Research Article - Current Pediatric Research (2021) Volume 25, Issue 4
Prognostic significance of Cytokine Receptor-Like Factor 2 (CRLF2) gene expressions in pediatric acute lymphoblastic leukemia.
Maha Youssef Kamal1*, Neven Lewis2, Mona Wagdi Ayad2, Omnya Sabri2
1 Departments of Paediatrics, Alexandria University, Alexandria, Egypt
2 Department of Clinical Pathology, Alexandria University, Alexandria, Egypt
Corresponding Author:
- Maha Youssef Kamal
Department of Pediatrics Faculty of Medicine University of Alexandria Egypt
Tel: 00201223106023
E-mail: elsayedamr@yahoo.com
Accepted date: April 19, 2021
Abstract
Background: The Cytokine Receptor-Like Factor 2 (CRLF2) genes play an important role in early Bcell development. Aberrations in CRLF2 activate the JAK-STAT signaling pathway that contributes to B-cell Acute Lymphoblastic Leukemia (B-ALL). The prognostic significance of CRLF2 overexpression in various B-ALL risk subgroups has not been well established. Methods: Thirty patients with newly diagnosed childhood B-ALL were enrolled from the Egyptian population. All the patients in this study were assessed by detailed history taking, a complete clinical examination required radiological and routine laboratory investigations, and flow cytometry to detect the subtype of ALL. Peripheral Blood and bone marrow samples were taken from patients, and the CRLF2 expression was measured using RT-PCR. Results: The prevalence of CRLF2 overexpression status was analyzed, and the prognostic impact of CRLF2 overexpression on B-ALL was evaluated by assessing their influence on overall survival and event-free survival. CRLF2 overexpression was found in 56% of the whole cohort. The results showed that CRLF2 overexpression was associated with a poor outcome in unselected B-ALL. Conclusion: From the present study's findings, we can conclude that CRLF2 gene expression at diagnosis has a significant prognostic impact on the clinical outcome in childhood ALL.
Keywords
Acute lymphoblastic leukemia, Cytokine receptor-like factor 2, Paediatrics, Genetic aberrations.
Introduction
B-cell Acute Lymphoblastic Leukemia (B-ALL) is the most common type of ALL in children; it is characterized by clonal genetic aberrations. Lots of have become fat in treating B-ALL by risk-adapted approach. 20%-25% of B-ALL fails to respond to current therapies, with an increased relapse incidence [1].
Recent advances in the molecular characterization of B-ALL offer chances to identify genetic aberrations as new prognostic indicators for better risk stratification and new targeted antileukemia agents [2].
The Cytokine Receptor-Like Factor 2 (CRLF2) genes encodes a subunit of the Thymic Stromal Lymphopoietin (TSLP) receptor that heterodimerizes with the Interleukin-7 Receptor (IL7R) to form the type I cytokine receptor. The type I cytokine receptor is a functional receptor for TSLP that is an important physiologic signal for early B-cell development [3,4]. Upon ligand binding, the type I cytokine receptor interacts with JAK proteins, which in turn phosphorylate certain targets of STAT5 to promote transcription of proliferation and antiapoptotic factors CRLF2 plays a role in early B-cell development, and CRLF2 aberrations activate the JAK-STAT signaling pathway leading to increased cell proliferation [5-7]. CRLF2 rearrangements have been reported in about 5%–10% of childhood B-ALL cases and 50% of B-ALL patients with down syndrome CRLF2 genetic aberrations are mainly due to an interstitial deletion of the pseudoautosomal region on the sex chromosomes (Xp22/ Yp11), resulting in the P2RY8-CRLF2 fusion gene or a cryptic translocation between Xp22 or Yp11 and 14q32/IGH, resulting in the IGH-CRLF2 fusion gene [6]. Other aberrations such as the CRLF2 mutation F232C are rare in BALL [8].
The prognostic impact of CRLF2 aberrations on B-ALL is still undetermined with a presumptive assumption that an association between overexpression of CRLF2 and adverse prognosis, while a correlation between P2RY8-CRLF2 and an inferior outcome has been demonstrated [8,9]. No well-defined prognostic value of CRLF2 overexpression on B-ALL with different risk features has been well established yet. The aim of the present study is to assess the relationship and prognostic significance of CRLF2 overexpression in childhood ALL.
Patients and Methods
The study was conducted on two groups; Group 1: 30 pediatric newly diagnosed for ALL patients; and Group 2: 10 healthy pediatric patients as a control group.
