Research Article - Journal of Fisheries Research (2023) Volume 7, Issue 1
Potential of Ocean Calcifiers to Sequester Atmospheric Carbon in Quantity and Even Reverse Climate Change
David Moore1*, Matthias Heilweck2, William Burton Fears3, Peter Petros4, Samuel J Squires5, Elena Tamburini6 and Robert Paul Waldron7
1Department of Biology, Medicine and Health, School of Biological Sciences, The University of Manchester, UK
2Private address, independent researcher, Kaysersberg, France
3Department of Medicine, Southwestern Medical School, Dallas, Texas, & Founding Fellow of the American College of Endocrinology, USA
4Kaapa Biotech Oy, Teilinummentie 4, 09120 Karjalohja, Finland
5Department of Biology, Medicine and Health, The University of Manchester, UK
6Department of Environmental and Prevention Sciences, University of Ferrara, Ferrara, Italy
7Private address, independent researcher, Mandeville, Louisiana, USA
- *Corresponding Author:
- David Moore
ORCID ID: https://orcid.org/0000-0003-3968-0587
E-mail: david@davidmoore.org.uk
Received: 31-Dec-2022, Manuscript No. aajfr-22-85180; Editor assigned: 05-Jan-2022, PreQC No. aajfr-22-85180(PQ); Reviewed: 21-Jan-2023, QC No. aajfr-22-85180; Published: 30-Jan-2023, DOI:10.35841/aajfr-7.1.132
Citation: Moore D, Heilweck M, Fears WB, et al. Potential of ocean calcifiers to sequester atmospheric carbon in quantity and even reverse climate change. J Fish Res. 2023;7(1):132
Abstract
Today’s marine calcifiers (coccolithophore algae, Foraminifera [protists], Mollusca, Crustacea, Anthozoa [corals], Echinodermata and some annelids) convert atmospheric carbon dioxide (CO2) into the solid calcium carbonate (CaCO3) shells which are left when they die. These organisms could be the biotechnological carbon capture and storage mechanism to control climate change. Two criticisms of this are: (i) ocean acidification has allegedly been shown to cause reduced shell formation in calcifiers; (ii) the calcification reaction that forms CaCO3 crystals is alleged to return CO2 to the atmosphere. Here, we review evidence about such criticisms and find reasons to doubt both. Experiments showing ocean acidification is damaging to calcifiers have all used experimental pH levels that are not projected to be reached in the oceans until the next century or later; today’s oceans are alkaline. Claiming precipitation of CaCO3 by calcification as net source of atmospheric CO2 might be true in open water environments in equilibrium with the atmosphere. Living calcifiers do not carry out the calcification reaction in such environments. Life’s chemistry is specifically isolated from open water; taking place on enzymatic polypeptide surfaces, within organelles with ion-selective phospholipid membranes, contained in a cell enclosed by phospholipid bilayer membranes. Ignoring what is known about the biology, physiology, and molecular biology of living calcifiers leads to erroneous conclusions and deficient advice about the potential for calcifier biotechnology to contribute to atmosphere remediation. We conclude that the world’s aquaculture industries already operate the biotechnology that, with massive and immediate global expansion, can sustainably control atmospheric CO2 levels at reasonable cost.
Keywords
Aquaculture, Biotechnology, Carbon sequestration, Carbonate biology, Carbonate chemistry, Climate change, Remediation.
Introduction
In several recent publications we have advocated that shellfish farmers should greatly expand their production specifically to generate more shell to sequester atmospheric carbon [1-10]. Our core conviction is that humankind must look to the oceans for the solution to the excess CO2 in the atmosphere that drives climate change, and that marine calcifiers (coccolithophores, Foraminifera, Mollusca, Crustacea, Anthozoa, Echinodermata and some annelids) are the tools that will provide that solution. We consider that the action plans we have suggested offer the good news message that if we act quickly to change our attitude to calcifier cultivation and, particularly, greatly magnify the global scale of this activity, we could make a serious contribution to ameliorating climate change in the foreseeable future [7].
Despite the positive messages of our publications referenced above, distinguished marine chemists have cast doubt on our claims by stating (we paraphrase and add emphasis) ‘marine shellfish aquaculture could not make a contribution to climate mitigation’; two reasons being offered for this point of view: (i) seawater has become more acidic and shellfish species are shrinking in size and the shells deform, and (ii) precipitation of calcium carbonate in shellfish shells is a source of carbon dioxide (CO2) and the major way by which CO2 is returned to the atmosphere (see ‘Frequently Asked Questions’ section in [7]).
In this paper, we attempt to provide a different, biological, viewpoint of the published data bearing on these two specific issues, which we hope will show why cultivating calcifiers in the short term would be advantageous. We also include some comments about the psychological paradox of why, when we know more than enough about the climate system, we do so little to control climate change [11-14], being satisfied merely with coping with its outcomes.
Our conclusion remains positive. The most recent Life Cycle Assessments (LCA; described and referenced below) demonstrate that the shellfish cultivation industry offers unique opportunities for permanently sequestering carbon while producing food, but if significant carbon capture is to be achieved, the paradigm (and the business model of shellfish farms around the world) must be changed from cultivating shellfish for food towards cultivating shellfish for their shells. If the level of finance and global effort that are willingly anticipated for forest management and CCS (Carbon Capture and Storage) flue gas treatments was applied to expansion of shellfish (and other calcifiers) cultivation around the world, significant amounts of CO2 could be removed from the atmosphere with much greater permanence and less cost than any other solution can offer [9]. Start now and by the end of this century the action plan could be contributing to returning the CO2 level in our atmosphere to its natural, pre-industrial level.
Basic carbonate chemistry
The chemistry involved in the process of shell-making, whether performed by planktonic algae (coccolithophores), single celled protists (Foraminifera) or multicellular animals (Anthozoa, Crustacea, Mollusca, Echinodermata, annelids) is described by the following scheme:
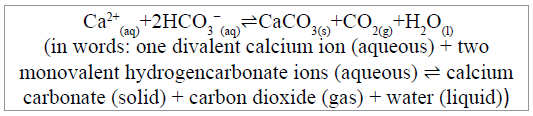
One molecule of CO2 from the hydrogencarbonate ions of seawater is released, together with a molecule of water, during the calcification (biomineralisation) reaction. Seawater is over-saturated with calcium ions and its concentration of hydrogencarbonate largely dominates that of carbonate and dissolved free CO2. In these conditions, the molecule of CO2 on the righthand side of the above scheme, if it is released to seawater during the biomineralisation of shells (which is very doubtful, as explained below), will react with water, forming carbonic acid which will dissociate forming hydrogencarbonate and hydrogen ions (protons) that would be available for marine calcifiers to form more CaCO3. Alternatively, the carbonic acid can dissociate to form a carbonate ion and two hydrogen ions. These electrolyte dissociations and associations are described by these schemes.
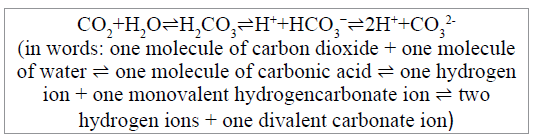
This release of hydrogen ions is usually interpreted as causing potentially damaging seawater acidification. Emerson and Hedges [15] describe carbonate dynamics (their Chapter 4, Carbonate Chemistry) in terms that can be paraphrased: “As organisms form their shells from Ca and carbonate, alkalinity is being removed. This causes the pH to drop which alters the speciation of the inorganic carbonate system to alter in favour of CO2. Thus, the CO2 concentration increases and any gradient driving the gas to the atmosphere increases.” Which is not so different from the following wording in Gattuso et al. [16].
“There are three pools of oceanic DIC [Dissolved Inorganic Carbon]: HCO3- (90%), CO3- (9%), and dissolved CO2 (1%). The latter pool is close to equilibrium with the atmosphere (present pCO2 ca. 360μatm). The carbon atom incorporated into CaCO3 is derived from the HCO3- pool, with the consequence that H+ is liberated and the water gets more acid. The acid pushes an additional amount of HCO3- across into the oceanic CO2 pool. There is then a physical equilibration between the seawater and atmosphere CO2 pools, and this physical equilibration pushes CO2 into the atmosphere”.
Represented by the following scheme:
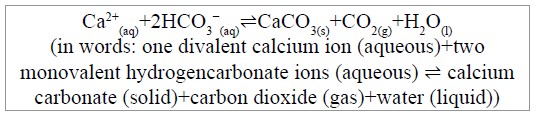
We want to make it clear that we do not doubt, or query in any way these chemical facts or interpretations as they apply to the progress of inorganic chemistry in the open water environment, in which environment it is doubtless perfectly true to say that: “Calcification is therefore a CO2-releasing process that can make water in equilibrium with the atmosphere degas, against the initial pCO2 gradient” but the number and range of reactions taking place within and between the atmosphere and ocean systems is enormous, so we believe that a perfectly respectable scheme can be made to the effect that the above quotation from reference may, in fact, describe the reverse of what actually happens (Pers. Commun., AB. McDonald, 2022) [16].
But our most important criticism of the interpretation expressed by reference is that living calcifiers do not carry out the calcification reaction in an open water environment which is ‘in equilibrium with the atmosphere’. The chemistry that we know as life takes place in a cell enclosed within a phospholipid bilayer membrane specifically to isolate its reactions from the open water environment. Many of the reaction trains upon which life depends take place within organelles that have their own phospholipid membranes within the cell [16].
Well known examples of such organelles are the mitochondria, that generate the chemical energy stored in adenosine triphosphate (ATP), and, in plants, the plastids, in which photosynthesis (or photosynthesis-related special metabolic activity, like starch storage) takes place. Transporters within these membranes control the movement of ions (including protons, electrons and inorganic ions), as well as molecules and macromolecules to and from the compartments the membranes enclose. Some metabolites may be allowed simply to diffuse across the phospholipid membrane or through pores in the membrane(s); in other cases, diffusion may be facilitated by highly specific and selective transporters; whilst linking a facilitated diffusion mechanism to an ATPase proton transporter produces an active transport system that can transport molecules against, often considerable, chemical diffusion gradients.
These selectively permeable phospholipid bilayer membranes isolate the cell from its environment and the compartments within the cell from one another as the ion-specific transporters across those membranes control the environments within cellular compartments to the benefit of the organism in the evolutionary war of natural selection.
A case in point is that Foraminifera actively pump hydrogen ions (protons) out from the site of calcification which is therefore surrounded by a low (acidic) external pH of their own making [17]. Foraminifera are amoeba-like, singlecelled protists that secrete a protective shell (called a ‘test’ because it is intracellular). The most primitive tests are made from cemented sand grains, but most are made of calcite or aragonite (CaCO3) crystals. Tests are found in globally extensive fossilized foraminifera limestones as old as the earliest Cambrian, about 545 million years ago (Mya), and planktonic and benthic Foraminifera are still abundant today, living in marine and brackish waters.
Kawahata, et al. [17] focus on the response of two major calcifiers, Foraminifera and corals, which together contribute significantly to global carbon sequestration in sediments and reefs. They demonstrate that the response to acidified seawater today depends on situations, species, and community structure and life-cycle stage. Some Foraminifera showed a positive response to low (acidic) pH conditions, while calcification in adult coral branches was not reduced by high CO2 concentrations. Direct visualization of pH distribution showed that proton (hydrogen ion) pumping by the cell which is associated with foraminiferal calcification during chamber formation in the tests is independent of initial seawater CO2 concentration or pH and produces a high internal pH (more than pH 9 within the membrane-enclosed site of calcification) and large internal-to-external pH difference (as much as 2 pH units).
Corals also regulate their internal pH at the tissue-to-skeleton interface to levels that could counteract ocean acidification [18,19]. Salinity, temperature, the amount of light and the amount of oxygen dissolved in the water are the most important factors that control living foraminifera and coral polyps. Higher ocean temperatures do induce bleaching of these marine calcifiers because their algal symbionts on which they depend for nutrition are temperature-sensitive (and this applies to corals, giant clams and Foraminifera). Loss of the nutrition contributed by the photosynthetic symbiont to its host animal results in malaise, reduced calcification and ultimately death of the host, although symbiotic Foraminifera are more robust and resilient than coral polyps at higher temperatures.
Given the ability of important calcifying organisms to modify their own internal environments in the ways indicated immediately above, our focus moves from a ‘water in equilibrium with the atmosphere’ viewpoint, to a view guided by what we know about the cellular biology of living things on Earth and in its oceans. It is our hope that we can build upon the work that has already been done on carbonate chemistry in open water environments and extend it to include the biological view of calcification chemistry.
Issue 1: Does ocean acidification have a harmful effect on the physiology of calcifying organisms?
Acidity is measured in terms of the pH where the pH of a solution = -log [H+], which is a logarithmic scale. A neutral solution has a pH of 7 and pH values less than 7 are considered acidic, whilst pH values above 7 are alkaline (or basic). The Encyclopeadia Universalis France (Tanhua, et al. 2015 [20]) states that.
“Since the industrial era, the ocean’s basic [alkaline] pH has fallen from 8.2 to 8.1. This drop of 0.1units corresponds to an increase in acidity of about 25% [because the scale is logarithmic]” [https://www.universalis.fr/encyclopedie/ acidification-des-oceans/].
To put these pH values into the context of our common experiences, the pH of fresh orange juice ranges from 3.3 to 4.2, and fresh cow’s milk about 6.7 to 6.9. Most black teas are in the range 4.9 to 5.5, with black coffees averaging pH 4.8 to 5.1. These beverages are considered weakly acidic, whilst the ‘safe’ pH level of drinks to avoid tooth damage is deemed to be 5.5.
Fassbender, et al. found that pH changes vary between the many domains of the world’s oceans, being constrained by geographical position, depth, temperature, salinity and current flows. They also highlight that pH provides a relative means for comparison but considered that the absolute hydrogen ion concentrations of areas may help better understand actual changes in our ocean. Nevertheless, the fact remains that today’s oceans are still generally alkaline in pH [21].
Mean pH of surface ocean waters is predicted under the IPCC ‘business-as-usual’ scenario [22] to decline by 0.3-0.4 units by 2100 AD [23,24], and Brewer (1997) [23] tabulated the evolving chemistry of surface seawater under this scenario as shown in Table 1.
| Year | Predicted oceanic pH |
| 1800 | 8.19 |
| 1996 | 8.10 (actual value = 8.2) |
| 2020 | 8.03 (actual value = 8.1) |
| 2040 | 7.97 |
| 2060 | 7.91 |
| 2080 | 7.85 |
| 2100 | 7.78 |
| Table adapted from Brewer (1997) [23] | |
Table 1: Estimated surface oceanic seawater pH between 1800 AD and 2100 AD1.
This decline in oceanic pH, both predicted and measured, (Table 1) is the very definition of “ocean acidification”. But in our view the description “acidification” (even though chemically and semantically accurate) over dramatizes the situation, which can cause a psychological response in individuals leading to group inaction, as the group perception is too alarming to resolve climate change/climate disruption [13,14,25]. When a solution is offered, we should DO something about it rather than dismiss the solution with illogical criticism.
The facts about ocean acidification are these:
• In 1800 AD the oceanic pH is estimated to have been a decidedly alkaline 8.2.
• In 2020 AD the oceanic pH was measured to be a decidedly alkaline 8.1.
• By 2100 AD the oceanic pH is predicted to be a decidedly alkaline 7.78.
The decreasing alkalinity of the oceans represented in these figures is not diminished in importance, and decreasing alkalinity on this scale is still
“A powerful reason, in addition to that of climate change, for reducing global CO2 emissions. Action needs to be taken now to reduce global emissions of CO2 to the atmosphere to avoid the risk of irreversible damage to the oceans” [25].
Our point is that the descriptive phrase “decreasing alkalinity” does not provoke wild fears of the White Cliffs of Dover fizzing away in an acid ocean by the end of the century like a lump of chalk thrown into a bowl of vinegar (try Googling “chalk in vinegar experiment”). It is important to control the phraseology to avoid thoughts that we might be faced by a circumstance we are powerless to control. According to Marshall (2015) [13]:
“There is some research evidence that people stop paying attention to climate change when they realize there is no easy solution for it” [13].
This is because of a subconscious human mechanism whereby we avoid uncomfortable emotions by rejecting facts that are too unpleasant to act on [14,27]. A more positive conclusion is implied in the following quotations from Stoknes (2015) [14].
“Evolutionary psychology highlights that imitating others is an efficient strategy. Among social animals, following the majority is good for learning and survival. But imitation isn’t fate. We can choose differently. So maybe there are smart ways to start harnessing the evolutionary force for imitation for climate action rather than the opposite” [14].
We maintain that humanity is not powerless to control climate change. It is an historical fact that we humans had the power to cause our current circumstance as we developed our global industrial muscle; and now, with the aid of 200 years of accumulated scientific knowledge, we have the power bestowed by that knowledge to apply our industrial muscle to change our current circumstances to alleviate some of the future consequences.
There is a readily implemented contribution to efforts to regulate climate change by combining two proven special natural talents the ability of calcifiers to remove carbon from the atmosphere immediately and permanently, and the ability of humans to get things done quickly. We can use the oceans of the planet to navigate our way out of the climate crisis of which we are now so aware [11,28,29].
Coccolithophores are the most prolific producers of CaCO3 in the oceans, accounting for almost half of the total CaCO3 produced in today’s oceans annually [30-32]. Evidence from the deep ocean indicates that over the past 220 years there has been a 40% increase in coccolith mass in the deep sea sediments [33]. Clearly, the coccolithophores have already reacted to the anthropogenic rise in atmospheric CO2 partial pressures by doing what they have done before: detoxifying their environment. The difference this time is that they are providing humanity with the service of detoxifying atmospheric CO2.
Study of subantarctic populations of the most abundant coccolithophore calcifying phytoplankton species, Emiliania huxleyi, found highly calcified morphotypes in more acidified high-CO2 conditions. Such observations challenge any claim that ocean acidification will necessarily be detrimental to algal calcifiers [34] even though it is also clear that ocean acidification and elevated temperatures in relatively shallow tropical waters adversely impact the viability of the symbiotic algae of Foraminifera, corals and giant clams alike. Challenging this positive notion, Doney, et al. (2009) [35] state that:
“Many calcifying species exhibit reduced calcification and growth rates in laboratory experiments under high-CO2 conditions”.
This is a fact that cannot be denied; but in laboratory experiments the pH used is in the hands of the experimenter and all have chosen pH values representative of the end of this century (or even later) rather than the present day. For example, Orr, et al. (2005) [24] reported that when live pteropods were exposed to conditions predicted for 2100AD in a two-day shipboard experiment, their shells showed notable dissolution. Pteropods are planktonic molluscs that contribute to pelagic food webs worldwide, so this is bad news for ocean biodiversity in 80 years’ time. Orr, et al. (2005) [24] use these data to argue that conditions detrimental to ocean ecosystems “could develop within decades, not centuries as suggested previously” (The emphasis is ours) but this detail may not be appreciated by those with deep fears for the here and now.
Other examples are experiments studying the effect of different pH treatments on shell properties of the blood cockle (or blood clam), Tegillarca granosa, that used experimental pH values of 7.1 and 7.5 and a pH of 7.81 as a control [36]. This clam occurs in the intertidal zone throughout the Indo-Pacific region, from South Africa through to Southeast Asia, Australia, and Japan. It is widely harvested in coastal and estuarine mudflats so the finding that “The shell weight and shell density of T. granosa was significantly reduced at pH 7.10” [36] could have severe economic consequences for the industry. However, this reduction in weight and density is in comparison with a control pH value (of 7.81) which is not expected to be reached in our oceans until 2100 AD. Further reassurance for the blood clam aquaculture industry for at least the next 300 years is that (again, the emphasis in this quotation is ours):
“However, the ocean acidification level of pH 7.50 which is predicted to occur by the year 2300 showed no significant decrease in shell weight and shell density of T. granosa compared to the control pH treatment (pH 7.81)” (Nithiyaa et al. 2021 [36]).
Fitzer, et al. (2016) [37] have demonstrated significant changes in the hydrated and dehydrated forms of amorphous CaCO3 in the crystalline layers of shells of the blue (or ‘common’) mussel (Mytilus edulis) cultured under experimental acidification conditions. This could be an important experimental observation as this edible marine bivalve mollusc has a global range and is the subject of a multi-million-dollar intensive aquaculture industry. However, these experiments used CO2 concentrations that were 2½ times higher than today’s observed natural levels. It is unreasonable to predict present day detrimental consequences for calcifiers on the basis of such extreme experimental procedures.
Another study with Mytilus, which used a similarly extreme upper CO2 level, found that the resultant acidification (or, as we prefer, reduced alkalinity) suggests a complex relationship between calcification and the various active components of climate change that might ease the negative effects of increased sea temperatures on biomineralisation in the mussel [38]. Even greater complexities become evident in organisms, like calcifying phytoplankton (coccolithophores) that bring photosynthesis into the mix of variables [39].
The publications reviewed so far illustrate the general trends in a copious literature into which we do not intend to delve further. Kroeker, et al. (2013) carried out a comprehensive meta-analysis of 155 studies examining biological responses to a 0.5-unit reduction or less in mean seawater pH, which approximates projected acidification by about 2100AD. They found “decreased survival, calcification, growth, development and abundance in response to acidification when the broad range of marine organisms is pooled together” but stressed variability:
“In species’ responses in multi-species assemblages, suggesting that it is important to consider indirect effects and exercise caution when forecasting abundance patterns from single-species laboratory experiments. Furthermore, the results suggest that other factors, such as nutritional status or source population, could cause substantial variation in organisms’ responses. Last, the results highlight a trend towards enhanced sensitivity to acidification when taxa are concurrently exposed to elevated seawater temperature” [40].
An additional source of variability that ALL these experimental studies fail to consider is that a broad range of calcifiers, particularly molluscs, have lifestyles and physiologies that have evolved to cope with tidal changes in their coastline habitats. At low tide the seawater within closed shells and, for mobile animals, remaining seawater in rock pools and crevices, increases in temperature, becomes anoxic and CO2-rich; conditions not far removed from those predicted for the wider ocean in the distant future. This emersion being a twice-daily occurrence, the animals have evolved physiological adaptations to suit.
Bivalves need to maintain a large volume of water inside the mantle cavity, which is enclosed by the shell, because the cavity functions as a respiratory chamber. The shell is secreted by the outer epidermal layers of the mantle tissue and during the adverse conditions of emersion there is survival advantage in continuing to calcify the now firmly closed, and possibly exposed-to-air shell, to reinforce the shell’s valves against predation.
The mantle cavity also contains the main body tissues of the animal (gills, foot and visceral mass of digestive tissues, reproductive organs, etc). In contrast to the shell, there is survival advantage in reducing the rates of body tissue growth under prolonged emersion. If the soft tissue continued to grow steadily and came to occupy a larger part of the space, there would be less water inside the shell to support the metabolic needs of the increased tissue mass [41-43]. Since the Moon was formed, its tidal effects on the Earth have been a key environmental factor in the evolution of life on our planet from its very earliest stages [44]. These are not recent adaptations of shore-dwellers, but they have within them the physiological tools to cope with at least some of the more recent environmental challenges.
Remembering that all this concern about acidification applies to the future-relevant pH levels of the next century, the commonly held view that anthropogenic CO2 in the world’s oceans have reduced the pH of seawater to levels likely to have a harmful effect on the physiology of calcifying organisms is not the case yet.
For oceans a lifetime into the future, acidification is a legitimate concern; but this is irrelevant for the present day and its dire predictions should not be allowed to influence our choice of biological mechanisms to control climate change today, nor our intent to put them into effect immediately. We are not alone in this conclusion. Connell, et al. (2017) [45] tested the effects of ocean acidification on a calcifying gastropod herbivore in a volcanic CO2 vent ecosystem with local CO2 levels close to those predicted for the world’s future oceans. They found that:
“Contrary to predictions, the abundance of this calcifier was greater at vent sites (with near-future CO2 levels). Furthermore, translocation experiments demonstrated that ocean acidification did not drive increases in gastropod abundance directly, but indirectly as a function of increased habitat and food (algal biomass) [45]”.
They concluded:
“The effect of ocean acidification on algae (primary producers) can have a strong, indirect positive influence on the abundance of some calcifying herbivores, which can overwhelm any direct negative effects [45]”.
The review paper entitled Rebuilding marine life [46] indicates that achieving the UN’s Sustainable Development Goal 14 (“to conserve and sustainably use the oceans, seas and marine resources for sustainable development”)
“Will require rebuilding the marine life-support systems that deliver the many benefits that society receives from a healthy ocean”. But they finally conclude that “Rebuilding marine life represents a doable Grand Challenge for humanity, an ethical obligation and a smart economic objective to achieve a sustainable future [46]”.
In the opinion of Duarte, et al. (2020) [46], recovery rates seen in past studies of conservation interventions suggest that:
“Recovery of the abundance, structure and function of marine life could be achieved by 2050, if major pressures including climate change are mitigated [46].”
In their brief letter to the journal Science, Gordon, et al. (2020) [47] asserted that “Marine restoration projects are undervalued” and in their final paragraph they concluded:
“The pessimistic view of marine restoration as a fruitless exercise differs from attitudes about the rehabilitation of forest habitats that suffer equivalent large-scale degradation. Generally, socioeconomic, ecological, and cultural values are appreciated in tree planting, whether it involves a few saplings or millions Political agreements for global reductions in atmospheric carbon have been slow to emerge. Relying on their implementation as the only solution to the degradation of tropical habitats is a major gamble. In the meantime, restoration projects could help maintain species survival and ecosystem services, ultimately providing humanity with the breathing space to stabilize the climate [47]”.
There is clearly a widely held view that protecting ocean health is important and overdue. If the roles that ocean calcifiers could play in atmospheric carbon capture and storage were to be factored into these arguments, it might increase the urgency with which ocean health is addressed.
Marshall (2015) [13] points out that science uses words like ‘uncertainty’ in a different way to the lay public. To avoid any thoughts of scientific uncertainty becoming a primary issue in this debate, we wish to emphasise that in relation to ‘ocean acidification’:
• The uncertainty lies in the doubts that exist about the future date at which the oceans will become acidified to the point at which calcifiers are grossly adversely affected by oceanic pH. Will it be 2050 AD, 2100 AD or 2150 AD?
• In contrast, it is certain that calcifiers in their natural environments will be adversely affected when the general ocean pH levels do reach the extreme levels that the experimenters choose to use in their laboratory experiments on the topic.
• It is equally certain that today’s ocean pH has no general adverse effect on the behaviour of our principle calcifiers, whether protist, animal, plant, unicellular or multicellular, which in many cases, where the tests have been done, showed a positive response to today’s less alkaline (but described as ‘acidified’) pH conditions.
Consequently, today’s calcifiers can be put immediately to the tasks of providing us with nutritious food, numerous ecosystem services (filtration, biodeposition, denitrification, enhanced biodiversity, reef building for shoreline stabilisation, wave management and coastal protection), whilst, incidentally, permanently sequestering CO2 from the atmosphere to the extent of at least half their mature body weight and depositing in the oceans as present-day fossilised limestone.
Issue 2: Does calcification make a net return of CO2 to the atmosphere?
CaCO3 and CO2 are produced from calcium ions and hydrogencarbonate ions by the calcification reaction that proceeds according to the following scheme:
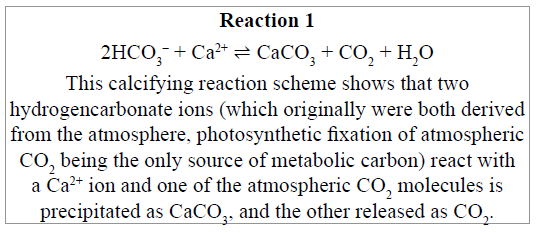
This reaction scheme is not disputed, rather it is the ongoing interpretation of this by marine inorganic chemists “as a net return of CO2 to the atmosphere” that is a concern. We want to emphasize at this point that the immediately following discussion does not deal with the ‘carbon footprint’ of bivalve farming (for which see Subsection ‘Life cycle assessments (LCA) of bivalve farming’ below) but deals specifically with the ‘carbon footprint’ of the formation of the shell material.
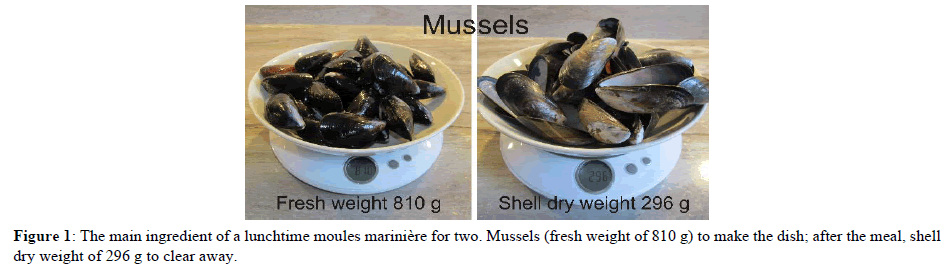
The proposition that shellfish offer no net removal of carbon from the atmosphere starts to worry us at the lunch table, when we discard all those CaCO3 shells that are left after our meals of moules marinière (Figure 1). Many of us enjoy shellfish foods, especially oysters, clams, mussels, lobster and crab, and all the other seaside treats, too. So, we must be aware from our own experiences of the amount of shell left over after the meal. For example, our average moules marinière for two (Figure 1), which uses 810 g fresh weight of mussels and, after the meal, leaves shells with a dry weight of 296 g.
If we assume that this shell ‘waste’ is all CaCO3, then this calculates to these two plates of food permanently removing about 36 g of carbon from the atmosphere. That may not be very much, but it is just two plates of food in one dining room on one occasion. Can you think of any other plate of food that demonstrably removes any carbon from the atmosphere, permanently? If we could arrange for every person on Earth to enjoy such a meal on just one day every week, about 7.5 million metric tonnes of carbon would be removed permanently from the atmosphere each year. We will think about scaling up these numbers more dramatically a little later.
For arithmetic convenience in what follows we will adopt the convention that the bivalve’s shell represents 50% of the fresh weight of the animal, though this is undoubtedly a gross underestimate of the amount of shell in shellfish harvests. The “shell to flesh” ratio is extremely variable between different cultivated bivalves. In addition, the shell, which is mineralised CO2 from the atmosphere, is the animal’s protective armour, of course, so a component of the variability lies in the individual animal’s response to its local environment by managing the physical density of its shell. Waldron (2019) [51] reminds us that the shell of the “Gulf oyster Crassostrea virginica, which has evolved to repel oyster drills, drumfish, oyster flatworms, raccoons, and crabs, can armor itself with 5 to 6 times its body weight in shell”. and he describes and illustrates a single individual “healthy, mature oyster, whose flesh weighed 3 ounces (85g), and whose shell weighed 1.1lbs (499g)” that appeared in a dredge sample during an oyster lease survey of bedded leases in Terrebonne Parish of Louisiana, USA, in September of 2018.
Shellfish tonnage (comprising oysters, mussels and clams) marketed in the European Union (EU) in 2019 had a live weight of 580,044 tonnes and yielded 458,700 tonnes of (waste) shells [52]. This Aquaculture Advisory Council (AAC) Recommendation to the European Commission and Member States [52] quotes the average meat percentages of harvested bivalve live weight as 8.5% (oysters), 25% (mussels) and 14% (clams). Importantly, the AAC report points out that in addition “to the volume of shells at consumer level, the volume of farmed shell debris must be added” [52]. This shell debris is part of the harvest and results from bivalve mortality during cultivation and, expressed as a percentage of live weight of the harvest, is estimated as 25% for oysters, 20% for mussels and 4% for clams. The total farmed shell debris harvested during 2019 being estimated as 118,230 tonnes. This makes the total farmed bivalve shell tonnage harvested in the EU in 2019 equal to 576,930 tonnes, which is further estimated to represent the sequestration of 45,124 tonnes of atmospheric carbon [52]. As the shells of dead molluscs are not digested and are chemically stable, they can contribute to offshore reefs that persist for geological periods of time.
Mulling this over after lunch, we realised that there are five major scientific reasons for doubting that shell calcification is “a net return of CO2 to the atmosphere”, so we decided to audit, in what follows, that which has been described as the Blue Carbon Account.
Two minus one cannot be a net return to atmosphere.
In shallow waters, where most shellfish are cultivated, CaCO3 is essentially insoluble and totally stable (limestone). Consequently, the biological calcification reaction removes from any further chemistry or biochemistry one of its two initial reactant hydrogencarbonate ions. As the ocean absorbs about 30% of the CO2 released into the atmosphere [53], the source of both of those hydrogencarbonate ions is atmospheric CO2, either through CO2 reacting with water to form carbonic acid (which dissociates), or from metabolism of food-derived organic carbon (ALL of which on this planet is derived from photosynthetic fixation of atmospheric CO2). Hence, Reaction 1 (scheme above) can be expressed as 2 atmospheric carbons+calciumone precipitated carbon+one potentially atmospheric carbon. Arithmetically, this cannot be claimed as a net return of CO2 to the atmosphere. Using only the stoichiometry of Reaction 1 to evaluate carbon fluxes gives a false impression and merely tells a small part of the story [53].
The calcification reaction is reversible in oceanic waters
We must also recognise the fact that Reaction 1 is chemically reversible (we deal with its enzymology below), the position of the equilibrium changing according to local, open water oceanic, conditions. The equilibrium shown above as the scheme for Reaction 1 refers to the chemistry of shallow waters. As water depth increases, changes in local conditions gradually change the causality to favour the reverse scheme, shown as Reaction 2, below.
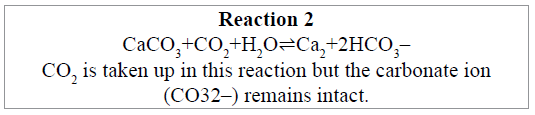
Solid shells of dead calcareous plankton and other calcifiers occur in the water column of the ocean, to depths of about 3,500 to 5,000 metres, which is the Calcite Compensation Depth (CCD) that separates calcareous from noncalcareous sediments [54]. At the depth of the CCD all CaCO3 dissolves to form hydrogencarbonate ions. High hydrostatic pressure at these depths, coupled with decreasing temperature and increasing amounts of dissolved CO2 derived from the respiration of organisms living in the habitat drives the equilibrium in the direction of hydrogencarbonate formation according to Reaction 2 (because gaseous CO2, as the only compressible reactant, cannot outgas from the solution).
If the seabed is above the CCD, bottom sediments consist of calcareous ooze, which accretes into a type of limestone or chalk in (geological) time. If the exposed seabed is below the CCD the sea floor sediment will be a layer of siliceous ooze or abyssal clay [55], because CaCO3 dissolves before reaching the ocean bottom, but this is a solvation, not dissociation; the carbonate ion remains intact.
Note that Reaction 2 (dissolution at high hydrostatic pressure) is the exact reverse of Reaction 1 (calcification). The gaseous atmosphere is not directly involved in either equilibrium direction, this calcification/dissolution equilibrium being a balanced oceanic CO2 cycle that depends on water depth. In the extreme case the solubilised hydrogencarbonate ions will be carried by the global thermohaline circulation and could take a thousand years to surface and interact again with the atmosphere.
Calcifying organisms use a highly conserved biomineralization toolkit to make the shell.
So far, we have dealt only with the reversible calcification reaction as though the chemical reactions were taking place in laboratory glassware. In the living cell, calcium has a key role in signalling and calcium homeostasis is strictly maintained. In coccolithophores calcium is transferred in vesicles, containing calcium-loaded particles that fuse with another “coccolith vesicle” in which coccolith calcification occurs [56]. Thus, to be returned to the seawater (one step prior to the atmosphere), CO2 molecules released by Reaction 1 would have to be transported across at least two ion-selective phospholipid membranes. Though, as we explain below, in all calcifying cells, Reaction 1 takes place on the surface of an evolutionarily conserved polypeptide complex, not in free solution.
If this CO2 were to be released from the calcification assemblage, it would dissolve in the first aqueous compartment it encounters in a matter of seconds [57] becoming a hydrogencarbonate ion which is a candidate for another round of calcification, and in illuminated coccolithophores, would most likely be harvested for photosynthesis. Even “dissolving in a matter of seconds” is too slow for metabolic processes. Waldron (2019) [51] has indicated that most of these debates about CO2/HCO3–/ H+ ignore the fact that the cascade of reactions giving rise to biogenic calcification is mediated by the enzyme carbonic anhydrase [58]. This indifference occurs despite the fact that this enzyme family is figuring increasingly in civil engineering biomimetic designs using immobilized enzymes for CO2 capture for industrial carbon capture and storage (CCS) processes from flue gases, where it is called microbially induced carbonate precipitation or MICP technology [59-64].
In living organisms, prokaryotes and eukaryotes alike, carbonic anhydrases (CAs) are so widely distributed that it is probably true to say they are universal enzymes. These zinc-containing polypeptides catalyse the reversible hydration of CO2 to hydrogencarbonate (‘bicarbonate’). At least five distinct CA families are recognized: α, β, γ, δ and ζ these families have no significant similarity in amino acid sequence (implying their convergent evolution) and vary in distribution across different organisms. Each family usually has numerous isoforms that may be differentiated for different functions in the cell, between organelles or between tissues and organs in more complex organisms. Sequence diversity of this magnitude demonstrates that enzymic control of the hydration of CO2 has been, and remains, of such crucial importance to life on this planet that the function has been endowed with exceptionally high positive selection pressure.
Physiologically the CA reaction contributes widely to normal metabolism: to control the acid-base balance of the cell, organelle or tissue; to metabolic respiration in aerobes (as well as gas transport and gas exchange ‘respiration’ in the complex organisms that ‘breathe’), and, in a chemically different environment, to anaerobic metabolism. CA is also essential in photosynthesis (for more information, view Science Direct at this URL: https://tinyurl.com/4h3thahn).
The essential contribution of carbonic anhydrase to calcification reactions of calcifier organisms is well documented. CA plays a key role in biomineralisation by the benthic foraminiferan; Amphistegina lessonii [65] and ten α-CAs were found in the mantle tissues of the Mediterranean mussel [66]. Comparative genomics of the sequence of the most abundant form (named, MgNACR) grouped MgNACR with oyster nacreins, suggesting to these authors that, like nacrein, the MgNACR protein “likely regulates mussel shell production” (Cardoso et al 2019 [66]). Miyamoto et al (1996) [67] had already demonstrated that nacrein, a soluble organic matrix protein in the nacreous layer of oyster pearls, contained two functional domains: a carbonic anhydrase domain which was split into two subdomains with what was suggested to be a calcium-binding domain between them. Seemingly, these domains participate in calcium carbonate crystal formation of the nacreous layer [67] and are components of a highly conserved biomineralization toolkit in shell-bearing bivalves [68] that has been described as “a complex bioceramic assembly process” [69].
For several reasons, therefore, but principally the wide distribution of carbonic anhydrases and the intimate connection of molecules having this enzymic activity with the structural assembly of the crystals that make up shell material, calcifying organisms will make no return of CO2 to the open atmosphere from their calcifying activities, but only controlled emissions of metabolic wastes, like CO2, to their local aqueous environment. Mussels on their rocky shore would fizz like sparkling wine if such calcification-related atmospheric emission really happened [53].
Life cycle assessments (LCA) of bivalve farming.
A growing amount of detailed and comprehensive data bearing on ‘shellfish for carbon sequestration’ has appeared in recent years, though use of quantitative units was confusingly variable in the early years [51,70-77]. The most recent of these studies make use of life cycle assessments (LCA) of mussel and clam farming in Mediterranean waters and conclude that the activity is a sustainable aquaculture practice as well as a carbon sink [58,78-81].
Alonso, et al. (2021) [82] estimated that the CO2 sequestration potential of bivalve aquaculture, using the then current value of 1 metric tonne of CO2 in the carbon market, to be over 25€ per tonne fresh weight of shellfish, which would represent a value of around 125 to 175 million € yr-1 to the European Union’s current bivalve aquaculture industry alone. A global overall assessment of the economic value of non-food ecosystem services provided by today’s bivalve aquaculture [77] estimated this to be worth about $US 6.5 billion per annum. This estimate did not include carbon sequestration, but the authors do claim that oyster shells have an additional global potential worth of $US 5.2billion, as they are widely seen as having great potential as a CaCO3 feedstock [52,83] primarily, perhaps, for the cement industry in which:
“Between calcination and energy use, the production of one metric ton of cement results in the approximate emission of 1 metric ton of CO2 into the atmosphere and according to the Zurich Polytechnic, something like 900 billion metric tons of it have been cast since the beginning of the industrial revolution” [84].
Data from FAO Fisheries and Aquaculture Information and Statistics Branch (as of 25 May 2019; https://www.fao.org/ fishery/en/statistics/en) show that over the years 2010-2017 aquaculture harvests across the globe totalled 53,512,850 metric tonnes of crustaceans and 122,527,372 metric tonnes of molluscs (a combined total of 176,040,222 metric tonnes in 8 years. If the shells represent an average 50% of the animal’s mass, total shellfish shell produced globally was 88 million tonnes over 8 years. An average of 11 million tonnes of shell per year [11].
Molluscan shell is composed of about 95%-99% CaCO3 with very small amounts of matrix proteins (responsible for directing species-specific crystal growth), whilst arthropod (crab, shrimp, lobster) exoskeletons are composed largely of chitin, but this is hardened after moulting by heavy depositions of calcium-magnesium carbonate nanocrystals. In either group of organisms, mature shellfish shell is about 95% crystalline calcium/calcium-magnesium carbonate. So, not much arithmetic precision is lost by assuming that the shells are made entirely from CaCO3. On a molar mass basis, carbon represents 12% of the mass of calcium carbonate. So, 11 million tonnes of shell per year is equivalent to 1.32 million tonnes of carbon per year being captured from the atmosphere by current aquaculture activities.
Moore, et al. (2022) point out that global carbon emissions from fossil fuel use were 9.8 billion tonnes in 2014 (equivalent to 35.9 billion tonnes of carbon dioxide) [source: https://www. co2.earth/global-co2-emissions], thus, the carbon captured by today’s world aquaculture is a very small contribution to compensating these emissions. However, these authors also calculated [11]:
“We estimate that 4.84 million tonnes of CO2 per year is being captured, and mineralised, from the atmosphere by current aquaculture activities around the world. In carbon-offset terms, that’s equivalent to one million business class return flights between London Heathrow and JFK New York (6 billion miles of flying per year, every year)” and that a single shellfish farm “designed to produce 10,000 tonnes of mussels per year would permanently remove from the atmosphere an annual total of 1,606 metric tonnes of CO2 [which could] offset 740 return business class tickets LHR-JFK, or offset driving 7,300,000 miles [in a 1.5 l petrol-engine family car]” [11].
Unfortunately, so little credence is given, mistakenly in our view, to the ability of shellfish calcifiers to sequester atmospheric carbon that none of this offsetting is possible. Aquaculture is not presently considered to be a valid carbon-offsetting scheme (for the reasons mentioned in the second paragraph of our Introduction, above), and the yield of captured atmospheric carbon by the world’s current aquaculture industry is considered pitifully small in the face of the annual emissions of CO2 through continued fossil fuel use. But the world’s current aquaculture industry is devoted to food production, and its scale, and the organisms cultivated are governed by the market forces applicable to a food delicacy; the atmospheric carbon that the activity captures and stores in the shells is a by-product (and too-often treated as a food waste needing some form of disposal).
Suppose we change the paradigm and cultivate shellfish for their shells, taking the meat produced as the nutritious byproduct. Then the market forces might dictate enhancing the scale of production towards a level that removes very significant quantities of carbon from the atmosphere. Moore et al. (2022) put it this way [11].
“A million mussel farms would permanently remove about 4.5% of the global CO2 emissions in each year. The call for a million mussel farms is by no means an extreme or unrealistic proposition. Imagine a mussel farm on every offshore wind turbine, every oil and gas rig, and every pier, wharf and jetty, every breakwater or harbour wall; imagine cultivating cockles (and other clams) in every shallow sandy/muddy bay. Imagine restocking and extending every fished-out oyster fishery, every fished-out scallop fishery. We could start tomorrow” [7,11].
To this, we would add that colocation of a range of aquaculture farming activities with wind farm installations has been demonstrated to be feasible and financially rewarding [85-87].
Another important point is that the LCAs show that as far as the aquaculture fishery industry is concerned, the diesel fuel consumption of diesel-powered fishing vessels and the electricity consumption of onshore industrial plant (refrigeration, processing plant, warehousing, road transport, etc) are the major contributors to the environmental burden (the carbon cost) of cultivating calcifiers like bivalves. This is no different from any other maritime industry and will be steadily reduced as fossil fuel energy is replaced by renewable resources across this sector.
We have dispelled the notion that the calcification reaction itself is a net CO2 source for the atmosphere earlier in this Section (above). But any adverse contribution of the respiratory flux of CO2 to promote production of the shell that might be suggested is also doubtful. This environmental burden is true for all living organisms and is an inescapable part of the natural carbon cycling generated by all the life processes of those organisms. Designs for atmospheric amelioration must concentrate on net additions of CO2 to the atmosphere resulting from use of fossilised resources. For an LCA about the aquaculture industry, although the carbon footprint of the boat’s diesel fuel is undoubtedly relevant, the release of CO2 as a metabolic waste by the shellfish is no more relevant to their ability to sequester carbon than the respiratory flux of CO2 of the boat’s crew; or even the respiratory flux of CO2 of those who write and read about it.
None of these caveats about the ability of cultivated molluscs to contribute to carbon trading schemes have any parallel in discussions about including forest trees, kelp forests, mangroves or seagrass meadows in carbon trading schemes. The common mantra there is ‘cultivate a plant to save the atmosphere/ biodiversity/ world’ and nobody seems to worry about the fact that plants also release respiratory CO2. Photosynthetic organisms only sequester carbon when illuminated. When the light goes out, they are net CO2 emitters; like the rest of us. Indeed, analysis of terrestrial forest carbon accounting indicates that, because of this, for more than two decades commercial forest carbon protocols have overestimated the carbon trading value of forest carbon by about 2½ times [88]. Another negative aspect for expectations that tree planting schemes can make a serious contribution to amelioration of our atmosphere is that “tree numbers have declined to nearly half since the start of human civilisation and over 15 billion trees are lost on an annual basis” [89].
The key aspect in any comparison between mariculture and forestry, though, is that photosynthetic organisms only sequester carbon whilst they remain alive; calcifier shells sequester atmospheric carbon permanently. Plant a billion trees [https://www.nature.org/en-us/get-involved/how-tohelp/ plant-a-billion/], and even though this number is only 7% of what is required to compensate for annual tree losses, all gains in terms of sequestered carbon are lost after the plant dies, being digested by the legions of animals, bacteria and, especially, fungi that are just waiting for the chance to consume the forest’s biomass and convert it back to atmospheric CO2 as quickly as possible. Cultivate a billion bivalves and when the animals die, they leave their shells as a legacy of solidified atmospheric carbon that can demonstrably stay sequestered for 500 million years. That’s a legacy worth cultivating.
Middens to be proud of!
Continuing the audit analogy, an audit trail (a sequential record of the history and details around an event) finds intact shellfish shells through the whole of early human evolution [4,5References -->
References
- Heilweck M, Moore D. Saving the planet with appropriate biotechnology: 3. The high seas solution. Mex J Biotechnol. 2021;6:92-128.
- Moore D. A biotechnological expansion of shellfish cultivation could permanently remove carbon dioxide from the atmosphere. Mex J Biotechnol 2020;5:1-10.
- Moore D. Saving the planet with appropriate biotechnology: 4. Coccolithophore cultivation and deployment. Mex J Biotechnol. 2021;6:129-55.
- Moore D, Heilweck M, Petros P. Saving the planet with appropriate biotechnology: 1. diagnosing the problems. Mex J Biotechnol. 2021;6:1-30.
- Moore D, Heilweck M, Petros P. Saving the planet with appropriate biotechnology: 2. Cultivate shellfish to remediate the atmosphere. Mex J Biotechnol. 2021;6:31-91.
- Moore D, Heilweck M, Petros P. Aquaculture: Ocean blue carbon meets UN-SDGS. Switzerland, Springer Nature. 2022.
- Moore D, Heilweck M, Petros P. What should be done. In: Aquaculture: Ocean blue carbon meets UN-SDGS . 2022;217-42.
- Petros P, Heilweck M, Moore D. Saving the planet with appropriate biotechnology: 5. An action plan. Mex J Biotechnol. 2021;6:1-60.
- Petros P, Moore D. Comparing industrial and biotechnological solutions for carbon capture and storage. In: Aquaculture: Ocean Blue Carbon Meets UN-SDGS. 2022;177-216.
- Moore D, Heilweck M, Petros P. Planetary bioengineering on earth to return and maintain the atmospheric carbon dioxide to pre-industrial levels: Assessing potential mechanisms. Front Astron Space Sci. 2022;9:797146.
- Moore D, Heilweck M, Petros P. Cultivate shellfish to remediate the atmosphere. In: Aquaculture: Ocean Blue Carbon Meets UN-SDGS. 2022;35-63.
- Fischhoff B. Hot air: The psychology of CO2-induced climatic change. In: Cognition, social behavior and the environment. NJ, USA, Lawrence Erlbaum Associates Inc, 1981.
- Marshall G. Don’t even think about it: Why our brains are wired to ignore climate change. New York, Bloomsbury Publishing Plc, 2015.
- Stoknes PE. What we think about when we try not to think about global warming: Toward a new psychology of climate action. White River Junction, VT, USA, Chelsea Green Publishing Co, 2015.
- Emerson S, Hedges J. Chemical oceanography and the marine carbon cycle. Cambridge UK, Cambridge University Press. 2012.
- Gattuso JP, Frankignoulle M, Smith SV. Measurement of community metabolism and significance of coral reefs in the CO2 source-sink debate. Proc Natl Acad Sci. USA. 1999;96:13017-22.
- Kawahata H, Fujita K, Iguchi A, et al. Perspective on the response of marine calcifiers to global warming and ocean acidification - behavior of corals and foraminifera in a high CO2 world “hot house”. Progr Earth and Planet Sci. 2019;6:5.
- Ohno Y, Iguchi A, Shinzato C, et al. An aposymbiotic primary coral polyp counteracts acidification by active pH regulation. Sci Rep. 2017;7:40324.
- McCulloch M, Falter J, Trotter J, et al. Coral resilience to ocean acidification and global warming through pH up-regulation. Nat Clim Change. 2012;2:623-7.
- Tanhua T, Orr JC, Lorenzoni L, et al. Monitoring ocean carbon and ocean acidification. WMO Bulletin. 2015;64:48-51.
- Fassbender AJ, Orr JC, Dickson AG. Technical note: Interpreting pH changes. Biogeosciences. 2021;18: 1407-15.
- IPCC Special Report on Emissions Scenarios. A report of working group III of the intergovernmental panel on climate change. Ed Nakicenovic N, et al. 2000. Cambridge, UK and New York, USA: Cambridge University Press.
- Brewer PG. Ocean chemistry of the fossil fuel signal: The haline signal of “business as usual”. Geophys Res Lett. 1997;24:1367-9.
- Orr JC, Fabry VJ, Aumont O, et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature. 2005;437:681-6.
- Newell BR, Pitman AJ. The psychology of global warming: Improving the fit between the science and the message. Bull Am Meteorol Soc. 2010;91:1003-14.
- Raven J, Caldeira K, Elderfield H, et al. Ocean acidification due to increasing atmospheric carbon dioxide. Royal Soc Policy doc 12/05, 2005.
- Cohen S. States of denial: Knowing about atrocities and suffering. New York, USA, Polity Press 2001.
- Wilson M, Daly M, Gordon S. The evolved psychological apparatus of human decision-making is one source of environmental problems. In: Evolutionary Perspectives on Environmental Problems. New York, USA, Routledge, 2007.
- Diamond JM. Collapse: How societies choose to fail or survive. London, Penguin Books, 2011.
- Westbroek P, Young JR, Linschooten K. Coccolith production (biomineralization) in the marine alga Emiliania huxleyi. J Protozool. 1989;36:368-73.
- Moheimani NR, Webb JP, Borowitzka MA. Bioremediation and other potential applications of coccolithophorid algae: A review. Algal Res. 2012;1:120-33.
- Moore D. Coccolithophore cultivation and deployment. In: Aquaculture: Ocean Blue Carbon Meets UN-SDGS. 2022;155-76.
- Iglesias-Rodriguez MD, Halloran PR, Rickaby REM, et al. Phytoplankton calcification in a high-CO2 world. Science 2008;320:336-40.
- Rigual-Hernandez AS, Trull TW, Flores JA, et al. Full annual monitoring of subantarctic Emiliania huxleyi populations reveals highly calcified morphotypes in high-CO2 winter conditions. Sci Rep. 2020;10:2594.
- Doney SC, Fabry VJ, Feely RA, et al. Ocean acidification: The other CO2 problem. Ann Rev Mar Sci. 2009;1:169-92.
- Nithiyaa N, Ho AX, Norhanis MR, et al. The effect of acidified seawater on shell characteristics of blood cockle, Tegillarca granosa. J Surv Fish Sci. 2021;7:133-42.
- Fitzer SC, Chung P, Maccherozzi F, et al. Biomineral shell formation under ocean acidification: A shift from order to chaos. Sci Rep. 2016;6:21076.
- Knights AM, Norton MJ, Lemasson AJ, et al. Ocean acidification mitigates the negative effects of increased sea temperatures on the biomineralization and crystalline ultrastructure of Mytilus. Front Mar Sci. 2020;7:567228.
- Bach LT, Mackinder LCM, Schulz KG, et al. Dissecting the impact of CO2 and pH on the mechanisms of photosynthesis and calcification in the coccolithophore Emiliania huxleyi. New Phytol. 2013;199:121-34.
- Kroeker KJ, Kordas RL, Crim R, et al. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob Chang Biol. 2013;19:884-96.
- Seed R. Factors influencing shell shape in the mussel Mytilus edulis. J Mar Biolog Assoc. 1968;48:561-84.
- Seed R. Shell growth and form in the Bivalvia. In: Skeletal Growth of Aquatic Organisms: Biological Records of Environmental Change, Ed Rhoads DC, 1980;23-68.
- Gimin R, Mohan R, Thinh LV, et al. The relationship of shell dimensions and shell volume to live weight and soft tissue weight in the mangrove clam, Polymesoda erosa (Solander, 1786) from northern Australia. NAGA, WorldFish Center Quarterly. 2004;27:3(4).
- Byrne HM, Green JAM, Balbus SA, et al. Tides: A key environmental driver of osteichthyan evolution and the fish-tetrapod transition? Proc Roy Soc A. 2020;476:20200355.
- Connell SD, Doubleday ZA, Hamlyn SB, et al. How Ocean acidification can benefit calcifiers. Curr Biol. 2017;27:95-6.
- Duarte CM, Agusti S, Barbier E, et al. Rebuilding marine life. Nature. 2020;580:39-51.
- Gordon TAC, Radford AN, Simpson SD, et al. Marine restoration projects are undervalued. Science. 2020;367:635-6.
- Mackinder L, Wheeler G, Schroeder D, et al. Molecular mechanisms underlying calcification in coccolithophores. Geomicrobiol J. 2010;27:585-95.
- Mejia R. Will ion channels help coccolithophores adapt to ocean acidification? PLoS Biol. 2011;9:e1001087.
- Monteiro FM, Bach LT, Brownlee C, et al. Why marine phytoplankton calcify. Sci Adv. 2016;2:e1501822.
- Waldron RP, Is Crassostrea virginica gulf oyster reef a sustainable resource subject to equivalent carbon credit trading on the world CAP and trade market? Natl Shellfisheries Assoc. 2019.
- AAC. Recommendation on Carbon Sequestration by Molluscs. A Recommendation to the European Commission and the Member States, published by the Aquaculture Advisory Council, 2022.
- Moore D, Heilweck M, Petros P. Diagnosing the Problem. In: Aquaculture: Ocean Blue Carbon Meets UN-SDGS. 2022;1-34.
- Bickert T. Carbonate compensation depth. In: Encyclopedia of Paleoclimatology and Ancient Environments, ed Gornitz V. Dordrecht, Springer, 2009.
- Berger WH. Calcite compensation depth (CCD). In: Encyclopedia of Marine Geosciences. Eds Harff J et al. Dordrecht, Springer, 2016.
- Gal A, Sorrentino A, Kahil K, et al. Native-state imaging of calcifying and noncalcifying microalgae reveals similarities in their calcium storage organelles. Proc Natl Acad Sci USA. 2018;115:11000-5.
- Mitchell MJ, Jensen OE, Cliffe K, et al. A model of carbon dioxide dissolution and mineral carbonation kinetics. Proc Math Phys Eng. 2010;466:1265-90.
- Tamburini E, Turolla E, Lanzoni M, et al. Manila clam and Mediterranean mussel aquaculture is sustainable and a net carbon sink. Sci. Total Environ. 2022;848:157508.
- Lee SW, Park SB, Jeong SK, et al, Trachtenberg MC. On carbon dioxide storage based on biomineralization strategies. Micron. 2010;41:273-82.
- Ferry JG. Carbonic anhydrases of anaerobic microbes. Bioorg Med Chem. 2013;21:1392-5.
- Penders-van Elk NJMC, Versteeg GF. Enzyme-enhanced CO2 absorption. In: Absorption-Based Post-combustion Capture of Carbon Dioxide, ed Feron PHM. Woodhead Publishing, 2016;225-58.
- Reddy MS, Joshi S. Carbon dioxide sequestration on biocement-based composites. In: Carbon Dioxide Sequestration in Cementitious Construction Materials, eds Pacheco-Torgal F, et al. Woodhead Publishing, 2018;225-43.
- Kelemen P, Benson SM, Pilorgé H, et al. An overview of the status and challenges of CO2 storage in minerals and geological formations. Front Clim. 2019;1: 00009.
- Hills CD, Tripathi N, Carey PJ. Mineralization technology for carbon capture, utilization, and storage. Front Energy Res. 2020;8:0042.
- Goeyse S, Webb AE, Reichart GJ, et al. Carbonic anhydrase is involved in calcification by the benthic foraminifer Amphistegina lessonii. Biogeosciences. 2021;18:393-401.
- Cardoso JCR, Ferreira V, Zhang X, et al. Evolution and diversity of alpha-carbonic anhydrases in the mantle of the Mediterranean mussel (Mytilus galloprovincialis). Sci Rep. 2019;9:10400.
- Miyamoto H, Miyashita T, Okushima M, et al. A carbonic anhydrase from the nacreous layer in oyster pearls. Proc Natl Acad Sci USA. 1996;93:9657-60.
- Marie B, Arivalagan J, Mathéron L, et al. Deep conservation of bivalve nacre proteins highlighted by shell matrix proteomics of the Unionoida Elliptio complanata and Villosa lienosa. J R Soc Interface. 2017;14:20160846.
- Evans JS. The biomineralization proteome: protein complexity for a complex bioceramic assembly process. Proteomics. 2019;19:1900036.
- Pelletier NL, Ayer NW, Tyedmers PH, et al. Impact categories for life cycle assessment research of seafood production systems: Review and prospectus. Int J Life Cycle Assess. 2007;12:414-21.
- Iribarren D, Moreira MT, Feijoo G. Life cycle assessment of fresh and canned mussel processing and consumption in Galicia (NW Spain). Resour Conserv Recycl. 2010;55:106-17.
- Lejart M, Clavier J, Chauvaud L, et al. Respiration and calcification of Crassostrea gigas: contribution of an intertidal invasive species to coastal ecosystem CO2 fluxes. Estuaries Coasts. 2012;35:622-32.
- Waldbusser GG, Powell EN, Mann R. Ecosystem effects of shell aggregations and cycling in coastal waters: an example of Chesapeake Bay oyster reefs. Ecology. 2013;94:895-903.
- Filgueira R, Byron C, Comeau L, et al. An integrated ecosystem approach for assessing the potential role of cultivated bivalve shells as part of the carbon trading system. Mar Ecol Prog Ser. 2015;518:281-7.
- Filgueira R, Strohmeier T, Strand Ø. Regulating services of bivalve molluscs in the context of the carbon cycle and implications for ecosystem valuation. In: Goods and Services of Marine Bivalves, eds Smaal A, et al. Cham, Switzerland, Springer Nature, 2019;231-51.
- Jansen H, van den Bogaart L. Blue carbon by marine bivalves: perspective of carbon sequestration by cultured and wild bivalve stocks in the Dutch coastal areas. Wageningen Marine Research report, No. C116/20, 2020.
- van der Schatte Olivier A, Jones L, Vay LL, et al. A global review of the ecosystem services provided by bivalve aquaculture. Rev Aquac. 2020;12:3-25.
- Martini A, Calì M, Capoccioni F, et al. Environmental performance and shell formation-related carbon flows for mussel farming systems. Sci Total Environ. 2022;831:154891.
- Tamburini E, Fano EA, Castaldelli G, et al. Life Cycle Assessment of oyster farming in the Po Delta, Northern Italy. Resources. 2019;8:170.
- Tamburini E, Turolla E, Fano EA, et al. Sustainability of Mussel (Mytilus galloprovincialis) farming in the Po River Delta, Northern Italy, based on a life cycle assessment approach. Sustainability. 2020;12:3814.
- Turolla E, Castaldelli G, Fano EA, et al. Life Cycle Assessment (LCA) proves that Manila Clam farming (Ruditapes philippinarum) is a fully sustainable aquaculture practice and a carbon sink. Sustainability. 2020;12:5252.
- Alonso AA, Álvarez-Salgado XA, Antelo LT. Assessing the impact of bivalve aquaculture on the carbon circular economy. J Clean Prod 2021; 279:123873.
- de Alvarenga RAF, Galindro BM, Helpa CF, et al. The recycling of oyster shells: An environmental analysis using Life Cycle Assessment. J Environ Manage 2012;106:102-9.
- Di Stefano D. Cement: The Most Destructive Material in the World or a Driver of Progress? Posting on the GreenBiz Group Inc. website (2022).
- Blanchard S. Colocation of aquaculture and wind farm in Liverpool Bay: Effective incorporation of finfish aquaculture to the Gwynt y Mor wind farm. LAP Lambert Academic Publishing, 2020.
- Maar M, Bolding K, Petersen JK, et al. Local effects of blue mussels around turbine foundations in an ecosystem model of Nysted off-shore wind farm, Denmark. J Sea Res. 2009;62:159-74.
- Stenberg C, Christoffersen M, Mariani P, et al. Offshore wind farms and their potential for shellfish aquaculture and restocking (one page poster). National Institute of Aquatic Resources, ICES Council Meeting Nantes, France, 2010.
- Marino BDV, Bautista N. Commercial forest carbon protocol over-credit bias delimited by zero-threshold carbon accounting. Trees, Forests and People. 2022;7:100171.
- Crowther TW, Glick HB, Covey KR, et al. Mapping tree density at a global scale. Nature. 2015;525:201-5.
- van Andel TH, Heath GR, Moore TC Jr. Cenozoic history and paleoceanography of the central equatorial Pacific Ocean. Mem Geol Soc Am 1975;143:1-134.
- van Andel TH, Thiede J, Sclater JG, et al. Depositional history of the South Atlantic Ocean during the last 125 million years. J Geol 1977;85:651-98.
- Preiß-Daimler I, Zarkogiannis SD, Kontakiotis G, et al. Paleoceanographic perturbations and the marine carbonate system during the middle to late Miocene carbonate crash-A critical review. Geosciences. 2021; 11:94.
- Ysebaert T, Walles B, Haner J, et al. Habitat modification and coastal protection by ecosystem-engineering reef-building bivalves. In: Goods and services of marine bivalves, eds Smaal A, et al. Cham, Switzerland, Springer Nature, 2019.
- Moore D, Heilweck M. Aquaculture: Prehistoric to Traditional to Modern. In: Aquaculture: Ocean Blue Carbon Meets UN-SDGS. Switzerland, Springer Nature, 2022;65-95.
- Moore D. Farming Giant Clams in 2021: A Great Future for the ‘Blue Economy’ of Tropical Islands. In: Aquaculture: Ocean Blue Carbon Meets UN-SDGS. Switzerland, Springer Nature, 2022;131-53.
- Jones AR, Alleway HK, McAfee D, et al. Climate-friendly seafood: the potential for emissions reduction and carbon capture in marine aquaculture. BioSci. 2022;72:123-43.
- Summa D, Lanzoni M, Castaldelli G, et al. Trends and opportunities of bivalve shells waste valorization in a prospect of Circular Blue Bioeconomy. Resources. 2022;11.
- Yesner DR, Ayres WS, Carlson DL, et al. Maritime hunter-gatherers: Ecology and prehistory [and comments and reply]. Curr Anthropol. 1980;21:727-50.
- Villagran XS. The shell midden conundrum: comparative micromorphology of shell-matrix sites from South America. J Archaeol Method Theory. 2019;26:344-95.
- Villagran XS, Klokler D, Peixoto S, et al. Building coastal landscapes: Zooarchaeology and geoarchaeology of Brazilian shell mounds. J Isl Coast Archaeol. 2011;6:211-34.
- Smith NF, Lepofsky D, Toniello G, et al. 3500 years of shellfish mariculture on the Northwest Coast of North America. PLoS ONE 2019;14:e0211194.
- Lepofsky D, Toniello G, Earnshaw J, et al. Ancient anthropogenic clam gardens of the Northwest Coast expand clam habitat. Ecosystems 2021;24:248-60.
- Zarrelli N. The Voluminous Shell Heaps Hidden in Plain Sight All Over NYC. Posting on the Atlas Obscura website, 2016.
- Kurlansky M. The Big Oyster: History on the Half Shell. Ballantine Books, 2006.
- Mitra S, Fazli P, Jana H, et al. Molluscan community: a potential sink of carbon. J Environ Carbon Credits. 2015;5(3):13-7.
- Hickey JP. Carbon sequestration potential of shellfish. In: Seminars in Sustainability, School of Natural and Built Environs, and University of South Australia. Published on and by The Fish Site, 25 October 2004.
- Lockwood R, Mann R. A conservation palaeobiological perspective on Chesapeake bay oysters. Phil Trans R Soc . 2019;374:20190209.
- Armstrong S, Hull S, Pearson Z, et al. Estimating the carbon sink potential of the Welsh marine environment. Report No 428, published by Natural Resources Wales, Cardiff, 2020.
- Macreadie PI, Robertson AI, Spinks B, et al. Operationalizing marketable blue carbon. One Earth. 2022;5: 485-92.
- Campbell JS, Foteinis S, Furey V, et al. Geochemical negative emissions technologies: Part I. Review. Front Clim 2022; 4: 879133.
- Heilweck M. The High Seas Solution. In: Aquaculture: Ocean Blue Carbon Meets UN-SDGS. Switzerland, Springer Nature, 2022;97-130.
- Gokoglu N. Shellfish Processing and Preservation. Switzerland, Springer Nature, 2021.
- Goddek S, Joyce A, Kotzen B, et al. Aquaponics food production systems: Combined aquaculture and hydroponic production technologies for the future. Switzerland, Springer Nature, 2019.
- Lucas JS, Southgate PC, Tucker CS. eds, Aquaculture: Farming aquatic animals and plants. 3rd ed, Wiley-Blackwell; 2019.
- Hai FI, Visvanathan C, Boopathy R. Sustainable aquaculture (applied environmental science and engineering for a sustainable future). Switzerland, Springer Nature, 2018.
- Xiang J. Recent major advances of biotechnology and sustainable aquaculture in China. Curr Biotechnol. 2015;4:296-310.
- Spence A, Pidgeon N. Framing and communicating climate change: the effects of distance and outcome frame manipulations. Glob Environ Change. 2010;20:656-67.
- Spence A, Poortinga W, Pidgeon N. The psychological distance of climate change. Risk Anal. 2012:32, 957-72.
- Jones C, Hine DW, Marks ADG. The future is now: reducing psychological distance to increase public engagement with climate change. Risk Anal. 2017;37:331-41.
- Carranza A, zu Ermgassen PSE. A global overview of restorative shellfish mariculture. Front Mar Sci. 2020;7:722.
- Sea MA, Hillman JR, Thrush SF. The influence of mussel restoration on coastal carbon cycling. Glob Change Biol. 2022;28:5269-82.
- Sea MA, Hillman JR, Thrush SF. Enhancing multiple scales of seafloor biodiversity with mussel restoration. Scientific Reports. 2022;12:5027.
- Zhang H, Che H, Xia J, et al. Sedimentary CaCO3 accumulation in the deep west Pacific Ocean. Front Earth Sci. 2022;10:857260.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref