Research Article - Journal of Dermatology Research and Skin Care (2023) Volume 7, Issue 3
Positive effects of Nourkrin® Woman with Marilex® on hair growth and appearance leads to a high level of patient satisfaction-A Clinical study on women with diffuse hair loss from the Arabian countries of the Gulf Cooperation Council.
Jan Wadstein1*, Fahad Abdulaziz Almutawa2, Haitham Al-Ramal3
1Research and Development, Ostra Ronneholmsvagen Malmo, Sweden
2Consultant dermatologist and Assistant professor, Kuwait University, Kuwait
3Department of Clinical Pharmacy, Rawdah COOP PH. Kuwait City, Kuwait
- *Corresponding Author:
- Jan Wadstein
Research and Development
Ostra Ronneholmsvagen Malmo, Sweden
E-mail: dr.jan.wadstein@gmail.com
Received: 25-May-2023, Manuscript No. AADRSC-23-99959; Editor assigned: 27-May-2023, PreQC No. AADRSC-23-99959(PQ); Reviewed: 11-Jun-2023, QC No AADRSC-23-99959; Revised: 15-Jun-2023, Manuscript No. AADRSC-23-99959(R); Published: 23-Jun-2023, DOI:10.35841/aadrsc-7.3.148
Citation: Wadstein J, Almutawa F A, Ramal H Al. Positive effects of Nourkrin® Woman with Marilex® on hair growth and appearance leads to a high level of patient satisfaction-A Clinical study on women with diffuse hair loss from the Arabian countries of the Gulf Cooperation Council. Dermatol Res Skin Care. 2023;7(3),148
Abstract
Proteoglycan replacement therapy (PRT) is an emerging anti hair loss treatment that utilises a targeted complex of bioactive proteoglycans (Marilex®) to stimulate anagen and maintain active growth in affected hair follicles with Female Pattern Hair Loss (FPHL) and Telogen Effluvium (TE). Nourkrin® with Marilex® increases the concentration of follicular proteoglycans and thereby prevents and treats hair loss and miniaturisation caused by Proteoglycan Follicular Atrophy (PFA). PFA is known to be a key pathology behind diffuse hair loss. The current study aimed to evaluate the treatment satisfaction rate of PRT with Nourkrin® in female patients residing in the Arab countries of the Gulf Cooperation Council. 108 female individuals with diffuse hair loss (average age = 34.31 years) were enrolled into a longitudinal cohort study. The participants voluntarily started a 6-month course of monotherapy with Nourkrin® Woman (600 mg Marilex® per day) and were monitored every 3 months using a semi-structured questionnaire. Enrolment and follow-ups were carried out in collaboration with the World Hair Council. 84% of volunteers were presented with moderate to severe diffuse hair loss of whom more than half had not received medical treatment before. 84.26% and 68.52% of subjects reported improvements in their hair growth and appearance, and 86.11% felt more confident with their hair after 3 months of using Nourkrin® Woman. Patient satisfaction rate was at 89.81% at study mid-point. Out of the 101 participants who had finished the study, 95.05% found their hair growth and appearance improved, which also enhanced their self-confidence. Continuing Nourkrin® therapy for 6 months enhanced treatment satisfaction rate by 8%, denoting the importance of time in achieving optimal clinical results. Overall, our findings confirm that PRT with Nourkrin® is effective in improving both hair growth and appearance as reported by hair loss patients. Nourkrin® efficacy and satisfaction rate was comparable with the previously studied European and Latin American populations.
Keywords
Female pattern hair loss, Proteoglycans, Proteoglycan Replacement Therapy, Nourkrin®, Marilex®, Patient outcome assessment, Patient satisfaction, Self-confidence.
Introduction
Hair is an emblem of femininity and is closely linked to the self-identity of women. Indeed, bountiful hair is considered an asset of a woman, and thus no wonders any disturbance to its integrity may have profound impact on her psychosocial wellbeing [1]. Several distinct forms of hair growth disorders are recognised in women, Female Pattern Hair Loss (FPHL) and Telogen Effluvium (TE) being the most common diagnoses. Both FPHL and TE may present clinically as diffuse and progressive shedding of telogenic hair from large scalp areas on forehead and vertex. The importance of understanding and treating hair conditions is growing because of the emphasis that modern society lays on 'looking good and youthful'.
Diffuse hair loss is a common complaint of women being referred to dermatology and trichology clinics. Epidemiological data indicates that FPHL occurs in more than 55% of women, while the true prevalence of TE is not known [2]. However, according to available data, the prevalence of alopecia is substantially variable in different parts of the world. It is generally believed that specific environmental conditions in Middle Eastern Arab nations may cause higher incidence of hair loss. For example, experts believe that hair loss in United Arab Emirates (UAE) is rampant, i.e., nearly half of the dermatology patients in daily practice make inquiries about losing their hair. During the summer, this already high prevalence further increases by at least 10% [3]. A survey was conducted on 100 women (between the ages of 25 and 60) living in Dubai and Abu Dhabi by an independent market research company. Researchers have reported that more than two-thirds of participants were struggling with some form of hair loss. The role of environmental factors is evident since a large number of expatriates complain from hair loss after moving to UAE [4]. As shown by a retrospective study on adult females from Makkah region, Saudi Arabia, around 2% of women with diffuse hair loss had TE of which 79% was chronic [5].
As of today, both FPHL and chronic TE pose a grand clinical challenge to healthcare professionals and medical scholars. Numerous unanswered questions still exist with regard to the underlying processes that cause diffuse hair loss in women and effective approaches to address them. During the past two decades, however, new light has appeared at the end of the tunnel with the discovery of follicular proteoglycans as crucial aetiological factors in different forms of hair loss [6]. This original knowledge has led to targeted utilisation of marinederived proteoglycans, marketed as Nourkrin® with Marilex® (produced by Pharma Medico Aps, Aarhus, Denmark), in the clinical treatment of both FPHL and TE. Oral administration of this unique formulation of natural proteoglycans is called Proteoglycan Replacement Therapy (PRT), indicated for the treatment of pattern hair loss and TE. Clinical trials and subjective studies have verified the safety and efficacy of PRT with Marilex® in patients from European and Latin American populations [7-9]. The present study has been conducted to investigate the efficacy and patient acceptability of Nourkrin® therapy in Middle Eastern countries of the Gulf Cooperation Council.
Materials and Methods
Study design
This was an observational multi-centre, cohort study with a concurrent, 6-month follow-up period. Study protocol and onsite execution were approved and continuously supervised by members of the World Hair Council (WHC). WHC operates as a non-profit organization of hair loss experts including trichologists and dermatologists dedicated to improving the lives of people living with hair growth disorders (https,// worldhaircouncil.com).
The under-study treatment was PRT with Nourkrin® Woman (Pharma Medico Aps, Aarhus, Denmark) administered as 2 tablets per day delivering 600 mg of Marilex®. This active complex is a proprietary natural extract rich in ‘hair growth stimulating’ proteoglycans such as versican and decorin. All participants have voluntarily decided to start a 6-month course of monotherapy with Nourkrin® Woman.
Outcome evaluation
Study primary outcome was subjective satisfaction with Nourkrin® treatment. This outcome was evaluated after 3 and 6 months from the start of the treatment using a structured, self-administered, 2-point (yes or no) questionnaire. Questions concerning the changes in the growth, appearance and quality of hair compared to baseline as well as the effect of Nourkrin® Woman on hair confidence and overall treatment satisfaction of the participants were included in the study questionnaire. At each follow-up evaluation, targeted questions were asked about potential side effects and newly-onset symptoms that may have been related to the treatment.
Study participants
All study participants met the following eligibility criteria, females of 18-64 years of age; living in a country of the Gulf Cooperation Council; and having a physician-based diagnosis of FPHL or TE. The initial screening phase was carried out by a number of collaborating dermatologists practicing in outpatient clinics in Countries of the Gulf Cooperation Council. 142 eligible individuals were screened and underwent a more detailed clinical interview. Understandable information regarding the aim of the survey, efficacy, and safety of Nourkrin® Woman and other available options was provided to each potential participant. At the end of the screening phase, 108 patients were enrolled into the study from whom an informed consent was obtained. The stage of hair loss at baseline was assessed using Ludwig classification scale for FPHL [10].
Study investigators have instructed the patients against using any form of anti-hair loss medications or supplements, undergoing laser treatment, hair transplantation or other elective surgical procedures involving the scalp. Additionally, taking medications known to affect hair growth (e.g., contraceptive pills, anabolic steroids, immunomodulators and cytotoxic or cytostatic drugs) was not allowed during the study or shortly before enrolment. The participants also agreed to maintain their usual hairstyling practices for the duration of the study. Violating any of these preconditions was considered a basis for elimination. Pregnant and breastfeeding women as well as the individuals with a known allergy to fish or shellfish were not enrolled. In this paper, data from all the enrolled subjects is analysed and reported in an intention-totreat fashion.
