Review Article - Biomedical Research (2022) Volume 33, Issue 2
Polymeric nanoparticle based oral insulin delivery system
Rishab Roy Choudhury1*, Payel Mukherjee2
1Gurunanak Institute of Pharmaceutical Science and Technology, 157/F, Nilgunj Rd, Sahid Colony, Panihati, Kolkata, West Bengal, India
2M.R. College of Pharmaceutical Sciences and Research, Kolkata, West Bengal, India
- Corresponding Author:
- Rishab Roy Choudhury
Gurunanak Institute of Pharmaceuticl Science and Technology
157/F, Nilgunj Rd, Sahid Colony, Panihati,
Kolkata,West Bengal
India
Accepted date: February 24, 2022
Abstract
Diabetes is one of the most lethal silently killing diseases because of its massive prevalence and wide numbers of secondary effects. Insulin proves it as a game changer in the treatment of diabetes. Oral route would be most favourite for the administration of such medications as it is the most conventional and comfortable route, however, due to bioavailability consideration, insulin is administered through parenteral route. Oral absorption of insulin is restricted by various absorption, chemical and enzymatic barriers and provides a critical scientific challenge. In recent years polymer based nanoparticles are showing promising results for oral insulin delivery, though such formulations are not marketed yet, they are still under development. In this review we shall discuss about recent developments in nanoparticle based oral insulin drug delivery using various polymers like poly (lactide-co-glycolic acid), Polyurethane-Alginate, cashew gum etc.
Keywords
Diabetes, Insulin, Oral delivery, Polymer, Nano-particle, Bioavailability.
Introduction
Diabetes is one of the most wide spread pandemic for complete human population at present decades. Due to its massive prevalence and wide numbers of secondary effects, diabetes is one of the most dangerous silent killer and it cause almost three million deaths per year throughout the world, as mentioned by the World Health Organization [1]. The International Diabetic Federation indicated that almost 366 million people were affected by different types of diabetes in 2011 and estimated that the sufferer may raise up to 552 million by 2030 [2]. In an average, the life expectancy of people with type 1 diabetes is reduced to about 20 years and for type 2 diabetes the number is about 10 years [3].
In 1922, the revelation of insulin from canine pancreas was a milestone in the history of diabetes therapy with a Nobel Prize victoriously triumphing, which had a great paramountcy in biomedical research [4]. Insulin proves it as a game changer in the treatment of diabetes. From the time of this revelation, peptides and proteins have been used as biopharmaceuticals because of high activity, specificity and effectiveness compared to conventional system [5]. Oral route would be most favourite for the administration of such medications as it is the most conventional and comfortable route [6,7] but suitable oral formulations for oral insulin delivery are still under development [5]. Hence, insulin is administered through parenteral route [8].
The utilization of biocompatible and biodegradable nanoparticles is showing promising advantages toward oral administration of proteins and peptides [3]. For the enhancement of the oral bioavailability of insulin, and to provide a stable and biocompatible environment to the encapsulated drug, polymeric nanoparticles have been claimed to be the impeccable candidates for oral insulin delivery [9].
In this review I shall discuss about different challenges to oral insulin delivery, attributes of oral insulin carriers, benefits and drawback on oral insulin delivery, transport mechanism of insulin loaded NPs and recent developments to deliver insulin through oral routes by using polymeric nano formulations.
Challenges to Oral Insulin Delivery
Insulin delivery through oral route is a big challenge because of several obstacles which limit its effect. When we administer insulin orally, it gets exposed to the chemical and enzymatic barriers of the GI tract. Apart from it, the intestinal transport of insulin is restricted because of the presence of absorption barrier in the intestinal epithelium [10].