All the patients in this study were assessed by detailed history taking, complete clinical examination, required radiological and routine laboratory investigations, and flow cytometry to detect the subtype of ALL. Peripheral Blood and bone marrow samples were taken from patients, and the CRLF2 expression was measured using RT-PCR.
Results
In the present study, ALL group included 9 (30%) females and 21 (70%) males, while the control group consisted of 4 (40.0%) females and 6 (60%) males, and there was no statistically significant difference detected between the two groups as regards the gender (p=0.700).
The age of ALL patients ranged from 1 to 18 years with a mean of 5.30 ± 3.32 years, while the age of controls ranged from 2 to 9 years with a mean of 5.08 ± 2.20 years. No statistically significant difference was detected between the two studied groups as regards the age (p=0.847)
The most frequent clinical presentation was hepatomegaly in 25 (83.3%) patients followed by fever (80%), then splenomegaly (73.3%), lymphadenopathy (50.00%), bone pain, pallor (53.3%), and the least frequent was a bone fracture, Deviation of mouth, Eye puffiness and testicular swelling (2.63%).
Bone marrow aspiration was done following the end of induction chemotherapy to assess response to treatment. 21 (70%) patients achieved complete remission (negative MRD), and 9 (30%) patients had induction failure (positive MRD) CRLF2 gene expression was compared between the cases and control using 2-ΔΔct. There was a statistically significant difference between ALL patients and controls, with 17 (56.7%) of the cases showed significantly high gene expression compared with the control group with a mean expression level of 253.77 ± 764.34 (Table 1).
| All patients (n=30) | Control (n=10) | ||||
|---|---|---|---|---|---|
| n | % | n | % | ||
| CRLF2 (× 104) normal | 13 | 43.3 | 10 | 100 | FEp=0.002* |
| Over | 17 | 56.7 | 0 | 0 | |
| Min-Max. | 0.0-3914.32 | 1.0-1.0 | p=0.011* | ||
| Mean ± SD | 253.77 ± 764.34 | 1.0 ± 0.0 | |||
| Median | 5.54 | 1 |
Table 1. CRLF2 comparison between the two studied groups. n: Number of patients; Min: Minimum; Max: Maximum; SD: Standard Deviation; FE: Fisher Exact correction; *: Statistically significant.
Overall survival analysis was performed using the Kaplan- Meier method, and the differences were compared using the log-rank test. Overall survival was defined as the time from the diagnosis to death from any cause or the last follow-up (Table 2).
| CRLF2 | p | ||||
|---|---|---|---|---|---|
| Normal (n=13) | Over (n=17) | ||||
| n | % | n | % | ||
| Risk stratification | |||||
| High risk | 3 | 23.1 | 12 | 71 | p=0.010* |
| Standard risk | 10 | 76.9 | 5 | 29 | |
| Age (years) | |||||
| Min-Max | 1.50-9.0 | 1.0-13.0 | p=.863 | ||
| Mean ± SD | 5.42 ± 2.49 | 5.21 ± 3.91 | |||
| Median | 5.5 | 3.5 | |||
| WBCs (/µL) | |||||
| <50000 | 12 | 92.3 | 8 | 47 | FEp=.017* |
| >50000 | 1 | 7.7 | 9 | 53 | |
| End of induction BM | |||||
| Negative | 12 | 92.3 | 9 | 53 | FEp=.042* |
| Positive | 1 | 7.7 | 8 | 47 | |
| Death within one year | |||||
| Died | 0 | 0 | 3 | 18 | FEp=.042* |
| Alive | 13 | 100 | 14 | 82 | |
| Relapse | |||||
| No | 13 | 100 | 16 | 94 | FEp=1.000 |
| Yes | 0 | 0 | 1 | 5.9 | |
| Remission after indication | |||||
| No | 1 | 7.7 | 8 | 47 | FEp=.042* |
| Yes | 12 | 92.3 | 9 | 53 | |
Table 2. Relation between CRLF2 and different parameters in the cases group. n: Number of patients; Min: Minimum; Max: Maximum; SD: Standard Deviation; FE: Fisher Exact correction; *: Statistically significant.
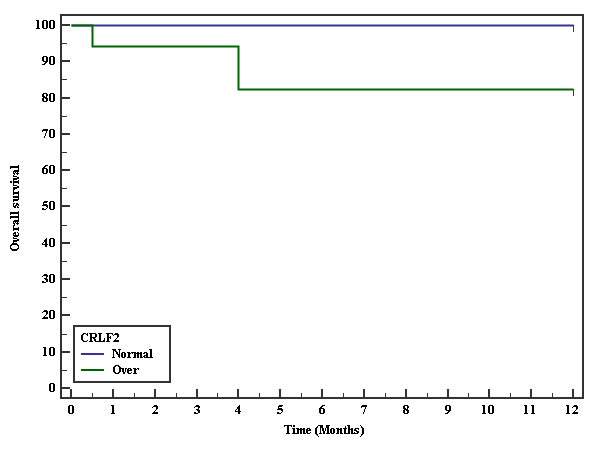
The period of follow-up was twelve months. 100% of patients with normal expression survived, while 82.4% of patients with overexpression survived. There was no statistically significant difference (p=0.118) (Figure 1).
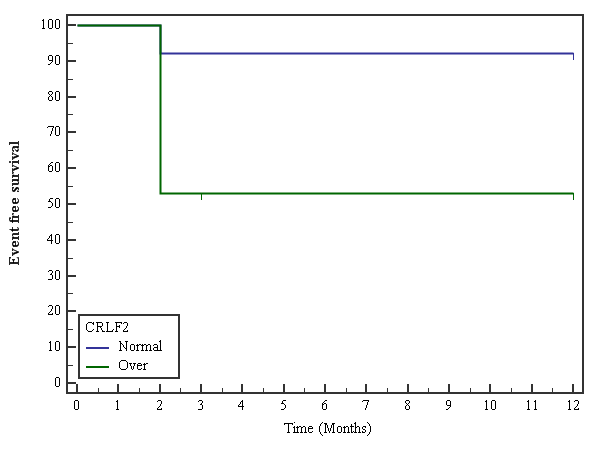
Event-free survival was defined as the time from diagnosis to induction failure or relapse. The initial induction therapy response was 52.9% of patients with overexpression compared to 92.3% of patients with normal gene expression. This difference was statistically significant (p=0.022) (Figure 2).
Discussion
In this study, there was a significant difference between the case and control group regarding CRLF2 gene expression; we found that CRLF2 mRNA level was significantly higher in cases compared to normal control. In accordance with our findings, Ge et al., observed the same difference between the patients and control groups [10].
Differences in CRLF2 overexpression incidences seen in different groups are likely due in part to other methods used to define the cut-off for CRLF2 overexpression. Some set cut-off at the lowest level of CRLF2 expression in the patients with known CRLF2 rearrangement, and others described the threshold as over specific folds of overall median expression value.
In our study, ALL patients were categorized according to NCI into the standard risk group (50%) and high-risk group (50%).
It was observed that high CRLF2 expression was more prevalent in patients with HR-ALL (70%, 12/15) than in the patients with SR-ALL (29%, 5/15).
Agreeing with us, a study performed by Yamashita et al., who performed their study on 194 patients, found higher CRLF2 gene expression in HR-ALL than in SR-ALL. The contradictory results reported by Asai et al., in which CRLF2 gene overexpression was more prevalent in the standard-risk group (10/41) than the high-risk group (6/50) [11,12]. MRD monitoring based on a specific marker can help us predict leukemia relapse and determine the best-individualized treatment. In the present work, we measured MRD at the end of induction therapy and relate their value to CRLF2 gene expression.
We found that the patients' higher CRLF2 gene expression at diagnosis was associated with significantly higher MRD (P=0.02), as ALL patients who had a positive MRD after induction were nine patients, eight of whom had elevated gene expression, and only one case with normal gene expression. Similarly, Chen et al. studied the association of high CRLF2 expression with end induction MRD and observed that HR– ALL cases with high CRLF2 gene expression are associated with higher MRD level and poor RFS at the end of induction therapy. Palmi et al., who classified his group of patients into a high-risk group, intermediate-risk group, and standard-risk group, found that statically significant difference between CRLF2 overexpression and MRD across the different risk subgroup (p=0.09) [13,14].
In the present work, patients were followed up for 12 months, and the influence of CRLF2 gene expression was determined using Kaplan-Meier. OS was defined as the time from the diagnosis to death from any cause or the last follow-up. There was no significant between CRLF2 gene expression and overall survival. These results go hand in hand with the findings with Palmi et al., who studied the prognosis of P2RY8-CRLF2 fusion CRLF2 overexpression in pediatric ALL who found that CRLF2 overexpression did not affect overall survival (p=0.35), but the patients with P2RY8-CRLF2 fusion have a very poor outcome [14].