Results
Baseline characteristics of study participants are summarised in Table 1. 3 months into the study, 7 subjects were dropped out of the study for personal reasons leaving a final study population of 101. It is worth noting that most of the participants were in their young to middle-aged adulthood and were diagnosed with moderate stages of diffuse hair loss. Surprisingly, more than half of our patients (54.6%) have never sought medical attention and were suffering in silence; and one in every three patients complained of being under notable psychological stress at the time of enrolment.
| Number of participants | 108 |
| Age (years), mean (range) | 34.31 (24-57) |
| Grade of hair thinning/loss (number) | |
| 1 (Mild) | 17 |
| 2 (Moderate) | 72 |
| 3 (Severe) | 19 |
| Duration of hair thinning/loss (months), mean (range) | 17.4 (2-108) |
| Positive history of previous therapy (%) | 45.37 |
| Participants with a recent stressful period (%) | 27.78 |
Table 1. Baseline demographics of study participants
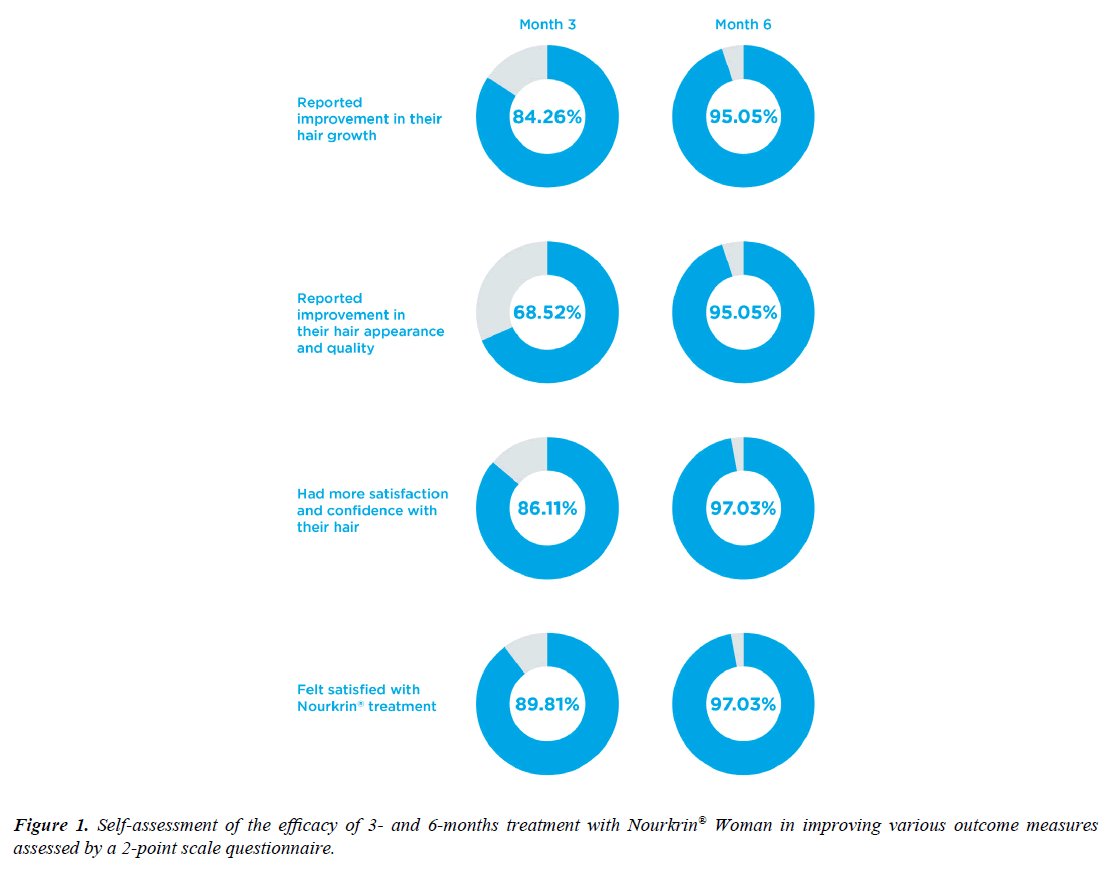
After 3 months of Nourkrin® therapy, 84% of subjects expressed their positive impression with the efficacy of PRT for improving the growth and density of their hair and 69% believed their hair had better quality and appearance. As depicted in Figure 1, these improvements had caused 86% of Nourkrin® users to feel more confident with their hair. At this point, 9 out of 10 hair loss patients were satisfied with their treatment and were willing to continue taking Nourkrin® Woman.
At the end of the 6-month assessment period, the number of patients who experienced improvements in the growth and quality of their hair increased by 11% and 27%, respectively, and reached 95%. All participants, except for three, felt better and more confident with their hair and expressed their satisfaction with Nourkrin® monotherapy (see Figure 1).
The collaborating dermatologists examined each participant for any symptoms that may have occurred after starting the treatment at each follow-up visit. Reportedly, no new-onset symptoms have been observed, which could be associated with Nourkrin® use; and none of the dropouts in this study were caused by treatment intolerance.
Discussion
In the current study, we have examined the efficacy of a novel hair loss treatment, PRT with Nourkrin®, in a less-studied population from the Gulf Cooperation Council in the Middle East. The safety and efficacy of Nourkrin® has rigorously been studied from both objective and subjective standpoints. Quantitative measurement of hair density in individuals with diffuse hair loss has revealed a 35.7% increase after 6 months of monotherapy with Nourkrin® [8]. Patient satisfaction and treatment acceptability have also been studied previously in the United Kingdom (UK) and Brazil [11]. However, hair loss disorders are known to have characteristic prevalence rates, patterns, contributing factors and gene polymorphisms in different populations, and these variations can affect patients’ response to treatment [12]. It is, therefore, of clinical importance to assess regional treatment outcomes and patient satisfaction rates with PRT in different parts of the world.
Our findings provide evidence that the efficacy of Nourkrin® monotherapy in hair loss patients from the Gulf countries is comparable with that of the European and Latin American populations. Published literature indicates that more than 90% of subjects from both the UK and Brazil found Nourkrin® Woman effective in improving the growth and quality of their hair after a 6-month course of treatment. The treatment satisfaction rate of 97% with Nourkrin® in this cohort is on par with 98% in the UK [9] and 97% in the Brazilian hair loss study. ‘Treatment satisfaction rate’ is an important, but neglected, outcome measure that gives useful insights into the patient’s perspective on their current treatment and helps clinicians with making an insightful selection among alternative therapeutic options. This important index is suggested to be a sensitive measure of ‘treatment effectiveness’ in clinical drug trials [13].
A notable observation in our patients was the progressive enhancement of hair growth and quality over time. As stated previously, comparing the final with the 3-month results unveils a significant increase in all outcome measures over time. This time-dependent efficacy improvement with Nourkrin® was expected considering the data from published clinical trials. Both objective hair density and patient satisfaction rates (measured by a visual analogue scale) have surged from 3 to 6 and 6 to 12 months of PRT. Based on this, it appears justified to conclude that a minimum of 6 months of full-dose treatment with Nourkrin® is needed for optimal results, while clinical outcomes can be further improved by continuing the treatment for another 6 months.
Scientific evidence shows that hair loss leaves pervasive negative effects on self-confidence and self-image of the majority of affected women [14]. This specific complication of hair loss may reasonably be the most important aspect that clinicians are expected to adhere to. PRT with Nourkrin® has evidently been successful in addressing the psychological presentations of diffuse hair loss as demonstrated by Kingsley, et al. [15]. Self-esteem together with the other subcategories of ‘quality of life’ has been significantly augmented after a 6-month course of treatment with Nourkrin® (P<0.001) in women with hair loss [15]. Our observations have confirmed these findings by showing that 86% and 97% of patients felt higher satisfaction and more confidence with their hair after 3 and 6 months of taking Nourkrin®.
Contemporary research efforts have shed light on the pharmacological mechanisms via which PRT with Nourkrin® with Marilex® stimulates hair growth and prolongs the active phase of the hair cycle. It is well demonstrated that specific proteoglycans, such as versican and decorin, are periodically expressed in highly active parts of the hair follicle and support the anabolic activities of proliferating and stem cells [16-18]. The expression level of these bioactive proteoglycans appears to be declined in susceptible hair follicles of patients with pattern hair loss and TE. This phenomenon is called Follicular Hypoglycania (FHG), which gradually leads to follicular hypotrophy and atrophy, known as Proteoglycan Follicular Atrophy (PFA). FHG and PFA are significant underlying pathologies that disturb the normal regulation of the hair growth cycle and cause clinical hair loss and miniaturisation. Marilex® is specifically formulated to improve the concentration of active proteoglycans and thereby normalise the reduced anagen/telogen ratio and stimulate dormant hair follicles to start producing new hairs. Yet, this novel approach possesses an additional advantage of a desirable safety and tolerability profile as confirmed by former clinical experiments and the current study. For more detailed information, we refer the reader to a comprehensive review by Wadstein, et al. [6].
Conclusion
The findings of the present study have verified that PRT with Nourkrin® Woman with Marilex® is both safe and effective in a population of Arab descent, residing in members of the Gulf Cooperation Council. A 6-month course of treatment enhanced hair growth and appearance in 95% of the hair loss patients and substantially improved their confidence and self-esteem. Treatment satisfaction rate was very high after both 3 and 6 months of Nourkrin® therapy and almost all participants were satisfied with the outcomes. These results are comparable with findings of similar studies conducted in the UK and Brazil and showed no considerable ethnicitybased variations. In conclusion, Nourkrin® Woman with Marilex® has positive effects on both the growth and quality of scalp hair that produces high levels of treatment satisfaction in patients with diffuse hair loss.
Acknowledgements
Our gratitude goes to all the clinicians and associates who participated in this project. Authors would also like to appreciate the dedicated contribution of the members of World Hair Council, who provided professional counselling and supervision during the whole time period of the current study. We acknowledge Pharma Medico Aps for supplying the Nourkrin® Woman tablets.
Disclosures
The authors have no relevant conflict of interest to disclose.
References
- Hadshiew IM, Foitzik K, Arck PC, et al. Burden of hair loss: Stress and the underestimated psychosocial impact of telogen effluvium and androgenetic alopecia. J Invest Dermatol. 2004;123(3):455-7.
- Gan DC, Sinclair RD. Prevalence of male and female pattern hair loss in Maryborough. J Investig Dermatol Symp Proc. 2005 Dec;10(3):184-9.
- https://www.thenationalnews.com/uae/health/summer-heat-and-a-bad-diet-lead-to-hair-loss-in-the-uae-1.391557
- https://www.emirates247.com/news/emirates/arab-asian-women-have-worst-hair-loss-2012-03-06-1.446934
- Fatani MI, Mahdi AH, Alafif KA, et al. Prevalence and factors associated with telogen effluvium in adult females at Makkah region, Saudi Arabia: A retrospective study. J Dermatol Surg. 2015;19(1):27-30.
- Wadstein J, Thom E, Gadzhigoroeva A. Integral roles of specific proteoglycans in hair growth and hair loss: mechanisms behind the bioactivity of proteoglycan replacement therapy with Nourkrin® with Marilex® in pattern hair loss and telogen effluvium. Dermatol Res Prac. 2020;2020.
- Thom E. Efficacy and tolerability of Hairgain® in individuals with hair loss: A placebo-controlled, double-blind study. J Int Med Res. 2001;29(1):2-6.
- Thom E. Nourkrin®: Objective and subjective effects and tolerability in persons with hair loss. J Int Med Res. 2006;34(5):514-9.
- Thom E, Wadstein J. Treating female diffuse hair loss using nourkrin® woman (with Marilex®)-An open-label, subjective, outcome study on hair growth and appearance, self-confidence and treatment satisfaction. J Clin Dermatol Ther. 2019;5:037.
- Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97(3):247-54.
- Mattos Simoes M, Thom E, Wadstein JN. Woman with Marilex® enhances hair growth and appearance and improves hair confidence in women with diffuse hair loss from Brazil: An investigator-initiated Clinical Study. J Clin Investigat Dermatol. 2020;8(1):4.
- Lee WS, Lee HJ. Characteristics of androgenetic alopecia in asian. Ann Dermatol. 2012;24(3):243-52.
- Rosen R, Goldstein I, Huang XY, et al. The Treatment Satisfaction Scale (TSS) is a sensitive measure of treatment effectiveness for both patients and partners: Results of a randomized controlled trial with vardenafil. J Sex Med. 2007;4(4_Part_1):1009-21.
- Van Der Donk J, Hunfeld JA, Passchier J, et al. Quality of life and maladjustment associated with hair loss in women with alopecia androgenetica. Soc Sci Med. 1994;38(1):159-63.
- Kingsley DH, Thom E. Cosmetic hair treatments improve quality of life in women with female pattern hair loss. J Appl Cosmetol. 2012;30(2):49-59.
- Malgouries S, Thibaut S, Bernard BA. Proteoglycan expression patterns in human hair follicle. Br J Dermatol. 2008;158(2):234-342.
- Sano M, Shang Y, Nakane A, et al. Salmon nasal cartilage proteoglycan enhances growth of normal human dermal fibroblast through Erk1/2 phosphorylation. Biosci Biotechnol Biochem. 2017;81(7):1379-85.
- Kashiwakura I, Takahashi K, Takagaki K. Application of proteoglycan extracted from the nasal cartilage of salmon heads for ex vivo expansion of hematopoietic progenitor cells derived from human umbilical cord blood. Glycoconj J. 2007;24:251-8.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref