Research Article - Current Pediatric Research (2025) Volume 29, Issue 2
Prevalence, associated factors and antibiogram of streptococcal upper respiratory tract infections in children under 5 years at holy innocents children's hospital, Mbarara city.
Kenyange Ritah, Abonga Emmanuel, Ebenezer Felex Sikon*, Ainomugisha Mathias, Lwanga Justus, Muwanguzi Enoch
Department of Medical Laboratory Science, Mbarara University, Mbarara, Uganda
- Corresponding Author:
- Ebenezer Felex Sikon
Department of Medical Laboratory Science, Mbarara University, Mbarara, Uganda,
Email: siikoniebenezer97@gmail.com
Received: 22-Jul-2024, Manuscript No. AAJCP-24-142747; Editor assigned: 24-Jul-2024, AAJCP-24-142747 (PQ); Reviewed: 07-Aug-2024, QC No. AAJCP-24-142747; Revised: 08-Feb-2025, Manuscript No. AAJCP-24-142747 (R); Published: 15-Feb-2025, DOI: 10.35841/0971-9032.29.02.1-8
Abstract
Objectives: This study aimed at understanding the prevalence, associated factors and antibiogram of streptococcal upper respiratory tract infections in children under 5 years at Holy Innocents Children’s Hospital, Mbarara.
Methods: This was the hospital-based cross-sectional study at Holy Innocents Children’s Hospital (HICH), Mbarara city in Uganda from August to September 2022. The study included children aged 5 years below presenting with signs and symptoms of upper respiratory tract infections. Clinical and demographic data was collected using a well-structured questionnaire and also by use of patient logbooks. The study included 236 participants whose oropharyngeal swabs were collected in Stuart transport media and transported to the Mbarara Diagnostic Laboratory Center (MDLC) for culture to isolate the Streptococci species. Culture was done by standard microbiology procedure for isolation of the Streptococci species from the samples and identification done using gram staining, catalase test, optochin, bacitracin test and bile solubility test. Antibiotic susceptibility testing was done in accordance with the Clinical and Laboratory Standard Institute (CLSI) guidelines. The collected data was entered in excel, cleaned and exported to SPSS version 22 for final analysis using the appropriate analysis models.
Results: The prevalence of streptococcal URTIs was 36.6% of the 236 studied participants, most 119/236 (50.4%) were females. The mean age of the children was found to be 31.5 months (SD ± 16.2 months (95% CI 29.4-33.6)). Most 175/236 (74.2%) of the children had been weaned from breast milk and antibiotic usage in at least the last one month was reported to be low 26/236 (11.02%). Among the caretakers, the mean age was found to be 33.3 years (SD ± 8.7 years (95% CI 32.2-34.4)) and most who presented to hospital were found to be female 161/236 (71.6%). Among the children, the prevalence of Streptococcal URTIs was found to be 86/236 (36.4%). Viridan streptococci accounted for the most 59/86 (68.6%) isolated, followed by Streptococci pyogenes which accounted for 25/86 (29.1%). Streptococci pneumoniae and Streptococci pyogenes were found to be 100% susceptible to ceftriaxone. However, few 4/59 (6.8%) of the Viridans streptococci were observed to be resistant. Very high resistance for observed for penicillin (100%) and ampicillin (above 80%). Variables that were significantly associated with having an URTI among our study population were education of the caretaker (P=0.022), living with other siblings (P=0.048), anyone smoking a cigarette (P=0.036) and age of the child (P=0.037).
Conclusion: The study found that the prevalence of Streptococcal URTIs among children admitted at the hospital was high mostly associated with Viridan streptococci. The following variables that were significantly associated with having an URTI among our study population; education of the caretaker, living with other siblings, anyone smoking a cigarette and age of the child. Resistance to the studied penicillin by the different streptococcal isolates was found to be high. The most effective drug that most streptococcal isolates were susceptible to was found to be ceftriaxone.
Keywords
Mbarara diagnostic laboratory center, Upper respiratory tract infections, Viridan streptococci, Streptococci pneumoniae, Penicillin, Ampicillin.
