Research Article - Journal of RNA and Genomics (2025) Volume 21, Issue 1
Prospects in sustainable lead pollution management: A microbiological perspective.
Momodu Oshiomane, Esther Omoye*, Ehis-Eriakha Chioma Bertha, Akaharaiyi Fred Coolborn, Akemu Stephen Eromosele
Department of Microbiology, Edo State University Uzairue, Edo State, Nigeria
*Corresponding Author:
- Esther Omoye
Department of Microbiology, Edo State University Uzairue, Edo State, Nigeria
E-mail: azetaesther@gmail.com
Received: 21-Aug-2023, Manuscript No. RNAI-23-110799; Editor assigned: 23-Aug-2023, RNAI-23-110799 (PQ); Reviewed: 06-Sept-2023, QC No. RNAI-23-110799; Revised: 20-Jan-2024, Manuscript No. RNAI-23-110799 (R); Published: 27-Jan-2025, DOI: 10.35841/2591-7781.21.1000217
Citation: Oshiomane M, Omoye E, Bertha EEC, et al. Prospects in sustainable lead pollution management: A microbiological perspective. J RNA Genomics. 2025;21(1):1-9.
Keywords
Heavy metal, Bioremediation, Lead, Bacteria, Molecular mechanism, Lead toxicity, PGPR.
Introduction
Various anthropogenic activities have caused environmental contamination to reach its apex. Quarrying, as every mining operation, is a destructive development activity whose socioeconomic benefits may be unable to compensate for the overall detrimental effects on natural ecosystems. Blasting and crushing of rocks and use of explosives and heat releases particulate matter and dust of different metallic constituents from the machineries and blasting processes. Through this process, a large quantity of heavy metals is released into the environment as dust particles, especially into surrounding soils [1]. The most prevalent environmental pollutant and very harmful substance is heavy metal [2]. A few heavy metals occur naturally in soil but can however cause major harm when they are in higher concentrations. Pollutions caused as a result of increased concentrations of heavy metals have become a concern and threat to the environment and living organisms [3]. There is an urgent need for a remediation solution for heavy metal contamination, which is a significant environmental issue that affects the entire planet [4].
Lead (Pb) pollution has gotten the most attention of all the heavy metals due to its widespread industrial use and exceedingly hazardous properties. Lead is very valuable and easily available, which causes a lot of lead-containing waste and puts all living creatures in danger. Lead was put on the environmental potential agency agenda and 20-26 October was designated as National Lead Poisoning Prevention Week (NLPPW). The National Lead Poisoning Prevention Week (NLPPW) seeks to unite people, groups, businesses, states and local governments in order to raise awareness of the health risks posed by lead pollution and to take action to reduce lead exposure to humans and other living things.
Common physical and chemical techniques have failed to eliminate lead pollution in a way that is long-lasting. As a result, attention has been drawn to bioremediation methods that provide long-term remedies for lead pollution. Utilizing biological processes, bioremediation cleans up pollution in an efficient manner without adding more pollution. Bioremediation can be defined as a process of employing any biological entity to remediate pollutants. It can be achieved with the help of bacteria, fungus, algae, plants or any part of a living being like enzyme, or exopolysaccharide. Among all living organisms, bacteria are widely recognized for their bioremediation efficacy due to versatility and adaptability. Bacteria are capable of growing in adverse conditions by developing adaptable mechanisms. Several bacterial species such as Acinetobacter, Pseudomonas, Bacillus, Gluconacetobacter, and Serratia have been reported to solubilize and/or tolerate lead. However, reports on the ability of Bacillus infantis strain K66, Halopseudomonas xiamenensis strain B13, Lysinibacillus fusiformis strain KAF67 and Pseudomonas spp. strain A27 to bioremediate lead polluted soils are scarce.
In this study, Bacillus infantis strain K66, Halopseudomonas xiamenensis strain B13, Lysinibacillus fusiformis strain KAF67 and Pseudomonas spp. strain A27 have shown to be lead resistant bacteria. Therefore, the present work was designed to study the ability of lead-resistant bacteria in alleviating lead pollution in soil.
Materials and Methods
Bacteria collection and culture condition
The Bacillus infantis strain K66, Halopseudomonas xiamenensis strain B13, Lysinibacillus fusiformis strain KAF67, and Pseudomonas spp. strain A27 were previously isolated from the contaminated soil of Okpella mining site, screened for lead resistant potential, plant growth promoting traits and identified based on 16S rRNA sequencing and were preserved in 20% glycerol stock at 4°C and were refreshed in nutrient agar for 24 hours at 37°C before use in this study.
Functional gene analysis of lead resistant organisms
DNA extraction and high-throughput sequencing Bacterial community succession in soil samples during remediation were determined. Briefly, the total DNA in samples collected on days 1, 7, and 35 were extracted with the FastDNA® SPIN Kit for soils (Mpbio, USA) according to manufacturer’s protocols. The V3-V4 hypervariable regions of the 16S rRNA gene were amplified with the primers PbrA, PbrB, PbrC, and PbrF. After purification, quantification, and pooling, a clone library was constructed using the TruSeq® DNA PCR-Free sample preparation kit (Illumina, USA) and the amplified DNA samples were sequenced by Illumina NovaSeq 6000 (Novogene, Beijing). All the raw sequence data was filtered using Qiime quality filters (Version, 1.9.1) to remove the lowquality sequence reads. After that, the remaining sequences were clustered by using Uparse software (Uparse v. 7.0.1001) as assigned to Operational Taxonomic Units (OTUs) at similarities of 97%. Alpha diversity was applied in analyzing the complexity of species diversity through Chao1, Shannon, and Simpson indices, and beta diversity analysis was conducted to evaluate the difference of samples in species complexity using Qiime software. Additionally, Un-weighted Pair-Group Method with Arithmetic means (UPGMA) Clustering was performed as a type of hierarchical clustering method to interpret the distance matrix using average linkage and was conducted by QIIME software (Version, 1.9.1).
Collection of soil sample
For the purpose of collecting soil samples, Neboh et al. methodology was used. The samples were taken early in the day, between 9:00 and 11:00 am, when activity was at its height. To allow for the best bacterial activity, the samples of soil were dug out at a depth of 0 to 30 cm and collected using aluminum foils for both an organic chemical analysis and a microbiological examination. In ice jackets, the soil samples were delivered to the lab at Edo State University while the maize seedling was collected from ministry of agriculture.
Soil physiochemical analysis
Soil pH value and Electric Conductivity (EC) was measured at a soil/water mass ratio of 1:2.5 by using a pH meter and conductance meter, respectively. Soil Organic Matter (SOM) content was determined via K2Cr2O7 oxidation and FeSO4 titration. The water extractable organic carbon in soils was extracted with deionized water at a 1:20 (w/v) and measured with a total carbon analyzer (TOC-VCSH, Shimadzu, USA). Additionally, the available P, available K, and inorganic N (NO3 and NH4) in soils were analyzed using UV Spectrophotometer (UVmini-1240, Shimazu, Japan), flam spectrophotometer (6400A, Shjingmi Inc. China) and flow injector auto-analyzer (AA3. SEAL Analytical Inc. USA), respectively, after extraction. In terms of metals, the total Cd content was acid digested with a mixture of HCl-HNO3- HClO4, according to NY/T1613-2008 (MOA, 2008), and the available lead were extracted with Diethylenetriamine Pentaacetic Acid (DTPA) solution (1:10, w/v; pH ¼ 7.3). The lead contents in digestant and leaching solutions were measured using a flame atomic absorption spectrometry (FAAS, PerkinElmer, Aanalyst 700, USA) [5].
Total culturable heterotrophic species count
The soil sample was weighed at 1 g into a beaker and mixed with 9 ml of distilled water homogenously and test tubes were arrange in test tube rank, 9 ml of pure water was measured into the test tubes and 1 ml of mixed sample was measured from the beaker into the test tubes one with the aid of syringe and from test tube one, 1 ml was measured into test tube two and from two to three until the last fold [6]. The soil samples after serial dilution were suspended in nutrient agar. Plate was hatched at 37°C for 24 hours and colonies with morphological characteristic were counted [6].
Experimental design for bioremediaton of lead contaminated soil
Bioremediation study was carried out in a greenhouse condition using block randomized design for the experiment. Contaminated (spiked soil) and uncontaminated (control) soil samples were used in the bioremediation investigation. The experiment consists of five treatments; pot 1 treated with Bacillus infantis strain K66, pot 2 treated with Halopseudomonas xiamenensis strain B13, pot 3 treated with Lysinibacillus fusiformis strain KAF 67, pot 4 treated with Pseudomonas spp., pot 5 without bacteria (control experiment). 10% (v/v) of microbial inoculum was added to 5 g of contaminated soil. Inoculums for each treatment contained 3 × 109 CFU/mL. The experimental set up lasted for 56 days and lead bioremediation was monitored biweekly using bacteria count and lead concentration reduction respectively. The soil was irrigated with 250 ml of Water to keep the moisture level at 60-65% [7].
Spiking of soil with leas solution
In a laboratory, a solution of binary metal salt (PbCl) was used to intentionally contaminate the soil. Zero point five grams (0.5 g) of PbCl salt was incorporated into 250 ml of distilled water to achieve a concentration of 500 mgl-1 of lead and was added to the soil sample. The level of lead in the spiked soils was based on the “Soil environmental quality-risk control standard for soil contamination of agricultural land (GB15618-2018)” (MEEPRC, 2018), where risk management value is 0.5 mgl-1.
Establishing a microbial consortia for lead bioremediation in polluted soil
Prior to being inoculated into nutrient broth and cultured for 24 hours, individual strains were initially cultivated in nutrient agar for 24 h at 37°C in automatic orbital shaker fixed phase at 150 rpm. The various strains were pooled out in equal proportions at a wavelength of 600 nm after reaching a growth of 1.3 ABS [8-10].
Statistical analysis
Results from this study are shown as means with Standard Deviations (SD). The difference between many therapies was done utilizing one-way Analysis of Variance to assess (ANOVA), and Duncan's multiple comparisons were performed to ascertain the variation's relevance between various treatments at p<0.05 [11]. Utilizing origin 2020, the data were plotted. The Bioaccumulation Factors (BCF) that come after the following equations were used to quantify the transport of lead from soil to maize plants:
BCF1/4 M1=M2
Where M1 represents the lead content in maize plants (mg kg-1 dry weight) and M2 represents the metal occurrence in soil (mg kg-1 dry weight).
Results
The results show in Table 1.
| Primers | ||||
|---|---|---|---|---|
| Organisms | PbrA | PbrB | PbrC | PbrT |
| Halopseudomonas xiamenensis | + | + | + | - |
| Lysinibacillus fusiformis | + | + | + | + |
| Pseudomonas spp. | + | + | + | - |
| Bacillus infantis | + | + | + | - |
Table 1. Functional genes clusters present in lead resistant bacteria.
Base line physiochemical parameters of the sample soil
The base line result of the soil physiochemical properties revealed that the lead concentration in the soil was above permissible level by WHO and also other parameters were at variance which is an indication of the consequence of lead pollution (Table 2).
| Parameters | Soil sample | Normal soil |
|---|---|---|
| Moisture content (%) | 1.23 | 10-14 |
| pH | 8.44 | 5.8-6.2 |
| Electrical conductance (mS/cm) | 47 | 0.8-2.4 |
| Organic carbon (%) | 0.58 | 02-10 |
| Organic matter (%) | 0.96 | 03-6 |
| Phosphate (mg/kg) | 75.16 | 25-50 |
| Nitrate (mg/kg) | 4.06 | 10-50 |
| Pb (mg/kg) | 40.86 | 0-17 |
Table 2. Base line physicochemical data of lead polluted soil sample.
Base line of total culturable heterotrophic and lead resistant bacteria count
The result revealed that the lead resistant bacteria population was higher than the total culturable heterotrophic bacteria which underscore a long term input of lead in the soil (Table 3).
| Total heterotrophic bacteria | Lead resistant bacteria |
|---|---|
| 6.5 x 105 | 2.5 x 107 |
Table 3. Base line count of heterotrophic bacteria and lead-resistant bacteria.
Monitoring during bioremediation using microbiology indices
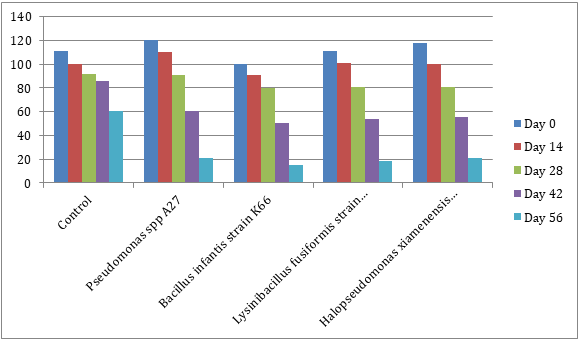
Monitoring during the bioremediation across different days revealed that there was a decrease in the bacteria population both the total heterotrophic bacteria and lead resistant bacteria as the time progresses. This could be as a result of environmental conditions (Table 4).
| Isolates | Day 0 | Day 14 | Day 28 | Day 42 | Day 56 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| THB | LRB | THB | LRB | THB | LRB | THB | LRB | THB | LRB | |
| Control | 2.6 ×107 | 3.3 × 107 | 2.2 × 107 | 3.7 × 107 | 1.1 × 107 | 2.0 × 107 | 1.3 × 107 | 2.1 × 107 | 3.7 × 106 | 1.3 × 107 |
| Pbr1 | 1.0 × 108 | 3.0 × 108 | 9.7 × 107 | 2.4 × 108 | 9.0 × 107 | 1.6 × 108 | 8.3 × 107 | 1.8 × 108 | 7.1 × 107 | 1.5 × 108 |
| Pbr2 | 1.5 × 108 | 3.2 × 108 | 1.0 × 108 | 2.6 × 108 | 8.4 × 107 | 2.0 × 108 | 7.8 × 107 | 2.9 × 108 | 6.5 × 107 | 1.6 × 108 |
| Pbr3 | 9.6 × 107 | 1.9 × 108 | 9.0 × 107 | 1.8 × 108 | 7.7 × 107 | 1.5 × 108 | 7.6 × 107 | 1.5 × 108 | 6.7 × 107 | 1.4 × 108 |
| Pbr4 | 1.4 × 108 | 2.1 × 108 | 9.6 × 107 | 1.4 × 108 | 9.7 × 107 | 1.7 × 108 | 9.0 × 107 | 1.9 × 108 | 7.8 × 107 | 1.5 × 108 |
| Note: THB: Total Heterotrophic Bacteria; LRB: Lead Resistant Bacteria | ||||||||||
Table 4. Mean values of bacterial count across different monitoring days for THB and LRB.
Spiking of soil with lead solution
The soil sample was spiked using lead solution to increase the lead concentration and also for an effective monitoring (Table 5)
| Treatments | Before | After |
|---|---|---|
| (mg/kg) | (mg/kg) | |
| Control | 40.803 | 110.86 |
| Pbr1 | 50.531 | 120.58 |
| Pbr2 | 30.476 | 100.33 |
| Pbr3 | 40.825 | 110.91 |
| Pbr4 | 40.678 | 117.74 |
Table 5. Lead concentration in the soil before and after spiking (mg/kg).
Monitoring using lead concentration degradation across different days
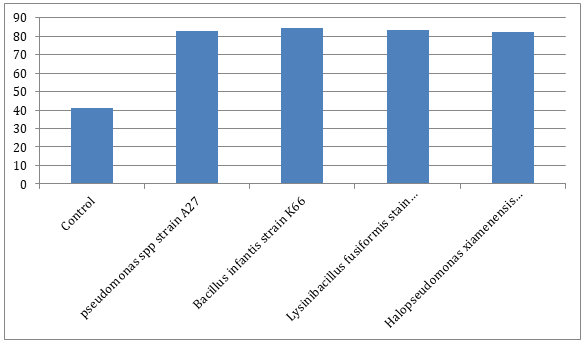
Monitoring of lead concentration degrediation revealed that Bacillus infantis strain K66 had the highest percentage degredation which was closely followed by Lysinibacillus fusiformis strain KAF67, Pseudomonas spp. strain A27, and then Halopseudomonas xiamenensis strain B13 while control pot had the lowest lead percentage degredation (Figure 1).
Lead percentage degradation during bioremediation
The percentage of lead degrediation revealed that Bacillus infantis strain K66 had the highest percentage degredation which was closely followed by Lysinibacillus fusiformis strain KAF67, Pseudomonas spp. strain A27, and then Halopseudomonas xiamenensis strain B13 while control pot had the lowest lead percentage degradation (Figure 2).
Shoot length of maize plant
It was observed that the shoot length of maize plant in pot 2 had the highest shoot length of (35.62 cm), also it maintained the highest shoot length throughout the cultivation period. It was closely followed by pot 3 (33.71 cm), pot 1 (32.99 cm) and then pot 4 (30.99 cm) while the control pot had the lowest growth (17.91 cm) (Table 6).
| Days | Control pot (cm) | Pot1 (cm) | Pot2 (cm) | Pot3 (cm) | Pot4 (cm) |
|---|---|---|---|---|---|
| 7 | 4 | 7 | 9 | 7 | 8 |
| 14 | 18 | 27 | 31 | 30 | 29 |
| 21 | 24 | 49 | 56 | 53 | 48 |
| 28 | 47 | 84 | 92 | 86 | 81 |
| Standard deviation | 17.91 | 32.99 | 35.62 | 33.71 | 30.99 |
Table 6. Shoot lenght of maize plant at day 7, 14, 21 and 28 (cm).
Root length of maize plant
The root length of maize plant revealed that maize plant in pot 2 had the highest shoot length (20.09 cm), followed by pot 3 (18.71 cm), pot 1 (17.78 cm) and then pot 4 (16.82 cm) while the control pot had the shortest root length (8.74 cm) (Table 7).
| Days | Control pot (cm) | Pot1 (cm) | Pot2 (cm) | Pot3 (cm) | Pot4 (cm) |
|---|---|---|---|---|---|
| 7 | 1.4 | 4 | 4 | 5 | 5 |
| 14 | 4 | 7 | 7 | 8 | 8 |
| 21 | 7 | 15 | 18 | 17 | 16 |
| 28 | 21 | 41 | 48 | 46 | 44 |
| Standard deviation | 8.74 | 16.82 | 20.09 | 18.71 | 17.78 |
Table 7. Root length of maize plant at day 7, 14, 21 and 28 (cm).
Fresh and dry root weight of maize plant
It was observed that pot 2 had the highest fresh root weight which was (3.18 cm) and dry root weight (1.03 cm) of maize plant followed by pot 3 (3.07, 1.01 cm), pot 1 (2.84, 0.96 cm) and then pot 4 (2.76, 0.89 cm) while the control pot had the lowest fresh and dry root weight (0.82, 0.25 cm) of the maize plant (Table 8).
| Treatment | Fresh root weight | Dry root weight |
|---|---|---|
| Control pot | 0.82 | 0.25 |
| Pot1 | 2.84 | 0.96 |
| Pot2 | 3.18 | 1.03 |
| Pot3 | 3.07 | 1.01 |
| Pot4 | 2.76 | 0.89 |
Table 8. Fresh and dry root weight of the maize plant after cultivation (cm).
Chlorophyll content of maize plant
It was observed that pot 2 had the highest chlorophyll content (63%) in the maize plant followed by pot 3 (60%), pot 1(59%) and then pot 4 (58%) while the control pot had the lowest chlorophyll content (24%) (Table 9).
| Treatment | Percentage (%) |
|---|---|
| Control | 24 |
| Pot1 | 59 |
| Pot2 | 63 |
| Pot3 | 60 |
| Pot4 | 58 |
Table 9. Chlorophyll content of the maize plant (%).
Lead uptake by maize plant
It was observed that only the control pot had lead uptake of (48%) in the maize plant while lead uptake was not detected in other pots after 21 days of cultivation (Table 10).
| Treatment | Percentage (%) |
| Control pot | 48 |
| Pot1 | not discovered |
| Pot2 | not discovered |
| Pot3 | not discovered |
| Pot4 | not discovered |
Table 10. Lead uptake in maize plant (%).
Residual lead in soil after maize cultivation
It was observed that only control pot had residual lead content of (53%) present while other pots had no residual lead content at the end of the cultivation period (Table 11).
| Treatment | Percentage (%) |
|---|---|
| Control | 53 |
| Pbr1 | not discovered |
| Pbr2 | not discovered |
| Pbr3 | not discovered |
| Pbr4 | not discovered |
Table 11. Residual lead content present in soil after cultivation of maize (%).
Discussion
This study involves the use of different strains of lead resistant bacteria combined with plant growth promoting bacteria; Lysinibacillus fusiformis strain BT3 to achieve the bioremediation and restoration of lead polluted soil. The lead resistant bacteria were obtained from previous studies after screening using different parameters to assess their tolerance potential to lead and identified with the 16S rRNA typing method. In this study, the genetic potential of these organisms were further assessed to confirm their resistance capability using PbrABCT gene clusters responsible for lead bioprecipitation from the environment [12]. The lead polluted soil sample prior to bioremediation was analysed for physicochemical properties and subsequently treated with the different lead resistance bacteria for a period of 56 days with periodic monitoring of lead concentration and bacterial count. At the end of the treatability study, strain BT3 was introduced to the soil as biofertilizer for the cultivation of maize to support the restoration of the lead treated soil [13].
At the end of the functional gene analysis Pseudomonas spp. strainA27, Bacillus infantis strain K66, and Halopseudomonas xiamenesis B13 harbored three of the gene clusterPbrA, PbrB and PbrC while Lysinibacilus fusifumis strain KAF67 harbored all of the genes in the cluster: PbrA, PbrB, PbrC and PbrT ascertaining their potential to tolerate lead using the bioprecipitation mechanisms which has been established according to Utami et al. [14]. The lead resistant gene cluster pbrTRABCD in Cupriavidus metallidurans CH34 has been implicated in the molecular mechanism of lead resistance in previous studies [15]. According to Sevvat et al., where PbrT encode a putative lead uptake which help to reduce the lead concentration in the soil, PbrB/PbrC creates integral membrane proteins having characteristics like signal peptidase responsible for phosphatase and PbrA produces an Adenosine Triphosphate (ATPase) transporter that aids in the cytoplasmic export of lead. As a result of the inorganic phosphate reaction produced by undecaprenyl pyrophosphate phosphatase, which is encoded by PbrB, the lead is further sequestered in precipitated form while to stop lead from being reintroduced into the microbes, PbrA transfers lead out of the cell, where it is precipitated by the inorganic phosphate that PbrB releases. PbrC creates essential membrane proteins which perform the role of signal peptidase responsible for phosphatase while PbrT encodes the potential lead uptake and lead-binding proteins. PbrABC also referred to as phosphate ATP-Binding Cassette (ABC) transporters have been established to be expressed by bacteria which aids in the extracellular production of phosphate needed to precipitate lead and these set of genes were present in strains A27, K66 and B13, respectively [16]. All of these genes in the cluster are connected and work together inside the bacterial cell to promote lead removal through the bioprecipitation mechanism. However, other kinds of molecular defenses are available to bacteria to deal with lead toxicity such as biosorption, efflux mechanism, siderophore production and others [17].
The physicochemical characteristics of the soil sample were at variance when compared with pristine soil. This served as baseline result to ascertain the prevailing inherent soil properties prior to bioremediation. In the soil sample, there was a greater level of lead compared with pristine soil which shows the effect of the mining activities in the area. According to Liu et al., crushed rock mining activities generates considerable amount of dust and wastes, which significantly increases the concentration of lead in the soil. These lead are mobilized or dissolved into the soil which tends to increase the concentration of the natural deposits and alter other physiochemical properties in the soil [18]. Different authors have pointed out the contribution of mining activities to the soil such as sincrease in lead concentration, reduction of soil fertility and biodiversity, decreased output and plant growth and increase in soil alkilinity. The higher concentrations of lead recorded in the soil sample within the mining site also confirms the relationship between lead concentration and mining activities and the results generated from this study are in line with the work of Nwovu et al. Other parameters analyzed such as nitrate and phosphate showed alterations in the physicochemical structure when compared to pristine soil, also an evidence of the effect of long term lead pollution. The phosphate content was higher in the contaminated soil than the pristine soil while nitrate was lower in the polluted soil sample (Table 4). The result may look incorrect or inaccurate but as earlier stated microorganisms responsible for bioprecipitation as lead toxicity reduction mechanism make use of Phosphate-Solubilizing Bacteria (PSB) which play a crucial part in soil lead bioremediation by releasing the phosphate needed for the lead bioprecipitation reaction from insoluble phosphate molecules like Ca3(PO4)2 [19]. Now that phosphate is bioavailable, it can interact with lead to create an insoluble lead phosphate, which will make lead less mobile. Also, the THB population in the mining site is lower than LRB which infers a possible reduction in the nitrogen fixing bacteria and nitrifying bacteria population responsible for producing nitrates and other nitrogen-based derivatives as a result of the toxic effect of lead contamination, this could be the reason for the low nitrate content in the soil sample. According to Raghad et al., heterotrophic microbes found in contaminated soil were observed to be very low as this is due to the sensitivity of nitrifying bacteria and other non-lead resistant bacteria to high lead concentrations.
Since lead pollution is known to affect the biotic and abiotic components of the environment, bioremediation has been established to be the most effective ecofriendly and cost effective approach. In this study, the bioremediation of lead was achieved employing the lead tolerant bacteria as remediation agents in order to remove lead from the sample soil. At the end of the 56 days period, strain K66 caused the highest percentage of lead reduction which was closely followed by strain KAF67, A27 and B13, all samples treated under same experimental conditions. These organisms individually caused >80% lead reduction. According to Kuddus et al., bioremediation of heavy metals can be scored successful when ≥ 65% or more of the metals are removed from a polluted environment and this indicates that the lead resistant bacteria displayed highly proficient capacity to precipitate and remove lead from the sample. This was achieved based on the functional gene analysis which showed the bacteria harbored gene clusters responsible for bioprecipitation of lead from soil. As reported by researchers, some bacteria have a stronger affinity and sensitivity to lead than others, which may explain the variance in the amount of lead removed by each treatment. When compared to a similar study by Fauziah et al., which investigated remediation of leadcontaminated soil using microbe isolated from a closed dump site, the removal activities of lead in the soil samples were very high. They found that adding microbes, specifically, introducing proteobacteria to leachate-contaminated soil may significantly reduce the heavy metal concentration, and adding bacterial groups to contaminated soil can remove metals from the environment more effectively. Although strain K66 harbored three of the gene clusters, it scored the highest remediation percentage which supports the fact bacteria responds to lead contamination differently, not just based on their genetic resistance signatures but also natural affinity and sensitivity. However, some Bacillus strains are known to be lead resistant with a high potential to resist a wide range of lead derivatives/ lead -based compounds. According to Qiao et al., Bacillus subtilis X3 bioprecipitates lead in a variety of forms, including Pb5(PO4)3OH, Pb10(PO4)6(OH)2, and Pb5(PO4)3Cl which supports the above statement.
No Significant Difference (SD) was recorded between the treatments however there was SD between treatment and control at p<0.05. The control had the least recorded lead removal and this could be attributed to the absence of any treatment or inoculant to enhance and sustain the remediation process which could be due to the presence of indigenous LRBs.
The increased level of lead in the environment has significantly affected the overall microbial activity and community as well as population size. Several research projects, based on the isolation-based protocol applied, have demonstrated that lead contamination gave rise to shifts in microbial population which is consistent with the findings from this study [20]. As seen in Table 4, after introducing the bacteria into the soil during bioremediation, the results revealed that higher populations of culturable LRB were obtained compared to THB pre and during bioremediation across all treatments and sampling days which shows a modification or shift in the bacterial population as a result of lead contamination. Also, the control pot which was un-inoculated showed a higher count of LRB consistently throughout the treatability study although both populations decreased across different days of investigation. From this study, it implies that the bioremediation of lead in soil samples were very successful.
Conclusion
Lead is a priority contaminant and highly hazardous. There have been numerous reports of lead pollution around the world, demonstrating the urgent need for environmentally friendly lead cleanup technologies. Lead resistance has been documented in a wide range of microorganisms. Different defense mechanisms have been established by lead-resistant bacteria to combat lead poisoning. Furthermore, based on several bacterial bioremediation methods, the potential applicability of lead-resistant bacteria was deduced. Combined application of lead reducing bacteria holds great promise in gaining environmental sustainability.
References
- Al-Ani RA, Adhab MA. Bean Yellow Mosaic Virus (BYMV) on broadbean: Characterization and resistance induced by Rhizobium leguminosarum. J Pure Appl Microbiol. 2013;7(1):135-42.
- Koul B, Singh S, Dhanjal DS, et al. Plant Growth-Promoting Rhizobacteria (PGPRs): A fruitful resource. Microbial Interventions in Agriculture and Environment: Volume 3: Soil and Crop Health Management. 2019:83-127.
- Bharagava RN, Mishra S. Hexavalent chromium reduction potential of Cellulosimicrobium sp. isolated from common effluent treatment plant of tannery industries. Ecotoxicol Environ Saf. 2018;147:102-9.
[Crossref] [Google Scholar] [PubMed]
- Bouquet D, Lepinay A, Gaudin P, et al. A new assay of bacterial selection with Pb reveals an unexpected effect of Pb on bacterial behavior: Implications for remediation. Environ Chem Lett. 2020;18:983-92.
- Brink HG, Horstmann C, Peens J. Microbial Pb (II)-precipitation: The influence of oxygen on Pb (II)-removal from aqueous environment and the resulting precipitate identity. Int J Environ Sci Technol. 2020;17:409-20.
- Briao GD, De Andrade JR, da Silva MG, et al. Removal of toxic metals from water using chitosan-based magnetic adsorbents. A review. Environ Chem Lett. 2020;18:1145-68.
- Brütting C, Crava CM, Schafer M, et al. Cytokinin transfer by a free-living mirid to Nicotiana attenuata recapitulates a strategy of endophytic insects. Elife. 2018;7:e36268.
[Crossref] [Google Scholar] [PubMed]
- Chen B, Fang L, Yan X, et al. A unique Pb-binding flagellin as an effective remediation tool for Pb contamination in aquatic environment. J Hazard Mater. 2019;363:34-40.
[Crossref] [Google Scholar] [PubMed]
- Das S, Dash HR, Chakraborty J. Genetic basis and importance of metal resistant genes in bacteria for bioremediation of contaminated environments with toxic metal pollutants. Appl Microbiol Biotechnol. 2016;100:2967-84.
[Crossref] [Google Scholar] [PubMed]
- Dixit R, Wasiullah X, Malaviya D, et al. Bioremediation of heavy metals from soil and aquatic environment: An overview of principles and criteria of fundamental processes. Sustainability. 2015;7(2):2189-212.
- Saha M, Sarkar S, Sarkar B, et al. Microbial siderophores and their potential applications: A review. Environ Sci Pollut Res Int. 2016;23:3984-99.
[Crossref] [Google Scholar] [PubMed]
- Bertha EE, Ezeanya-Bakpa CC, Akemu SE, et al. Assessment of Indigenous Rhizospheric Soil Microbes from Zea mays and Manihot esculenta for Plant Growth Promoting (PGP) Traits.
- Sabol J. Major analytical methods for determining lead in environmental and biological samples. Lead in Plants and the Environment. 2020:1-3.
- Rehan M, Alsohim AS. Bioremediation of heavy metals. Environmental chemistry and recent pollution control approaches. 2019;145-58. [Crossref]
[Google Scholar] [PubMed]
- Figueiredo MD, Seldin L, de Araujo FF, et al. Plant growth promoting rhizobacteria: Fundamentals and applications. Plant growth and health promoting bacteria. 2011:21-43.
- Egamberdiyeva D. The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Appl Soil Eco. 2007;36(2-3):184-9.
- Goteti PK, Emmanuel LD, Desai S, et al. Prospective zinc solubilising bacteria for enhanced nutrient uptake and growth promotion in maize (Zea mays L.). Int J Microbiol. 2013;2013(1):869697.
[Crossref] [Google Scholar] [PubMed]
- He C, Gu L, Xu Z, et al. Cleaning chromium pollution in aquatic environments by bioremediation, photocatalytic remediation, electrochemical remediation and coupled remediation systems. Environ Chem Lett. 2020;18:561-76.
- Nadarajah KK. Induction of systemic resistance for disease suppression. Crop Improvement: Sustainability Through Leading-Edge Technology. 2017:335-57.
- Msimbira LA, Smith DL. The roles of plant growth promoting microbes in enhancing plant tolerance to acidity and alkalinity stresses. Frontiers Sustain Food Syst. 2020;4:106.