, and while a pulmonary infection occurs, the secretion of IL-6 increases due to the activation of lymphocytes by the local injury of various inflammatory cells, so that the level of IL-6 can reflect the severity of pulmonary infection; in addition, IL-6 can also increase the production of TNF-α and other inflammatory factors to aggravate the inflammatory injury [15]. IL-1 is also one of cytokines involved in inflammations, inducing changes in the physiology and metabolism similar to those induced by TNF-α, and producing a synergistic effect with TNF-α [16]. IFN-γ is derived from activated NK and T cells, and can activate macrophages to release oxygen free radicals and increase the expression of MHC class II transactivator, thereby promoting the development of inflammatory responses [17]. The results showed that after the infection with Streptococcus pneumoniae, compared with those in the model group, TNF-α, IL-6, IL-1 and IFN-γ levels in the mice’s lung tissue at the different time points were significantly lower in NFCG-treated groups (P<0.05), except that the IFN-γ level in the lung tissue of mice in the low-dose NFCG group showed no significant change on the 1st day (P>0.05), indicating that the protective effect of NFCG on the lung tissue of mice with AURI should be related to its anti-inflammatory effect.
Innate immunity and acquired immunity are the complex immune defense mechanisms in the body [18]. The innate immunity is the first defensive line of the body, and the data have showed that the microbial identification mechanism of human innate immunity may be attributed to Toll-like receptors (TLRs). TLRs can not only activate the innate immunity, but also be involved in the initiation of specific immune response, and are an important part that plays the role of innate immunity in anti-infection immune responses in the body [19]. TLR2 and TLR4 are the core of a bacterial infection, in which TLR2 is the main recognition receptor of gram-positive bacteria lipoproteins and TLR2 activated by lipoteichoic acid can induce the secretion of pro-inflammatory cytokines [20]; TLR4 is the main receptor of LPS, a component of gram-negative bacteria cell wall, with the function of recognizing viral proteins [21]. MyD88 molecule is an adaptor of most TLRs signal transductions, in which TLRs can transmit signals to the adaptor molecule MyD88 and the MyD88 adaptor molecule can activate NF-κB, thereby controlling the secretion of inflammatory factors [22]. There are several ways in the signal transduction pathway between TLRs and NF-κB, and NF-κB is located in the downstream hub of the TLRs signal transduction pathway, which is an ideal inflammatory signal pathway regulation target [23]. In this study, Immunohistochemistry and western blot were respectively applied to detect the effect of NFCG on the expression of TLR2, TLR4, MyD88 and NF-κB proteins in the lung tissue of mice with AURI, and the results showed that NFCG could decrease the expression of TLR2, TLR4, MyD88 and NF-κB proteins in the lung tissue of the model mice, indicating that NFCG can protect the lung tissue in the mice with AURI through the regulation of TLR2/4- MyD88 signaling pathway.
In conclusion, NFCG has a protective effect on the acute lung tissue injury caused by AURI, which may be related to its improvement on the immunity, anti-inflammation and regulation of TLR2/4-MyD88 signaling pathway.
Acknowledgment
The authors are grateful to the support by Key Research Program of the Ministry of Science and Technology in China (No. 2012ZX09103201-033) and Science and Technology Research Project of Education Department in Jilin Province (No. 2016018).
References
- Lai K, Lin L, Liu B. Eosinophilic airway inflammation is common in subacute cough following acute upper respiratory tract infection. Respirology 2016; 21: 683-688.
- Tandon P, Abraldes JG, Keough A. Risk of bacterial infection in patients with cirrhosis and acute variceal hemorrhage, based on child-pugh class, and effects of antibiotics. Clin Gastroenterol Hepatol 2015; 13: 1189-1196.
- Mahapatra A, Panda PK, Sagar R. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection treated successfully with a course of oral antibiotics. Asian J Psychiatr 2017; 25: 256-257.
- Hamoya T, Miyamoto S, Tomono S. Chemopreventive effects of a low-side-effect antibiotic drug, erythromycin, on mouse intestinal tumors. J Clin Biochem Nutr 2017; 60: 199-207.
- Mortensen EM. Deescalating antibiotics in pneumonia: laying the foundation. Ann Am Thorac Soc 2017; 14: 168-169.
- Uddin Z, Song YH, Curtis-Long MJ. Potent bacterial neuraminidase inhibitors, anthraquinone glucosides from Polygonum cuspidatum and their inhibitory mechanism. J Ethnopharmacol 2016; 193: 283-292.
- Chen L, Wang X, Liu Y. Dual-target screening of bioactive components from traditional Chinese medicines by hollow fiber-based ligand fishing combined with liquid chromatography-mass spectrometry. J Pharm Biomed Anal 2017; 143: 269-276.
- Yue DH, Wang HL, Gong XY. Effects of fuzheng chuyi granule on H1N1 influenza a virus infection in murine T cell subsets. J China Tradit Med Pharm 2014; 29: 704-707.
- Su X, Gong XY. Effects of new fuzheng chuyi granule on TLR2 and TLR4 mRNA in mice pulmonary tissue infected with streptococcus pneumonia. J China Tradit Med Pharm 2016; 31: 3302-3304.
- Obringer E, Chen JL. Acute Mastoiditis Caused by Streptococcus pneumoniae. Pediatr Ann 2016; 45: e176-179.
- Houri H, Tabatabaei SR, Saee Y. Distribution of capsular types and drug resistance patterns of invasive pediatric Streptococcus pneumoniae isolates in Teheran, Iran. Int J Infect Dis 2017; 57: 21-26.
- Cui J, Ma C, Ye G. DnaJ (hsp40) of Streptococcus pneumoniae is involved in bacterial virulence and elicits a strong natural immune reaction via PI3K/JNK. Mol Immunol 2017; 83: 137-146.
- Mortensen R, Nissen TN, Blauenfeldt T. Adaptive immunity against streptococcus pyogenes in adults involves increased IFN-γ and IgG3 responses compared with children. J Immunol 2015; 195: 1657-1664.
- Chitul A, Voiosu AM, Marinescu M. Different effects of anti-TNF-alpha biologic drugs on the small bowel macroscopic inflammation in patients with ankylosing spondylitis. Rom J Intern Med 2017; 55: 44-52.
- Nadeem A, Ahmad SF, Al-Harbi NO. GPR43 activation enhances psoriasis-like inflammation through epidermal upregulation of IL-6 and dual oxidase 2 signaling in a murine model. Cell Signal 2017; 33: 59-68.
- Alcaraz-Quiles J, Titos E, Casulleras M. Polymorphisms in the IL-1 gene cluster influence systemic inflammation in patients at risk for acute-on-chronic liver failure. Hepatology 2017; 65: 202-216.
- Ramalingam TR, Gieseck RL, Acciani TH. Enhanced protection from fibrosis and inflammation in the combined absence of IL-13 and IFN-γ. J Pathol 2016; 239: 344-354.
- Elahi A, Singh MP, Ali S. Antigen specific immune enhancement of innate and acquired immunity by pearl in ashed form. Int Immunopharmacol 2014; 21: 82-93.
- Turner ML, Cronin JG, Healey GD. Epithelial and stromal cells of bovine endometrium have roles in innate immunity and initiate inflammatory responses to bacterial lipopeptides in vitro via Toll-like receptors TLR2, TLR1, and TLR6. Endocrinology 2014; 155: 1453-1465.
- Li Y, Yang H, Wu X. Pretreatment with TLR2 and TLR4 ligand modulates innate immunity in corneal fibroblasts challenged with Aspergillus fumigatus. Invest Ophthalmol Vis Sci 2013; 54: 4261-4670.
- Zhang Y, Zhu X, Feng Y. TLR4 and TLR9 signals stimulate protective immunity against blood-stage Plasmodium yoelii infection in mice. Exp Parasitol 2016; 170: 73-81.
- Syeda S, Patel AK, Lee T. Reduced photoreceptor death and improved retinal function during retinal degeneration in mice lacking innate immunity adaptor protein MyD88. Exp Neurol 2015; 267: 1-12.
- Liu Z, Qu Y, Wang J. Selenium deficiency attenuates chicken duodenal mucosal immunity via activation of the NF-κb Signaling pathway. Biol Trace Elem Res 2016; 172: 465-473.