Enzymatic barrier
The environment of GIT for proteins and peptides are very stern because our digestive system is designed to breakdown them without doing any difference [11]. Protein and peptide drugs are degraded in stomach by pepsin after that in small intestine by trypsin, chymotrypsin, elastase and carboxypeptidases [12]. They are again digested by aminopeptidases in the brush-border membrane when passing across the intestinal enterocytes. Among all, insulin is mostly degraded by hymotrypsin, trypsin and carboxypeptidases in the intestinal lumen as well as in the mucus layer [13]. Insulin also can be degraded by insulin- degrading enzyme, present in cytosol [14]. After all, if some amount of insulin can successfully transported across the intestinal epithelium, it will further metabolised by liver first pass metabolism [15,16].
Chemical barrier
In oral insulin delivery the insulin must transport along the GIT, successfully transport across the intestinal epithelium and reach the systemic circulation. GIT has variation in pH environment which can destroy protein drugs like insulin. The GI pH is highly acidic in stomach (pH 1.2-3.0) and slightly basic in intestine (pH 6.5-8.0) [17]. This pH difference may cause pH-induced oxidatan, deamidation or hydrolysis of protein drugs; as a result the effect of such drugs gets deactivated [7].
Absorption barrier
The first barrier is mucus membrane, which act as enzymatic and diffusional barrier [18]. In every 4-5 hours mucus is continuously secreted and detached from epithelial surface [19]. Apart from all, the electrostatic repulsion between protein drugs and the mucus layer (negatively charged) obstruct the intimate contact between such drugs and the epithelial cells [18].
Intestinal epithelium is the next barrier. The main problem in the absorption of hydrophilic insulin is that it cannot easily cross through lipid bi-layered epithelial cell membrane to systemic circulation [10].
Dosage form stability
Protein drugs are susceptible to physical and chemical degradation during its manufacturing, transport and storage [20].
Attributes of Ideal Oral Insulin Carrier
An insulin carrier should:
(i) Have the ability to survive against Gastrointestinal (GI) enzymes and pH gradients and increase insulin permeability through GIT.
(ii) Provide a stable and biocompatible environment to make sure that the activity of insulin should remain unaffected during particle processing and insulin release [21,22].
(iii) Enhance its intestinal residence time, so that the permeability of the mucosal epithelium increased, as a result the absorption of drug increase and make available the intact drug to the systemic circulation.
(iv) Deliver required amount of insulin at specific rate to control the concentration of glucose in blood.
(v) Not provide any toxic effect [22].
To increase the oral bioavailability of insulin several strategies are introduced like use of polymeric delivery systems, protease inhibitors, chemical modification of proteins or permeation enhancers [23] but the bioavailability is still below the standard [24]. A combination of such strategies can solve the problem [25].
Benefits and Drawback of Oral Insulin Delivery
Though discovery of insulin is game changer in the field of diabetes treatment but there has no long lasting treatment and the conventional processes are associated with several adverse effects. Thus, many improvements are required among them the route of administration is very important. Parenteral administration of insulin is very uncomfortable for patients as it associated with pain and irritation but in case of oral administration, it is most favourite, comfortable and easy route of drug administration for the patients, self-medication is possible [26].
For physiological insulin, liver is most important target to control blood glucose level [27] but only about 20% of subcutaneously administered insulin can reach the leaver [28] but if we administer it through oral route, it will mimic the physiological condition as after passing the epithelial membrane of GIT, it first comes to the liver [8,5]. As the oral route can mimic the physiological condition, the side effects like risk of hypoglycemia episodes, immune responses, insulin resistance in type 2 diabetic patients will reduce [27]. Moreover irritation, redness, inflammation, infection at the site of injection will also be avoided in oral administration [3]. In other hand oral insulin will less costly, require less specialized person to prepare and easy to transport and store [29].
However still no oral insulin formulation is marketed, they are still under development. In GIT there are several barriers which highly reduce the bioavailability of orally administered insulin [3,8,30].
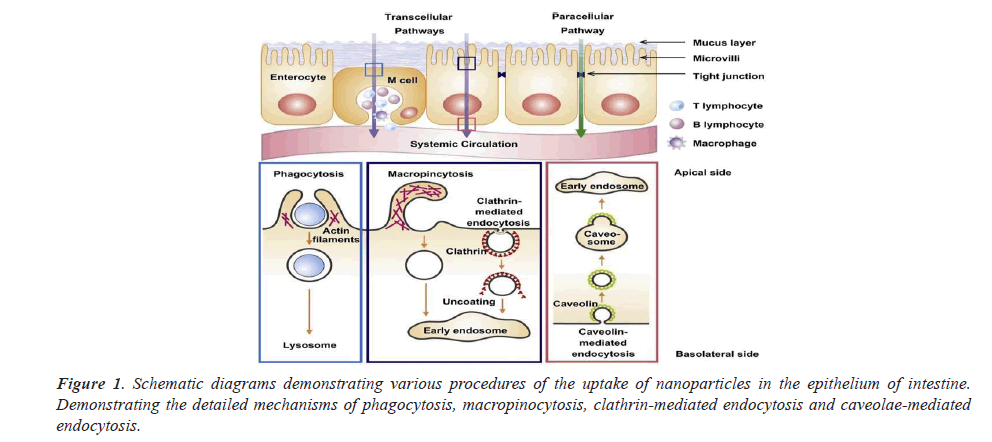
Transport mechanism of nano Particles across the intestinal epithelium
Figure 1 shows the schematic diagrams demonstrating various procedures of the uptake of nanoparticles in the epithelium of intestine.
Different Polymer Based Oral Insulin Delivery System
Polyurethane-Alginate (PU-ALG) nanoparticles
PU is prepared by reacting Bis (2-hydroxyethyl) Terephthalate (BHET), Polyethylene Glycol (PEG) and hexamethylene diisocyanate. In polyurethane- alginate nanoparticles inulin is encapsulated.
By using FT-IR comparative spectrum, proper blending of polyurethane and alginate is observed. It also determines the insulin encapsulation efficiency with polymer blend.
After doing XRD we can determine that after the addition of alginate the blended film becomes more amorphous than PU. It also confirms superior miscibility of blended film and due to intermolecular interaction, the deterioration of regularity of PU.
At weight ratio of 1.4:0.6:0.03 (PU: Alginate: Insulin) the average size of PU-ALG nanoparticles was 91 nm, whereas the average size of insulin loaded alginate nanoparticles having weight ratio of 0.6:0.03 (Alginate: Insulin) was 78 nm.
If the micro-particles have zeta potential values greater than +25 mV or less than -25 mV, then it has high electrostatical stability. Insulin loaded PU-ALG NPs have value less than -25 mV where before the loading of insulin blank PU-ALG NPs have zeta potential greater than -25 mV, which indicates insulin loaded PU-ALG NPs have high electrostatical stability.
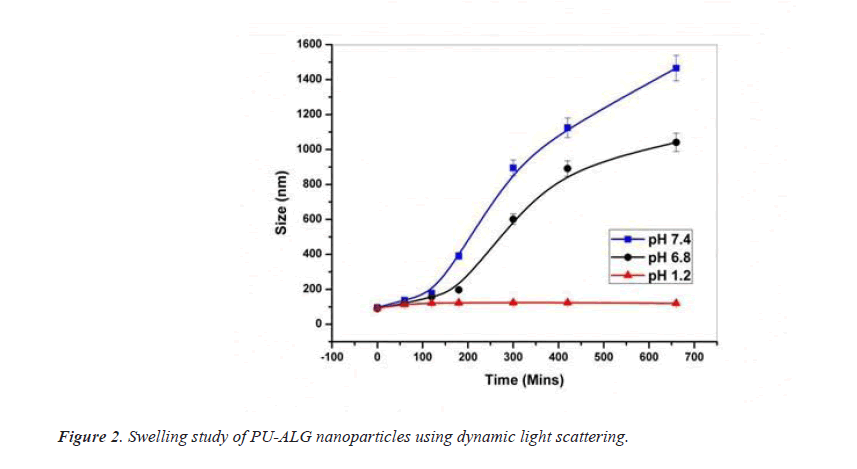
After doing dynamic swelling study, it indicates negligible variation in particle size at pH 1.2 which means encapsulated insulin should be protected from acidic environment of stomach, at pH 6.8 medium and sustained increase in particle size, at pH 7.4 noticeable increase of particle size with regular time interval clarify good swelling features [31] (Figure 2).
Maximum encapsulation efficiency was obtained while incorporating less quantity of insulin by maintaining same ratio of PU and alginate, when higher amount of insulin is incorporated, the encapsulation efficiency is reduce. NPs with PU and alginate ratio of 1.4 mg and 0.6 mg respectively, the encapsulation efficiency were 85%, 82% and 78% for consumed insulin quantity of 0.01 mg, 0.02 mg and 0.03 mg respectively. Different PU-ALG blend were studied and found that 7:3 ratio is most efficient. It is also found that NPs with 78% encapsulation efficiency have the ability to encapsulate maximum amount of insulin with minimum amount of polymer.
I experiment it is observed that maximum 14.4% of encapsulated insulin was release from PU- ALG NPs at pH 1.2 after 2 hours, at pH 6.8 it release 67.62% of encapsulated insulin after 10 hours, at pH 7.4 it release 96.5% of encapsulated insulin after 9 hours So PU-ALG NPs show sustained and prolong release of encapsulated insulin. Moreover at pH 1.2 NPs release very less insulin and after as the NPs were not further swelled, insulin release stop. In this way PU-ALG NPs protect maximum of its encapsulated insulin from acidic environment.
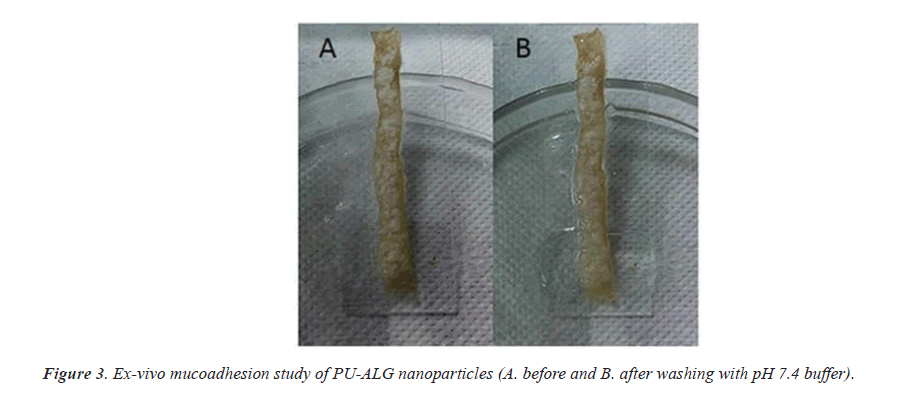
In animal testing it shows that the NPs are considerably wedged to the mouse intestinal limen, subsequent to washing with pH 7.4 buffers for half hour. This shows the good mucoadhesive property of PU-ALG NPs (Figure 3).
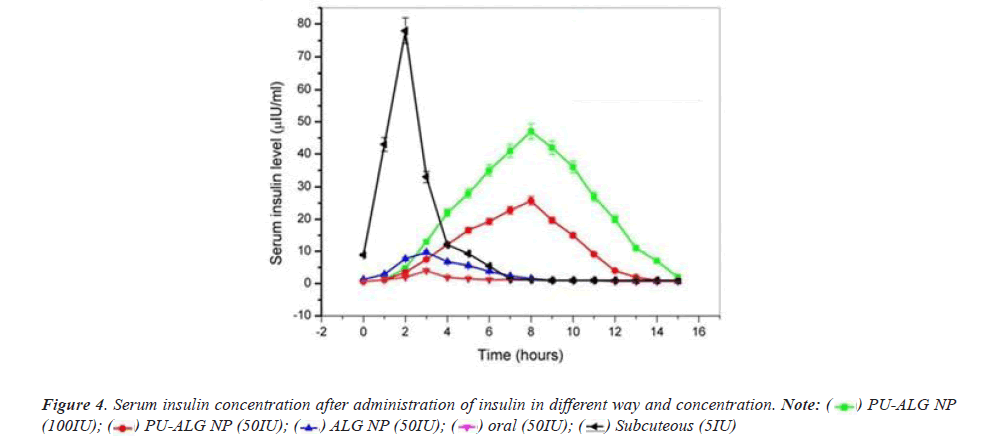
It is observed that 5IU/Kg/b.w. of insulin injection show great reduce in blood glucose level within 1 hour and the effect is maximum (blood glucose level 120 mg/dl) after 2 hours of administration, but the effect is short lusting as the blood glucose level return to the basal level within 6-7 hours. But it is observed that 50 and 100 IU/Kg/b.w insulin encapsulated PU-ALG NPs reduce blood glucose level to 121 and 101 mg/dl respectively after eight hours and it show along lusting effect of at least thirteen hours (Figure 4).
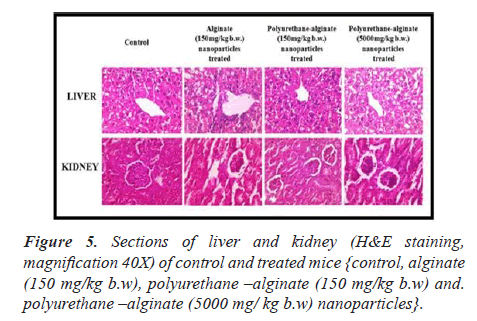
After doing liver function test it is confirm that there is no liver toxicity associated with oral insulin delivery using PU-ALG NPs. After doing nephron toxicity test it is confirm that PU-ALG NPs are safe polymeric carrier for oral delivery of insulin as it indicate no renal dysfunction. At animal model (mice), histopathological reports indicate no alteration in treated liver and kidney tissue [31] (Figure 5).
Cashew gum
The exudate of the plant Anacardium occidentale is the source of cashew gum which is a polysaccharide biopolymer composed of simple sugar and glucuronic acid. It is used to prepare nanoparticle having the ability to incorporate active ingredient. Ionotropic gelation technique is used to prepare this type of nanoparticles where cashew gum is integrated with dextran sulfate and poloxamer containing insulin stabilized with chitosan, poly (Ethyleneglycol) and which is coated with albumin.
Before coating the particle size was 156 nm and after coating it is increased to 5387 nm. The coated finished particles has poly dispersive index 0.2 which indicate good distribution of particle size.
Before coating i.e. the uncoated NPs have zeta potential -27 mv; after giving the coating of chitosan on NPs, they have zeta potential -6.8 mv; the finished NPs after coating with albumin, it show a zeta potential of -51 mv; this indicate a stable system. Due to high repulsive force between the particles, suspension stability allows less formation of aggregate.
To protect the encapsulated insulin from harsh gastric environment and to improve NPs stability, albumin coating is given on NPs. These NPs have insulin encapsulation efficiency of 92% [32].
L-Valin combined PLGA nanoparticles
In insulin loaded L-Valin combined PLGA (Poly Lactic co Glycolic Acid) nanoparticle, the L- Valin is used for carrier mediated transport of insulin. The use of PLGA is very common in preparation of nanoparticle for oral delivery. It is biocompatible, nontoxic, biodegradable and environment friendly. In the presence of water, PLGA undergo degradation by hydrolysis and form two monomers, lactic acid and glycolic acid, which are the by-product of many metabolisms in normal physiological condition of our body.
L-Valin conjugated PLGA nanoparticles can be prepared by Double emulsion solvent evaporation method. Carbodiimide coupling process is used to conjugate L-Valin and PLGA.
The particle size of ligand non-conjugated NPs are 209 ± 3.9 nm which is increased to 213 ± 1.9 nm when ligand is conjugated. Ligand non-conjugated NPs have zeta potential −23.03 ± 1.6 mV and after ligand conjugation, the zeta potential is decreased to −14.4 ± 2.1 mV. High absolute of zeta potential imply higher electric charge on the surface of NPs.
The drug entrapment efficiency of ligand non-conjugated NPs are 50.37 ± 5.9% which is increased to 52.02 ± 1.5% after ligand conjugation. In vitro release study shows parent cumulative drug release of 48.58 ± 7.3% after 24 h for ligand non-conjugated NPs where after ligand conjugation it is decreased to 45.81 ± 8.7% which imply that coupling of valine to PLGA give a sustained release property to the NPs.
When insulin loaded PLGA nanoparticles was given orally, at initial two hours the blood glucose level is not decrease from 265.4 ± 8.5 mg/dL, which after 4 hours further decreased to 246.6 ± 2.4 mg/dL, which further reduced to 198.7 ± 7.1 mg/dL value at 8th h, delimitate the hypoglycaemic effect extend for 4 hours. But when the L-Valin conjugated formulation are administered orally, it produced hypoglycaemic effect within 4 h, i.e, at initial two hours blood glucose level is reduced from 277.7 ± 5.3 mg/dL which is further decreased to 216.9 ± 1.9 mg/dL., the hypoglycaemic effect extend for 12 hours. So L-Valine conjugation produces a sustained release activity to PLGA NPs [33].
Biocompatible montmorillonite PLGA nanocomposites
The nanocomposite is made of food and drug administration approved layer of Montmorillonite (Mt) and biodegradable PLGA which is used for oral insulin delivery. The particles are reduced to their submicron size to maintain insulin’s bioactivity.
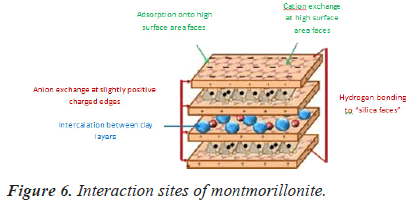
The insulin is protected from harsh acidic environment of GIT by montmorillonite, which is stable at acidic environment. During passing through the intestine the Mt-PLGA nanocomposites are retained in intestinal villi because of the submicron size of Mt-PLGA nanoparticles [34]. Mt provides mucoadhesive capabilities with increased intestinal/cellular absorption e?ciency in GIT [35,36]. Intestinal residence time and permeability of insulin is increased by their mucoadhesive property. This nanocomposites has encapsulation efficiency is up to 97% and can survive for up to 48 hours and the overall viability is above 65% for all concentration range (Figure 6).
The nanoparticles are biocompatible and don’t show any toxic effect on experimented cell line, so it is safe to use for insulin delivery [34].
Conclusion
The discovery of insulin in 1922 was truly a game changing strategy in the field of diabetes treatment as still now it is the most superior strategy of diabetes treatment. Though oral route is the most preferable route but there has no oral insulin formulation in market and still now parenteral route is used to deliver insulin, which associated with several disadvantages and side effects.
Though oral route of insulin delivery is associated with several advantages like patient compliance or can mimic physiological effect of insulin but the major problem is very poor bioavailability and polymeric toxicity. To overcome these problems; various natural, synthetic or semi synthetic polymers are used to prepare insulin. Encapsulated polymer based nanoparticles having advantages like non-toxicity, mucoadhesion, insulin stability etc.
Now a days the research programs are conducted in both academic and industrial field to develop a proper oral insulin delivery system. We have to be more focused to develop oral insulin nano carriers that would be more cost effective, have similar bio-potencial compared to parenteral route and should have proper safety consideration. A lot of works to do to develop and market a proper oral insulin formulation to help the mankind.
References
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes care 2004; 27: 1047-1053.
[Crossref] [Google Scholar] [PubMed]
- Whiting W, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011; 94: 311-321.
[Google Scholar] [PubMed]
- Hosseininasab S, Pashaei-Asl R, Khandaghi AA, Nasrabadi HT, Nejati-Koshki K, Akbarzadeh A. Synthesis, characterization and in vitro studies of PLGA-PEG nanoparticles for oral insulin delivery. Chem Boil Drug Des 2014; 84: 307-315.
[Crossref] [Google Scholar] [PubMed]
- Banting FG, Best CH. The internal secration of the pancreas. The journal of laboratory and clinical medicine. 1922; 7: 251-266.
- Herrero EP, Alonso MJ, Csaba N. Polymer-based oral peptide nanomedicines. Therapeutic delivery. 2012; 3: 657-668.
[Crossref] [Google Scholar] [PubMed]
- Goldberg M, Gomez-Orellana I. Challenges for the oral delivery of macromolecules. Nat Rev Drug Discov 2003; 2: 289-295.
[Crossref] [Google Scholar] [PubMed]
- Sood A, Panchagnula R. Peroral route: An opportunity for protein and peptide drug delivery. Chem Rev 2001; 101: 3275-3303.
[Crossref] [Google Scholar] [PubMed]
- Chaturvedi K, Ganguly K, Nadagouda MN, Aminabhavi TM. Polymeric hydrogels for oral insulin delivery. J Control Release 2013; 165: 129-138.
[Crossref] [Google Scholar] [PubMed]
- Morishita M, Peppas NA. Is the oral route possible for peptide and protein drug delivery? Drug Discov Today 2006; 11: 905-910.
[Crossref] [Google Scholar] [PubMed]
- Lin Y, Chen C, Liang H, Kulkarni AR, Lee P,Chen C, Sung H. Novel nanoparticles for oral insulin delivery via the paracellular pathway. Nanotechnology 2007; 18: 1-11.
- Fonte P, Andrade F, Araujo F, Andrade C, Neves JD, Sarmento B. Chitosan-coated solid lipid nanoparticles for insulin delivery. Methods in enzydomlogy 2012; 508: 295-314.
[Crossref] [Google Scholar] [PubMed]
- Bernkop-Schnurch A. The use of inhibitory agents to overcome the enzymatic barrier to perorally administered therapeutic peptide and proteins. J Control Release 1998; 52: 1-16.
[Crossref] [Google Scholar] [PubMed]
- Schilling RJ, Mitra AK. Degradation of insulin by trypsin and alpha-chymotrypsin. Pharm Res 1991; 8: 721-727.
[Crossref] [Google Scholar] [PubMed]
- Chang L, Bai JPF. Evidence for the existence of insulin-degrading enzyme on the brush- border membranes of rat enterocytes. Pharmaceutical research 1996; 13: 801-803.
[Crossref] [Google Scholar] [PubMed]
- Brogden RN, Hell RC. Human insulin: A review of its biological activity, pharmacokinetics and therapeutic use. Drug evaluation 1987; 34: 350-371.
[Crossref] [Google Scholar] [PubMed]
- Binder C, Lauritzen T, Faber O, Pramming S. Insulin pharmacokinetics. Diabetes care 1984; 7: 188-199.
[Crossref] [Google Scholar] [PubMed]
- Evans DF, Pye G, Bramley R, Clark AG, Dyson TJ, Hardcastle JD. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut 1988; 29: 1035-1041.
[Crossref] [Google Scholar] [PubMed]
- Cone RA. Barrier properties of mucus. Adv Drug Deliv Rev 2009; 61: 75-85.
[Crossref] [Google Scholar] [PubMed]
- Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol 2001; 280: G922-G929.
[Crossref] [Google Scholar] [PubMed]
- Balasubramanian J, Narayanan N, Mohan V, Anjana RM, Bindu MS. Nanotechnology based oral delivery of insulin-A retrospect. Int J Pharm Analy Res 2013; 2: 144-150.
- Park JW, Kim SK, Al-Hilal TA, Jeon OC, Moon HT, Byun Y. Strategies for oral delivery of macromolecule drugs. Biotechnology and bioprocess engineering 2010; 15: 66-75.
- Ramesan RM, Sharma CP. Challenges and advances in nanoparticle-based oral insulin delivery. Expert Rev Med Devices 2009; 6: 665-676.
[Crossref] [Google Scholar] [PubMed]
- Mahato RI, Narang AS, Thoma L, Miller DD. Emerging trends in oral delivery of peptide and protein drugs. Critical Rev Therapeutic Drug Carrier Syst 2003; 20: 153-214.
[Crossref] [Google Scholar] [PubMed]
- Allemann E, Leroux J, Gurny R. Polymeric nano- and microparticles for the oral delivery of peptides and peptidomimetics. Adv Drug Deliv Rev 1998; 34: 171-189.
[Crossref] [Google Scholar] [PubMed]
- Varum FJ, McConnell EL, Sousa JJ, Veiga F, Basit AW. Mucoadhesion and the gastrointestinal tract. Crit Rev Ther Drug Carrier Syst 2008; 25: 207-258.
[Crossref] [Google Scholar] [PubMed]
- Plapied L, Duhem N, Rieux AD, Preat V. Fate of polymeric nanocarriers for oral drug delivery. Current opinion in colloid & interface science 2011; 16: 228-237.
- Rekha MR, Sharma CP. Oral delivery of therapeutic protein/peptide for diabetes-future perspectives. Int J Pharm 2013; 440: 48-62.
[Crossref] [Google Scholar] [PubMed]
- Still JG. Development of oral insulin: Progress and current status. Diabetes metab res rev 2002; 18: S29-S37.
[Crossref] [Google Scholar] [PubMed]
- Pridgen EM, Alexis F, Farokhzad OC. Polymeric nanoparticle technologies for oral drug delivery. Clin Gastroenterol Hepatol 2014; 12: 1605-1610.
[Crossref] [Google Scholar] [PubMed]
- Chen M, Sonaje K, Chen K, Sung H. A review of the prospects for polymeric nanoparticle platform in oral insulin delivery. Biomaterials 2011; 32: 9826-9838.
[Crossref] [Google Scholar] [PubMed]
- Bhattacharyya A, Mukherjee D, Mishra R, Kundu PP. Development of pH sensitive polyurethane-alginate nanoparticles for safe and efficient oral insulin delivery in animal model. RSC advances 2016; 1-15.
- Silva ELV, Oliveira ACJ, Silva-Filho EC, Ribeiro AJ, Veiga F, Soares FLR. Nanostructured polymeric system based of cashew gum for oral administration of insulin. Ravista Material 2019; 24.
- Pramila V, Sanjay M, Sweta K, Abhilasha L. L-valin combined PLGA nanoparticles for oral insulin delivery. J Drug Deliv Ther 2018; 8: 93-101. [Crossref]
- Lal S, Perwez A, Rizvi MA, Datta M. Design and development of a biocompatible montmorillonite PLGA nanocomposites to evaluate in vitro oral delivery of insulin. Applied clay science 2017; 1-11.
- Dong Y, Feng S. Poly (D, L-lactide-co-glycolide) / montmorillonite nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005; 26: 6068-6076.
[Crossref] [Google Scholar] [PubMed]
- Feng S, Mei L, Anitha P, Gan CW, Zhou W. Poly(lactic)-vitamin E derivative / montmorillonite nanoparticle formulations for the oral delivery of docetaxel. Biomaterials. 2009; 30: 3297-3306.
[Crossref] [Google Scholar] [PubMed]