In the present study, the influence of CRLF2 overexpression on Event-free survival was significant (p=0.022). These findings are consistent with the results of Yamashita et al., who observed that 4-year EFS for the patients with high CRLF2 expression was significantly worse than that for those with low CRLF2 expression (p=0.003) and statistical difference between these groups was recognized only in patients with HR-ALL [11,12]. Also, Dou et al., found that patients with CRLF2 overexpression had shorter EFS (P=0.004) [15].
Conclusion
From the present study's findings, we can conclude that CRLF2 gene expression at diagnosis has a significant prognostic impact on the clinical outcome in childhood ALL.
References
- Pui C-H, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med 2006; 354(2): 166-78.
- Moorman AV. New and emerging prognostic and predictive genetic biomarkers in B-cell precursor acute lymphoblastic leukemia. Haematologica 2016; 101(4): 407-16.
- Pandey A, Ozaki K, Baumann H, Levin SD, Puel A, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nature Immunology 2000; 1(1): 59-64.
- Brown VI, Hulitt J, Fish J, Sheen C, Bruno M, et al. Thymic stromal-derived lymphopoietin induces proliferation of pre-B leukemia and antagonizes mTOR inhibitors, suggesting a role for interleukin-7Rα signaling. Cancer Research 2007; 67(20): 9963-70.
- Lin TS, Mahajan S, Frank DA. STAT signaling in the pathogenesis and treatment of leukemias. Oncogene 2000; 19(21): 2496-504.
- Mullighan CG, Collins-Underwood JR, Phillips LA, Loudin MG, Liu W, et al. Rearrangement of CRLF2 in B-progenitor and down syndrome-associated acute lymphoblastic leukemia. Nature Genetics 2009; 41(11): 1243-6.
- Russell LJ, Capasso M, Vater I, Akasaka T, Bernard OA, et al. Deregulated expression of cytokine receptor gene, CRLF2, is involved in lymphoid transformation in B-cell precursor acute lymphoblastic leukemia. Blood 2009; 114(13): 2688-98.
- Cario G, Zimmermann M, Romey R, Gesk S, Vater I, et al. Presence of the P2RY8-CRLF2 rearrangement is associated with a poor prognosis in non-high-risk precursor B-cell acute lymphoblastic leukemia in children treated according to the ALL-BFM 2000 protocol. Blood 2010; 115(26): 5393-7.
- Ensor HM, Schwab C, Russell LJ, Richards SM, Morrison H, et al. Demographic, clinical, and outcome features of children with acute lymphoblastic leukemia and CRLF2 deregulation: results from the MRC ALL97 clinical trial. Blood 2011; 117(7): 2129-36.
- Ge Z, Gu Y, Zhao G, Li J, Chen B, et al. High CRLF2 expression associates with IKZF1 dysfunction in adult acute lymphoblastic leukemia without CRLF2 rearrangement. Oncotarget 2016; 7(31): 49722-32.
- Yamashita Y, Shimada A, Yamada T, Yamaji K, Hori T, et al. IKZF1 and CRLF2 gene alterations correlate with poor prognosis in Japanese BCR-ABL1-negative high-risk B-cell precursor acute lymphoblastic leukemia. Pediatr Blood Cancer 2013; 60(10): 1587-92.
- Asai D, Imamura T, Suenobu S, Saito A, Hasegawa D, et al. IKZF1 deletion is associated with a poor outcome in pediatric B-cell precursor acute lymphoblastic leukemia in Japan. Cancer medicine 2013; 2(3): 412-9.
- Chen IM, Harvey RC, Mullighan CG, Gastier-Foster J, Wharton W, et al. Outcome modeling with CRLF2, IKZF1, JAK, and minimal residual disease in pediatric acute lymphoblastic leukemia: A Children's Oncology Group study. Blood 2012; 119(15): 3512-22.
- Palmi C, Vendramini E, Silvestri D, Longinotti G, Frison D, et al. Poor prognosis for P2RY8-CRLF2 fusion but not for CRLF2 over-expression in children with intermediate risk B-cell precursor acute lymphoblastic leukemia. Leukemia 2012; 26(10): 2245-53.
- Dou H, Chen X, Huang Y, Su Y, Lu L, et al. Prognostic significance of P2RY8-CRLF2 and CRLF2 overexpression may vary across risk subgroups of childhood B-cell acute lymphoblastic leukemia. Genes Chromosomes Cancer 2017; 56(2): 135-46.