Introduction
Upper Respiratory Tract Infections (URTIs can be defined as self-limiting irritations and swellings of the upper air ways (nose, sinuses, pharynx, larynx and the large air ways that are always associated cough with no proof of pneumonia or with no history of Chronic Obstructive Pulmonary Disease (COPD/ emphysema/chronic bronchitis [1]. Respiratory tract infections are important causes of mortality and hospitalization among children less than 5 years of age [2]. The latter are always categorized based on the area affect that is the upper or lower part of the respiratory system.
Over 9 million streptococcal diseases were reported in children below 5 years where more than 300 died with the majority of the cases occurring in low and middle income countries [3]. URTIs are one of the main health challenges among children for example in America, URTIs account for 40% to 60% of pediatric consultations [4]. In 2015, In sub-Saharan Africa, acute respiratory infections are a leading cause of mortality among children under 5 years of age. Reports from Uganda indicate that 80% of children that seek treatment from a health worker present with symptoms of an acute respiratory infection [5]. Common bacterial pathogens responsible for upper respiratory tract infections are Hemophilus influenza type B (Hib, Streptococcus pneumonia, Beta hemolytic Streptococcus, Corynebacterium diptheriae, Moraxella catarrhalis, Mycoplasma pneumonia and Bordetella pertussis [6].
Streptococci are frequent colonizers of the upper respiratory tract and the human nasopharynx is the only natural reservoir for transmission of the pathogen within the community. The pharynx is just a reservoir and thus colonization is a risk factor for CNS disease. These pathogens invade the mucosal lining of the upper respiratory tract and lead to the infections. The incubation period of most URTIs last from a few hours to 3 days after exposure while the symptoms may last for 7-10 days or even longer [7].
Ordinarily, Upper Respiratory Tract Infections (URTIs are recognized in the daily lives of people worldwide and can be characterized by several disorders such as tonsillitis, laryngitis, otitis media and common cold [8]. There are many sociocultural, demographic and environmental risk factors that predispose children less than 5 years to acquire respiratory tract infections [9]. Environmental sources and cross-infection from other people have been implicated in URTIs [10]. Microorganisms larger than 10 µm are trapped by nasal hair and cilia lining the epithelial layer. Coughing and sneezing are the reflexes that expel the microorganisms from the nose and mouth respectively.
URTIs are very common among children and therefore constitute a major target for inappropriate use of antibiotics, therefore WHO has advocated for the use of essential medicines including antibiotics. First-line antibiotics which used for the management of URTIs in children under 5 years include penicillin, amoxicillin, erythromycin and ampicillin. With the current looming bacterial resistance depicted in most bugs including the Streptococcal species, there is need to further study the latter bugs such that to inform policy makers so as to guide on a better treatment out-come. In the region, limited studies have reported on a focused Streptococcal etiology of URTIs. Therefore, it was plausible that the clinical assessment of children with respiratory symptoms was sufficient done so as to identify signs and symptoms that could help clinicians differentiate between common lower respiratory conditions, like pneumonia and asthma, requiring urgent treatment, versus URTIs and self-limiting viral infections for which supportive care was most important.
Material and Methods
Study area and study design
A hospital-based cross-sectional study was conducted at Holy Innocents Children’s Hospital (HICH), Mbarara city in Uganda where all children below 5 years of age who had signs of respiratory infection (severe cough or tachypnea with axillary temperature above 38°C) who were attending the HICH were enrolled during the study period. Study was conducted between August 2022 and September 2022. Sample size was 236, estimated using Kish Leslie formula for cross-sectional studies. The prevalence of infection of 19% was used. The minimum sample size obtained was 236. Children below 5 years that came to HICH during the study period who assented and whose caretakers consented to participate were included in the study. Children who were on antibiotic treatment for the last three weeks were excluded.
Data collection
Information on socio-demographic variables, living with health care workers, presence of young siblings in the family and exposure to passive smoking were collected from each participant through a face-to-face interview using a pre-tested structured questionnaire. Also, clinical information such as the status of previous history of a respiratory tract infection during the last month, sign of malnutrition, runny nose for the last two weeks, coryza for the last two weeks were collected through patient card reviews.
The throat swabs were collected by passing a sterile rayontipped (pre-packed sterile calcium alginate swabs on flexible aluminum shafts) by rotating 4-5 times both clockwise and counterclockwise directions before withdraw. Collection of the swabs was done according to the WHO guidelines for throat swab collection. Swabs were placed in Amie’s transport medium (Oxoid, UK) in a tube and transported to the microbiology laboratory at Mbarara diagnostic laboratory center for primary culture, isolation and identification of Streptococcus.
Laboratory procedures
The specimens were inoculated on sheep blood agar (Oxoid Ltd., CM0271) and on chocolate agar under 5% CO2 at 37°C for 24 hours. Bacterial isolates from culture positive plates that showed a characteristic growth of Streptococcal species were further followed for identification. Identification to species level was done by colony morphology, gram-staining and through standard conventional biochemical tests that included catalase test, bacitracin, optochin and bile solubility. Confirmed isolates where subjected antimicrobial susceptibility testing on Muller-Hinton supplemented with blood (Oxoid, UK) using the Kirby-Bauer disk diffusion method. The antimicrobial agents used were; penicillin, ceftriaxone, cotrimoxazole, ampicillin and gentamicin. The latter panel was selected since it was used for the daily management of patients who presented with respiratory infections. A 0.5 McFarland standard was used to standardize the turbidity of the inoculums suspension. The results of the zones of inhibition were interpreted in accordance with the clinical laboratory standard institute guidelines 32nd edition.
For quality control, each batch of newly prepared media was tested for sterility and performance tests. Reference strains of Streptococci species were used for quality control for antimicrobial susceptibility testing. A standardized bacteriological procedure was followed to maintain correct laboratory results.
Data analysis
Data was imported and analyzed using Statistical Package for Social Science (SPSS) version 22 (IBM Corp-Released 2022). The frequency of variables, prevalence of Streptococcus species and the antibiogram were presented in table formats and graphs. Distribution of the different streptococcal isolates among the participants was described by the fishers exact test and results presented in tables. Association between risk factors (independent variables) and streptococcus (dependent variables) URTIs were determined by first; the bivariate general model at a p<0.2 with a confidence interval of 80%. For the final analysis, a stepwise multiple logistic regression was done including factors that were significant in the earlier bivariate model and also those that were co-founding. A pvalue of less than 0.05 at 95% confidence interval was considered statistically significant.
Ethical consideration
Ethical approval was sought through submission to the department of Medical Laboratory Science for vetting and sent to Faculty Research Committee (FRC) for approval at Mbarara University of Science and Technology. Approved consent and assent from the parents and children respectively were requested before conducting any study inclusion of participants.
Results
Social demographic and clinical characteristics of both caretakers/children (Table 1).
| Characteristic | Parameter | Frequency (n)/N=236 | Percentage (%)/mean (±) |
| Parents/caretakers characteristics | |||
| Gender | Male | 67 | 71.60% |
| Female | 169 | 28.40% | |
| Employment status | Yes | 147 | 62.30% |
| No | 89 | 37.70% | |
| Education level | Tertiary | 85 | 36.00% |
| Secondary | 89 | 37.7% | |
| Primary | 46 | 19.5% | |
| Informal | 16 | 6.8% | |
| Age (years) | 33.3 years (SD ± 8.7 (95% CI 32.2-34.4)) | ||
| Children characteristics | |||
| Gender | Male | 117 | 49.60% |
| Female | 119 | 50.40% | |
| Antibiotic usage | Yes | 26 | 11.00% |
| No | 210 | 89.00% | |
| Known chronic disease | Yes | 40 | 16.90% |
| No | 196 | 83.1% | |
| Age (months) | 31.5 months (SD ± 16.2 (95% CI 29.4-33.6)) | ||
| Weight at birth (kg) | 3.3 kg (SD ± 0.6 kg) | ||
| HIV status | Negative | 134 | 57.00% |
| Positive | 0 | 0 | |
| Unknown | 101 | 43.00% | |
| Breastfeeding status | Yes | 14 | 5.90% |
| No | 175 | 74.20% | |
| Both | 47 | 19.90% | |
| Siblings with cough | Yes | 152 | 64.70% |
| No | 83 | 35.30% | |
| Note: n: Number of participants; N: Overall study population; %: Percentage | |||
Table 1: Demographic and clinical characteristics of both caretakers/children.
Description of bacterial growth patterns
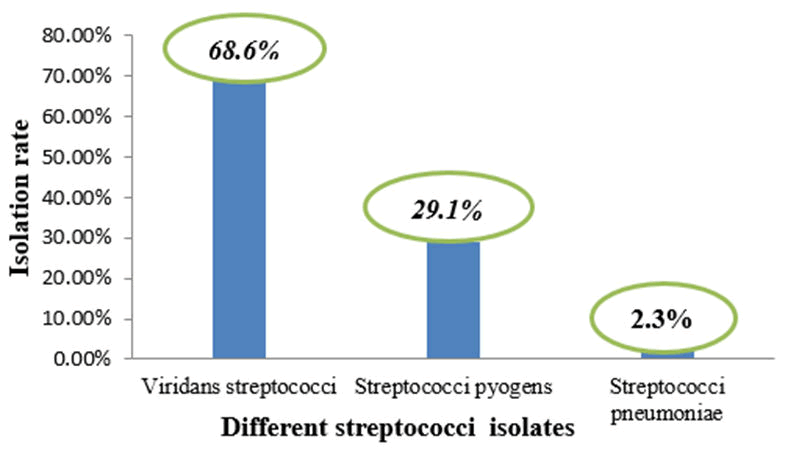
Overall significant growth of the organism of (Streptococci species) associated with URTI among the study participants was found to be 86/236 (36.4%). Viridan streptococci accounted for the most 59/86 (68.6%) isolated, followed by Streptococci pyogenes which accounted for 25/86 (29.1%), Staphylococcus species 88/236(37.3%), fungi 1/236(0.4%), as seen in Table 2 (Figure 1).
| Organisms | Frequency | Proportions |
| Streptococcus species | 86/236 | 36.40% |
| Staphylococcus species | 88/236 | 37.30% |
| Fungi | 1/236 | 0.40% |
| No growth | 61/236 | 25.90% |
Table 2: Overall significant growth of the organisms.
Figure 1. The different isolated Streptococci species.
Antibiotic resistance profiles of the different isolated Streptococci species
Streptococci pneumoniae and Streptococci pyogenes were found to be 100% susceptible to ceftriaxone. However, few 4/59 of the Viridans streptococci were observed to be resistant to the latter drug (Table 3). Moderate resistance was depicted by Streptococci pneumoniae (1/2 and Streptococci pyogenes (12/25 to gentamicin but most Viridans streptococci showed slightly higher 41/59 resistance to the latter drug. All the isolated Streptococci species were found to be resistant to penicillin. Very high resistance was observed across all the isolates for ampicillin. All organisms showed a moderate sensitivity to co-trimoxazole (Table 4).
| Antibiotic | SPN | SPY | SVI | Total sensitive | |||
| S | R | S | R | S | R | ||
| Ceftriaxone | 2/2 | 0/2 | 25/25 | 0/25 | 55/59 | 4/59 | 82/86 |
| Gentamicin | 1/2 | 1/2 | 13/25 | 12/25 | 18/59 | 41/59 | 32/86 |
| Co-trimoxazole | 1/2 | 1/2 | 15/25 | 10/25 | 32/59 | 27/59 | 48/86 |
| Ampicillin | 0/2 | 2/2 | 3/25 | 22/25 | 10/59 | 49/59 | 13/86 |
| Penicillin | 0/2 | 2/2 | 0/25 | 25/25 | 0/59 | 59/59 | 0/86 |
| Note: SVI: Viridans streptococci; SPY: Streptococci pyogenes; SPN: Streptococci pneumonia; S: Sensitive; R: Resistance | |||||||
Table 3: Antibiotic susceptibility patterns of the different isolated Streptococci species.
| Characteristic | Parameter | Organism | ||
| SPN (n/%) | SPY (n/%) | SVI (n/%) | ||
| Gender | Female | 0 | 15/25 (60.0%) | 29/44 (49.1%) |
| Male | 2/2 (100%) | 10/25 (40.0%) | 30/59 (50.9%) | |
| Breast feeding status | Yes | 0 | 1/25 (4.0%) | 5/ (8.5%) |
| No | 2/2 (100%) | 19/25 (76.0%) | 44/59 (74.5%) | |
| Both | 0 | 5/25 (20.0%) | 10/59 (17.0) | |
| Anyone smoking cigarette | None | 1/2 (50.0%) | 19/25 (76.0%) | 43/59 (72.9%) |
| Father | 0 | 0 | 4/59 (6.8%) | |
| Others | 1/2 (50.0%) | 6/25 (24.0%) | 12/59 (20.3%) | |
| School attendance | Yes | 2/2 (100%) | 13/25 (52.0%) | 27/59 (45.8%) |
| No | 0 | 12/25 (48.0%) | 32/59 (54.2%) | |
| Antibiotic usage | Yes | 1/2 (50%) | 2/25 (8.0%) | 5/59 (8.5%) |
| No | 1/2 (50%) | 23/25 (92.0%) | 54/59 (91.5%) | |
| Chronic disease | Yes | 1/2 (50%) | 3/25 (12.0%) | 10/59 (16.9%) |
| No | 1/2 (50%) | 22/25 (88.0%) | 49/59 (83.1) | |
| Siblings with cough | Yes | 2/2 (100%) | 19/25 (76.0%) | 35/59 (59.3%) |
| No | 0 | 6/25 (24.0%) | 24/59 (40.7%) | |
| Note: SVI: Viridan streptococci; SPY: Streptococci pyogenes; SPN: Streptococci pneumonia; %: Percentage | ||||
Table 4: Distribution of organisms with the different characteristics of the participants.
In this study, the distribution of the different organisms varied among the population. We found that the highest proportion of all organisms were isolated from participants who lived with siblings with a cough, had a history of a chronic disease and also among children who had not taken an antibiotic in the last month as seen in Table 4 above. Risk factors associated with streptococcal upper respiratory tract infections.
To assess for a general association between URTIs among the participants and factors/predictors, we inferred a bivariate analysis. As shown in Table 5 below, URTIs were found to be associated statistically significantly with caretakers’ employment status (p=0.166), caretakers’ education level (p=0.049), child birth weight (p=0.139), living with other siblings (p=0.114) and the children age (p=0.007). There was no significant association that was found between UTRIs with breast feeding status, children gender, HIV status of the child, history of antibiotic use, siblings with cough, school attending children and history of chronic disease with p>0.2.
| Dependent variable | Characteristic | OR | P* | 80% CI (2-tailed) | |
| Lower | Upper | ||||
| URTIs | Anyone smoking cigarate | 0.972 | 0.183 | 0.843 | 1.234 |
| Employment status | 1.103 | 0.166 | 1.007 | 1.21 | |
| Education of caretaker | 0.927 | 0.049 | 0.882 | 0.974 | |
| Breast feeding status | 0.996 | 0.948 | 0.912 | 1.087 | |
| Gender | 0.974 | 0.689 | 0.896 | 1.059 | |
| HIV | 0.938 | 0.34 | 0.861 | 1.022 | |
| History of antibiotic use | 1.006 | 0.956 | 0.874 | 1.158 | |
| Child birth weight | 0.917 | 0.139 | 0.851 | 0.989 | |
| Siblings with cough | 1.036 | 0.619 | 0.946 | 1.134 | |
| History of chronic disease | 1.026 | 0.773 | 0.914 | 1.153 | |
| Living with other siblings | 1.03 | 0.114 | 1.006 | 1.055 | |
| School attending children | 0.765 | 0.244 | 0.583 | 0.894 | |
| Children age | 1.004 | 0.071 | 1.001 | 1.007 | |
| Note: %: Percentage; CI: Confidence Interval; P*: Significant value; OR: Odds Ratio | |||||
Table 5: Bivariate model showing association between the Streptococcal URTIs and study participants characteristics.
Table 6 below showed the multivariable model where the variables significantly associated in the bivariate model and also were co-founding were included into the final analysis. Variables that were significantly associated with having an URTI among our study population were education of the caretaker (p=0.022), living with other siblings (p=0.048), anyone smoking a cigarette (p=0.036) and age of the child (p=0.037).
However, age of the child and living with other siblings were found to have higher odds of being associated with a child who presented with an URTI as seen in Table 6 above. In addition, history of antibiotic usage had a high odd of being associated with having an URTI though was not statistically significant as seen in the Table 5.
| URTIs | Characteristic | AOR | P* | 95% CI (2-tailed) | ||
| Lower | Upper | |||||
| Parameter | Anyone smoking cigarettes | Yes | 1.463 | 0.036 | 1.025 | 2.09 |
| No | 0.238 | |||||
| Employment status | Ref. | |||||
| Education of caretaker | 0.634 | 0.022 | 0.438 | 0.916 | ||
| Child birth weight | 0.637 | 0.084 | ||||
| History of antibiotic use | Yes | 1.289 | 0.463 | |||
| No | Ref. | |||||
| Living with other siblings | Yes | 1.173 | 0.048 | 0.993 | 1.386 | |
| No | Ref. | |||||
| Children age | 1.023 | 0.037 | 1.0005 | 1.041 | ||
| Note: %: Percentage; CI: Confidence Interval; P*: Significant Value; AOR: Adjusted Odds Ratios; Ref.: Reference parameter considered in the stepwise analysis | ||||||
Table 6: Multivariate logistic regression model showing association between the Streptococcal URTIs and study participants characteristics.
Discussion
The results from this study revealed that the overall prevalence of streptococcal URTIs from the study setting was found to be high 86/236 (36.4%) among the studied children presenting to the hospital. The finding was found to be similar to an earlier reported study in a Uganda conducted by Mbonye AK, which highlighted a prevalence of 37.4%. However, a study conducted in Northern Ethiopia by Zeru, et al. reported a prevalence of 52.6% which was higher than that of our study. Also, in a similar study about streptococcal UTRIs conducted in Turkey by Cinaroglu S, reported a slightly higher prevalence as compared to our current study. The difference in the reported prevalence could be owed to the fact that our study looked at a specific Streptococcal related URTIs. However, the reported prevalence in our study was higher than that reported by a study done in Atlanta, USA which reported 24%. This could be due to the difference in the settings by virtue that the USA is a very developed nation and suffers a less brunt of infections compared to our Uganda.
In this study, the most isolated Streptococcal pathogen associated with UTRIs was Viridan streptococci which accounted for the most 59/86 (68.6%) isolated, followed by Streptococci pyogenes which accounted for 25/86 (29.1%). This study finding is contrary to reports conducted elsewhere in Nepal and in a multi-country survey by Milucky, et al. that reported Streptococci pneumoniae to be the major cause of URTIs. The latter could have been attributed to by virtue that different population structures in different geographical areas are affected/colonized by bacteria differently.
Information from this study also provided insights into the problem of resistance in the streptococcal bacterial pathogens isolated from the study participants. Findings from the study demonstrated that, in general, streptococcal bacterial isolates associated with URTIs were resistant to most antibiotics that were commonly recommended for prescription in our country Uganda to treat respiratory related illness (Table 3). This study documented that there was an observed high resistance towards the penicillins for most of the isolated Streptococcal isolates. The finding is similar to that in a Ugandan study reported by Kateete, et al. However, this finding is contrary to other studies reported elsewhere in Kenya and Jima, Ethiopia, which indicated that streptococcal isolates were susceptible to penicillins. This might have been due to the presence of β-lactamase producing isolates from our study. Also, it could be owed to the fact that overuse of penicillins in Uganda among children is very common. Also, moderate resistance was observed among the isolates for co-trimoxazole and gentamicin. Furthermore, our study findings highlighted that the most effective drug which showed less resistance among all the different Streptococcal species was ceftriaxone. The latter could be due to the broad spectrum nature of ceftriaxone. In a report by Kisakye, et al. indicated that all the isolates studied were susceptible to Cefotaxime a drug in a similar class with ceftriaxone.
The study showed through the multivariate regression analysis that variables that were significantly associated with having an URTI among the study population were education of the caretaker (P=0.022), living with other siblings (P=0.048), anyone smoking a cigarette (P=0.036) and age of the child (P=0.037). The latter factors could have contributed to the child having an URTI by virtue that educated caretakers were informed of how to prevent the acquisition of the infection. Living with other children could contributed to a transmission pattern of the infection from one sibling to another. Lastly, age of the child could have contributed by virtue that the lower the age, the more prone to infection due to the yet still developing immune status. The findings of this study are similar to those reported in a study conducted in central Nepal by Yadav, et al. However, the latter study found age of the child not to be significantly associated with having an URTI which was contrary to our finding. The study findings on the association of the number of siblings living in the homestead were different from a report by Ghimire, et al. that found no association of URTIs with the former factor. Findings from the study also revealed that sex, birth weight, breastfeeding, presence of an URTI in sibling, and occupation were not significantly associated with having an URTI among the study participants. The finding was also reported by Ghimire, et al. that found no association of URTIs with the former factors. In contrast to the finding in this study, various studies documented elsewhere identified the factors to be significantly associated with having a respiratory infection among underfive children. This could be explained by virtue that different host-pathogen interaction aspects vary in different populations.
Conclusion
In conclusion, the study found that the prevalence of Streptococcal URTIs among children admitted at the hospital was 36.6%. Viridan streptococci was found to be the most predominantly isolated Strep among the children who presented with an URTI. The study found the following variables that were significantly associated with having an URTI among our study population; education of the caretaker, living with other siblings, anyone smoking a cigarette and age of the child. Resistance to the studied penicillin by the different streptococcal isolates was found to be 100%. The most effective drug that most streptococcal isolates were susceptible to was found to be ceftriaxone.
Acknowledgement
We are very gratefully for the parents who allow their children to participate in the study. We would like to acknowledge the assistance and guidance provided by all staff members of the Department of Medical Laboratory Science (Mbarara University), Holy Innocent Children’s Hospital and Mbarara Diagnostic Laboratory Center (MDLC).
References
- Stover CS, Litwin CM. The epidemiology of upper respiratory infections at a tertiary care center: Prevalence, seasonality, and clinical symptoms. J Respir Med 2014; 2014:469393.
- Bayu D, Mekonnen A, Mohammed J, et al. Magnitude of Streptococcus pneumoniae among under-five children with symptom of acute respiratory infection at Hiwot Fana specialized University Hospital, Harar, Ethiopia: Associated risk factors and antibacterial susceptibility patterns. Risk Manag Healthc Policy 2020; 13:2919-2925.
[Crossref] [Google Scholar] [PubMed]
- de Almeida-Winter DE, de Oliveira LH. Recommendations on the use of antimicrobials in upper respiratory tract infections in pediatrics. Resid Pediátr 2019; 9:284-289.
- Kiconco G, Turyasiima M, Ndamira A, et al. Prevalence and associated factors of pneumonia among under-fives with acute respiratory symptoms: A cross sectional study at a Teaching Hospital in Bushenyi District, Western Uganda. Afr Health Sci 2021; 21:1701.
[Crossref] [Google Scholar] [PubMed]
- Thapa S, Gokhale S, Sharma AL, et al. Burden of bacterial upper respiratory tract pathogens in school children of Nepal. BMJ Open Respir Res 2017; 4:e000203.
[Crossref] [Google Scholar] [PubMed]
- Josphat M, John M, Anthony K. Antimicrobial susceptibility patterns of bacteria associated with upper respiratory tract infections in Kitui, Kenya. Ethiop Med J. 2017; 55:121-127.
- Al-Badaii F, Al-taibi A, Al-shaeri H, et al. Isolation, identification and antibiotic susceptibility of bacteria from upper respiratory tract infections at Dhamar Governorate, Yemen. Int J Sci Res Biol Sci 2021; 8:12-19.
- Tazinya AA, Halle-Ekane GE, Mbuagbaw LT, et al. Risk factors for acute respiratory infections in children under five years attending the Bamenda Regional Hospital in Cameroon. BMC Pulm Med 2018; 18:7.
[Crossref] [Google Scholar] [PubMed]
- Kumar SV, Kumar GV, Kandati J, et al. A surveillance study of microbial pathogens and their antibiotic sensitivity of respiratory tract infections in a tertiary care hospital. Int J Curr Microbiol Appl Sci 2015; 4(11):35-44.
- Sumaila AN, Tabong PT. Rational prescribing of antibiotics in children under 5 years with upper respiratory tract infections in Kintampo Municipal Hospital in Brong Ahafo Region of Ghana. BMC Res Notes 2018; 11:443.
[Crossref] [Google Scholar] [PubMed]