Review Article - Biomedical Research (2017) Volume 28, Issue 3
Pivotal role of filler/matrix interface in dental composites: Review
Parisa Amdjadi1, Amir Ghasemi2, Farhood Najafi3 and Hanieh Nojehdehian1*1Department of Dental materials, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2Department of Restorative Dentistry, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran
3Department of Resin and Additives, Institute for Color Science and Technology, Tehran, Iran
- *Corresponding Author:
- Hanieh Nojehdehian
Department of Dental materials
School of Dentistry
Shahid Beheshti University of Medical Sciences, Iran
Accepted on July 12, 2016
Abstract
Dental material research has improved the application and performance of the polymer-based restorative dental composite materials. In spite of these advances, some deficiencies exist in their behaviour. Long term performance of composites is vulnerable due to its polymerization shrinkage and associated shrinkage stresses and also degradation of the filler/matrix interface. Development of low shrinkage resin systems that hinder the volumetric shrinkage and enhancing the quality of the filler/matrix interface has been the ultimate destination for the recent studies. A wide variety of coupling agent materials and techniques are currently available for surface treatment of inorganic fillers in composite materials, however there are still some concerns related to the best coupling agent at the interface that guarantee long term stability and crack resistance of these restorations. The surface modification of the inorganic part of a composite filling material possess a strong potential more than just a bond between the two different phases and can therefore be considered as a medium to compensate the deficits of dental composites. The objective of this article is to review available literatures regarding the improvements made in surface treatments of composite filler particles. Articles that were recently published on this subject focused on the polymer-grafted particles that can be designed with the preferred properties through an appropriate selection of grafting monomers and condition. Application of long chain hydrophobic polymers at the particle surface of composite materials, not only improve hydrolytic stability and uniform dispersion of fillers, but also reduce the polymerization shrinkage and associated stresses which would be very advantageous for restorative dental composites.
Keywords
Dental composite, Coupling agents, Hydrolytic stability, Filler dispersion, Polymerization shrinkage.
Introduction
The three major components of polymeric dental restorative composites are: 1) the matrix or continuous phase, 2) filler or the disperse phase and 3) Interphase or the coupling phase.
The continuous phase is a cross-linked matrix which is derived from the monomer system; and in most dental composites consists of a blend of various dimethacrylate monomers such as Bisphenol-A-Glycidyl Methacrylate (BIS-GMA) and Triethylene Glycol-Dimethacrylate (TEGDMA).
The dispersed inorganic filler particles are employed-to enhance the modulus, reinforce softer polymer phase and decrease polymerization shrinkage; the fillers usually include glass or ceramic particles of different sizes, compositions and size distribution. These particles are also appropriate material as vehicles for incorporating other components such as coupling agents [1-3].
Surface modification of inorganic nanoparticles has attracted great attention because it produces an excellent integration, improves the hydrophobic characteristics and prevents agglomeration resulting in a perfect homogenous distribution which is very important in the final material properties.
Nowadays, nanoparticles reflect the growing interest in the field of dental Composites as a result of enhanced wear resistance, aesthetic and polishing properties. The filler/matrix interface is a highly determinant factor in nano composites; this is because a higher surface area to volume ratio derived from the non-agglomerated nanoparticles will allow the incorporation of a higher percentage of coupling agents compared to composites with micrometre-sized fillers [4-6].
Surface Modification of Inorganic Fillers
Several physical and chemical approaches have been suggested to modify the filler surface for an optimal compatibility between two different phases. Physical surface modification methods include the use of ultraviolet laser and flame oxidation; these methods have limited application in the field of dental composites. Chemical modification techniques include those that involve chemical reaction that alter the surface of the particle. Chemical modifications are achieved by reaction with small molecules, such as silane coupling agents, or grafting polymeric chain or brushes through covalent bonding to the hydroxyl groups existing on the particles [7,8]. A proper surface modification of fillers actually alters its chemical structure, wetting ability and its optimal activity.
Even though the percentage of the coupling agent in the whole system is not very significant, the level of interaction at filler/ matrix interface influences the material performance and long term stability, especially for dental composites which are subjected to transient thermal and mechanical stresses [1,3,9-11]. An appropriate stress transfer from the matrix to the particle through a suitable filler/matrix coupling will establish optimum filler reinforcement. Hence, fracture toughness is also related to the quality of the interface. An appropriate coupling agent improves the compatibility of two different phases and interpenetration of coupling agent and matrix lead to their mixing on a molecular scale which can prevent leaching by limiting the penetration of water along the interfaces and decrease the hydrolytic degradation of composite material. The matrix degree of conversion, dispersion of nanoparticles, and amount of filler loading and the final mechanical properties of composite materials are all influenced by the quality of the interface; therefore, improving the interface properties in other to enhance the durability of the dental composite is an indispensable concern [12,13].
Chemical Surface Treatments
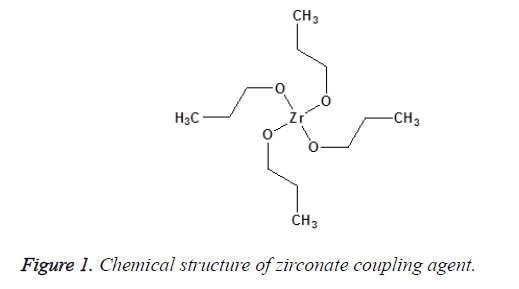
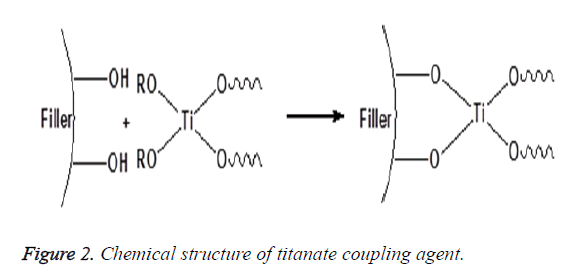
A chemical substance that is applied to inorganic filler and chemically binds it to the resin matrix is called a coupling agent. The amount of the coupling agent used the method of application and the chemical properties of the other two phases determine the performance of the interphase layer [14,15]. There are different coupling agents such as zirconate, titanate and silane that are used to improve the filler particles/polymer matrix interactions and to promote interfacial adhesion between these two phases. Zirconates are usually more reactive than silanes; hence, it is used particularly on surfaces that have no reactive hydroxyl groups as shown in Figure 1. Acidic zirconate has the capability to crosslink with polymer matrix by establishing donor–acceptor interactions and can also form a hydrogen bonding with the ceramic particles to promote shear bond strength (adhesion) and hydrolytic stability of the interface and so strengthens the polymeric composite material. The main advantage of the zirconate is great stability. The application of a zirconate coupling agent can establish a successful adhesion of the composite resin-to-zirconia surfaces [16-18]. Titanate coupling agents are known to chemically bind inorganic filler and organic polymer via proton co-ordination on non-silane reactive substrates without water for condensation and less void formation around the particles [19] as shown in Figure 2. Nanoparticles of TiO2 are modified with titanate coupling agent using ultrasonic wet method, decrease aggregation because of steric hindrance and improve the dispersion of TiO2 in the polymeric matrix [16,20].
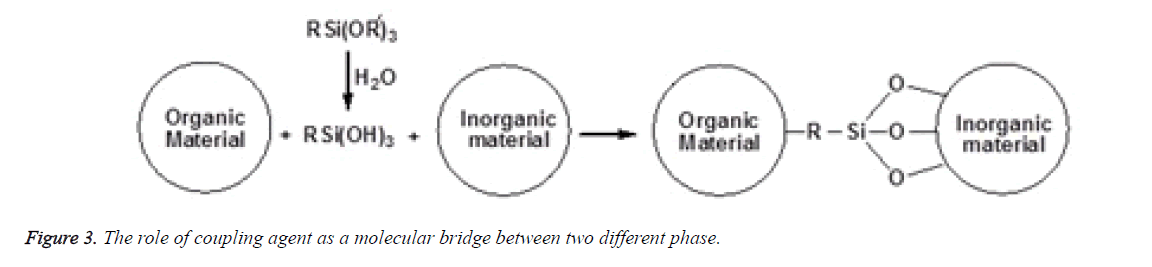
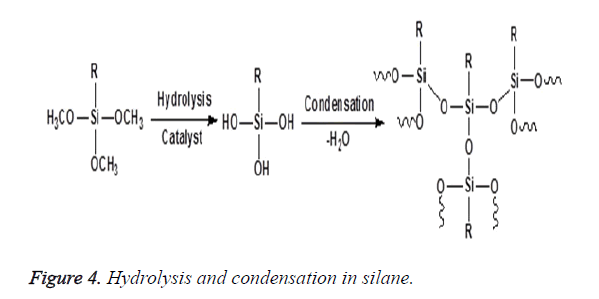
The concept of silane coupling agents was first reported by Plueddemann and its application has significantly increased to a large extent among the composite interface studies. Organofunctional silanes are widely used as coupling agents in the field of dental composites. The special property possessed by these coupling agents is due to the combination of the organic and inorganic functionality in a single molecule and as a result they can serve as molecular bridges between two different phases as shown in Figure 3. In the presence of moisture, the alkoxy groups of silanes is hydrolysed to form reactive silanols which either react with themselves to form oligomeric siloxanes or react with the hydroxyl group present at the mineral filler surface through a condensation reaction as shown in Figure 4. The most typical of these silanes are the methoxy and ethoxy substituents that improve adhesion and integrity on the surface of fillers [21,22]. The most prevalent silane used in dental composites is 3- Methacryloxypropyltrimethoxysilane (MPTS) that chemically binds to the hydroxyl group on the filler surface and other silane molecules via the methoxy group and also copolymerizes with the methacrylic polymer matrix by the methacryloxy functional groups [11,23,24].
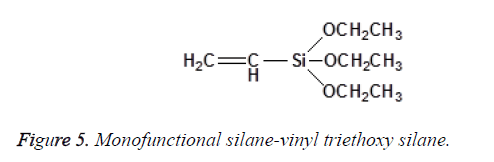
The chemical structure and degree of functionality of silane affect sorption behaviour and interface adhesion properties. Mono-functional Silanes possess one silicon (Si) atom with three alkoxy groups. Vinyltriethoxysilane is an example of a mono-functional silane as shown in Figure 5.
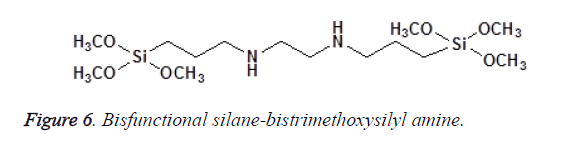
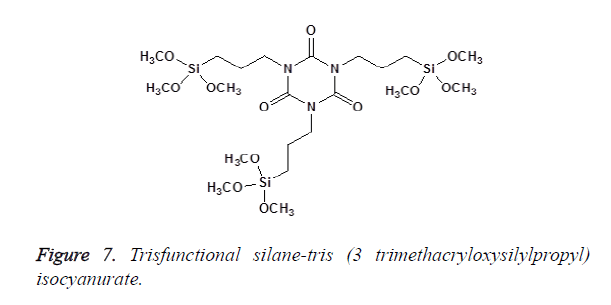
In Bis-functional silanes, there are two Si atoms, each with three alkoxy groups; an example is the bis (3-Trimethoxysilyl) propyletylenediamine as shown in Figure 6. Tris-functional silanes have three Si atoms (each attached to three alkoxy groups), e.g., tris (3-trimethoxysilylpropyl)-isocyanurate as shown in Figure 7 [11].
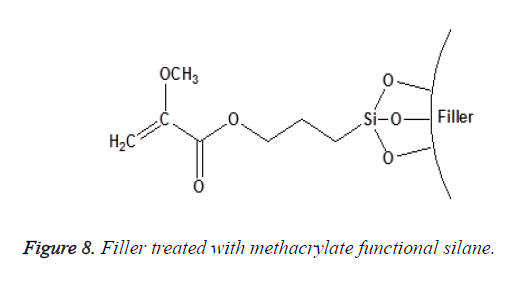
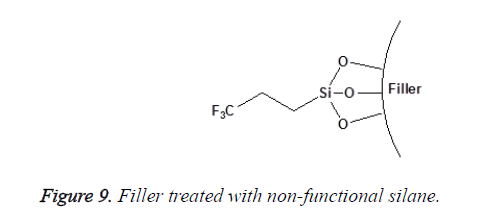
Non-functional silanes contain only Reactive Alkoxy (OR) functional groups that react with the surface hydroxyl groups of inorganic substrates after the hydrolysis of their silanol group [25]. This group of silanes (such as the aryl silanes) is not considered as coupling agents. They are only used to alter surface energy or wetting characteristics of the substrate as shown in Figures 8 and 9. The degree of functionality of silane also affects its reactivity toward water and their subsequent hydrolytic degradation; this will be discussed in detail later.
Substantial Role of Interface Layer in Composite System
Hydrolytic stability
A close investigation of the fatigue and crack growth behaviour of commercial micro hybrid (Z250) and nano filled (supreme plus) restorative composites under wet conditions revealed the evidence of interfacial deboning between the filler and matrix [26]. The filler-matrix interface seems to be the weakest part of the composite material and this is mainly due to the interaction of water at the interface and the hydrolytic degradation of silane coupling agent which accelerates filler deplugging and surface roughness. To overcome this problem a coupling agent with durable bonding and enhanced hydrolytic stability should be used at the interface [3,21,27,28].
The chemical structure of a coupling agent affects water sorption behaviour. MPTS is known to establish sufficient adhesion, but its hydrolytic stability is a great concern [29]. The production and concentration of OH-ions during hydrolytic degradation of siloxane layer leads to an autocatalytic reaction which can result to the following; degradation of the interface and subsequent leaching of ingredients, micro cracks at the interface, particle deboning and reduce fatigue resistance and final mechanical properties [15,28,30-32]. Salivary esterases and organic solvents existing in the oral cavity may cause more destructive effects than water, especially under cyclic loading [33,34]. Several studies have been conducted to verify the efficacy of a variety of functional groups and pre-hydrolysed silanes on adhesion at the interface for optimal performance. Some studies support the idea of using silanes with more reactive functional groups such as isocyanate (3- methacryloxypropyltriisocyanatosilane) and Mercapto functionalized silanes that improve crosslinking in the siloxane layer and show higher adhesion when compared to MPTS [35,36]. Nishiyama, Karabela and Sideridou also demonstrated that the presence of two functional groups (Urethanedimethacrylates) or even polyfunctional silanes promote the formation of a cross linked stable multisilane layer on the silica surface with higher hydrolytic stability at the interface between silica and polymeric resin. [29,30,37].
A great number of studies considered hydrophobic functional groups (e.g. vinyl, phenyl), as suitable resolution to improve the hydrolytic stability of silanes [38]. For instance, the hydrophobic phenyl group in 3-(3-methoxy-4- methacryloyloxyphenyl) Propyltrimethoxysilane (p-MBS) improved wear resistance because of the highly hydrophobic layer on the filler surface and also excellent affinity for the resin matrix. Hydrophobic 3-Methacryloxydecyl trimethoxysilane also improves interface durability [23,39]. Hydrophobic chlorosilane and fluoro alkylsilane also increase crosslinking and hydrolytic stability at the interface but the absence of a reactive double bond to react with the monomer limits their application [38].
In other studies, silanes with different polarity, flexibility, and reactivity were mixed and applied as a coupling agent to express the beneficial properties of each individual silane. The results indicated improved handling characteristics next to maintaining or promoting the mechanical properties of the cured composites. For example a mixture of non-functional fluorinated silane and MPTS exhibited an improved hydrolytic stability and increased tensile strength of the composites. Craig demonstrated that a combination of bis-trimethoxysilylethane with the highly hydrophobic fluorinated silane further increases its hydrolytic stability because of a greater degree of crosslinking in the silane interface. Research also demonstrated that a combination of MPTS and aromatic phenyl or vinyl triethoxysilane has the potential of a hydrophobic, matrix-resin compatible coupling agent [38,40-42]. The following were reported from studies (i) a mixture of aminoalkyl trialkoxysilane and 3-mercaptopropryltrimethoxysilane with MPTS improved silanization and high adhesion strength [43]; (ii) the combination of MPS with styryl functionalized silane, resulted higher modulus when compared with MPS treated control group [41]. (iii) The combination of a functional silane with a cross-linker silane like triethoxysilylethane or trichloroxysilylethane improved coupling agent durability and hydrolytic stability of the interfacial siloxane layer. The role of this cross-linker silane is to connect the silane molecules more extensively by forming three dimensional siloxane networks and interconnecting with the functional silanes. The penetration of water molecules into the inner interfacial layer becomes more and more difficult, as the degree of cross-linking siloxane network increases [44]. Lung and Matinlinna concluded that the present silane coupling agents are not ideal and do not accomplish the optimum requirements, so developments of novel surface conditioning methods and coupling agents are required to address the problem of bond durability [25]. Recently design of novel polymers with long hydrocarbon chain proved to be much more hydrophobic than those with short chain, and consequently was able to solve the quest for bond durability.
Filler dispersion
The dispersion of fillers in the polymeric matrix is one of the most important factors affecting the compatibility between the particles and the surrounding polymer. The presence of large agglomerates in the composite material is a drawback and the fracture resistance of composites containing aggregated particles drastically decreases with increasing number of aggregates [45,46]. The dispersion of nano sized inorganic particles in a polymer matrix, results in molecular level mixing between the polymeric chains and the particles. Furthermore, interfacial coupling of the nanoparticles through modification of the particle surface with chemical reactive groups reinforces the polymeric matrix and improves the mechanical properties of the composite material [47,48].
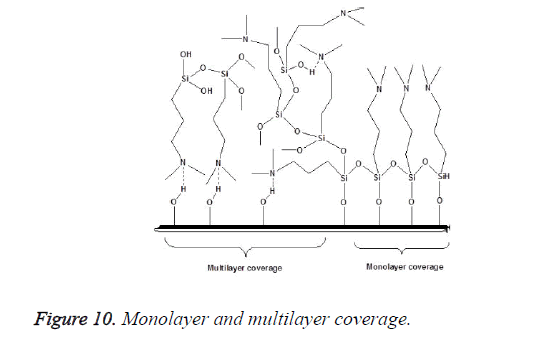
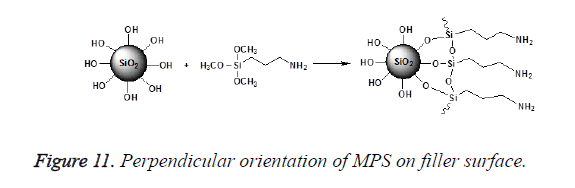
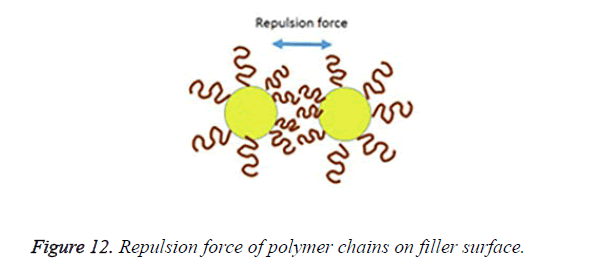
An incomplete filler surface coverage by the coupling agent may give rise to filler aggregation, insufficient bonding, increased viscosity and inferior mechanical properties of the resultant material, while an excess amount of coupling agent can also lead to deterioration of mechanical properties and should be avoided. To be effective the degree of silanization and silane layer orientation must be optimal [21]. Multilayer orientation of silane at the interface leads to immobilization of the suppressed functional groups and inefficient coupling between the two phases, since loosely adsorbed silane do not contribute to the bonding, excess amount of the coupling agent should therefore be removed to achieve an ideal monolayer coverage as shown in Figure 10 [14,49,50]. Furthermore due to the geometry, perpendicular orientation of silane molecules as shown in Figure 11 on the filler surface is thought to occupy less surface area, making it possible for more silane molecules to react with the filler surface [15,51]. Silane concentration determines its orientation; at lower silane concentration the silane molecule tends to form parallel orientation relative to the silica surface while at higher concentrations, they form random, parallel and perpendicular orientation [51]. Ranade demonstrated that the maximum coverage of particles do not imply the highest flexural strength; other factors like the uniform dispersion of particle which is achieved as a balance of attraction and repulsion forces between particles and particle-matrix are very important for idealized surface chemistry as shown in Figure 12 [52]. The uniform coverage of particles plays a more significant role and lead to an enhanced degree of spatial ordering which in turn affect dispersion of fillers in the polymeric matrix.
Compressive testing demonstrated improved mechanical strength in these ordered materials [41,53]. Allyltriethoxysilane treatment of titanium dioxide nanoparticles in a commercial composite (Z100) (3M ESPE Dental, united states) significantly improved dispersion of fillers, hardness and flexural strength [54]. Examples of non-functional silane showed large filler agglomerates in the resin matrix, while specimens of functional silane, and untreated fillers showed fewer agglomerates; also it is shown that composites with functional silane treated filler produced significantly less wear compared to non-functional and untreated filler [55]. Willson et al. also reported a more uniform dispersion of MPTS treated particles compared to the n-octyltrimethoxy silane which is a non-functional silane [42,56]. Hence it was resolved that the functional group acts as a stabilizer that prevents agglomeration and precipitation of filler particles.
Non-functional surface-modifier silanes are used as a hydrophilic compatibilizer rather than as a covalent adhesion promoter, that help to control rheological properties and reduce viscosity, thus allowing higher filler loading. Variations in organo-functional and hydrolysable groups also affect the quality of dispersion. The silane contained methoxy rather than ethoxy and methacrylate rather than acrylate organic functional groups have more influence on filler uniform dispersion [57]. Grafting of polymer chains to particle surface provide steric repulsion that helps disperse the particles, countering their tendency to aggregate due to van der Waals attraction. Masaki studied the influence of Polypropylene (PP) grafted to nanoparticles of silicon dioxide (SiO2) on the mechanical properties of Nano composites and showed that the grafted chains improved the dispersion of SiO2, and also the Young’s modulus and tensile strength because of the effective load transfer to SiO2 through the co-crystallization-mediated and direct bridging. The grafted PP chains also decreased the viscosity of the material [58]. The proper selection of grafted moiety and integration protocol improved the dispersion of particles and these composites improved in terms of storage modulus, tensile strength, and toughness which enhance the composite properties, leading to improvements that can be considered as novel achievement in the field of composites [59,60].
In a study, selected graft copolymers were examined on the effect of dispersing different concentrations of hybrid nanoparticles in a polymeric matrix. The result revealed that the modulus of the composite structure progressively increased as the hybrid grafted particle became uniformly dispersed in the matrix [61]. Ranade et al. also reported an ultra-high-molecular- weight polyethylene interface that improve particle dispersion and composite toughness [52]. The colloidal silica was modified with 3-aminopropyltrimethoxysilane (APTES), and then further treated with polyethylene glycol methacrylate based oligomer to enhance interfacial interaction and compatibility between silica and Poly Urethane Acrylate (PUA) matrix. The SEM showed that small PU-silica clusters were homogeneously dispersed in the PUA matrix; thus enhancing the Young modulus and tensile strength of the PUA Nano composites [62]. The quality of the interface and its effect on nanoparticle dispersion can also modify the matrix morphology which in turn can alter the deformation and failure of the matrix material.
Matrix degree of conversion
Studies on the effect of silane content on the conversion of resin matrix revealed that increasing the content of silane resulted in reduced conversion and for both functionalized and non-functionalized silanes; most of the bonds remain unreacted. The reason is related to the formation of a condensed interface at high concentration of silane which limits the mobility and penetration of methacrylate resin to the interphase. On the other hand, the concentration of reactive vinyl group (like MPTS) caused gelation at a lower degree of conversion and so, decreased the cross linking density and as a result impaired the mechanical properties. Therefore, non-reactive silanes with increased mobility are known to improve the degree of conversion [49]. Other studies support the idea that reactive functional groups are needed for free radical polymerization in the composite interphase, even though high concentration of those groups are not necessary [42]. An experimental composite material with polymer grafted nanoparticles demonstrated increased degree of polymerization and fracture toughness as a consequence of the existence of entanglements between surface-grafted chains that give rise to energy dissipation and delayed network gelation [63]. Ferdous demonstrated that the enhancement in mechanical properties of nano composites is largely dependent on the level of increase in overall matrix conversion, regardless of the condition of the interface [13]. It can be concluded that there is an indirect correlation between the coupling agent structure and overall mechanical properties of composite materials.
Polymerization shrinkage and contraction stresses
Polymerization stresses due to contraction in constrained conditions have been a determined problem in dental polymeric composites since their introduction and reduction of the shrinkage and associate stress has been a major problem in dental technology. The addition of high modulus fillers that are covalently bonded to the polymer matrix leads to additional contraction of the resin system [21,64-66].
Studies conducted with untreated fillers and fillers treated with non-functional silanes, that do not form stable bonds with the matrix phase, showed reduced stress development as compared with those that have covalent attachment with the resin.
The surfaces of such particles can serve as defect sites where localized free shrinkage can take place, consequently, the overall volumetric shrinkage and stress development is diminished. Since the interface between the filler and matrix phase have a potential in controlling the stress development in the composite, new strategies should include consideration of the resin-filler interface to improve material performance [67]. In the case of nano composites, the coupling phase plays an even more important role on the composite properties, since the nanoparticle fillers have an extremely high surface area-to-volume ratio and require a higher degree of surface treatment than the larger particulate fillers. Nano-composites with functionally silanized nanofiller particles showed a higher level of polymerization stress that can lead to marginal staining, caries, and sensitivity. Following this shortcoming, the idea of using non-functional silanes was developed. It has been shown that dental composites which include nano filler particles that are not treated with a functional agent to couple them to the resin matrix can result in lower stress levels. Silanized aerosol-type silicone dioxide with a non-functionalized silane has been formulated to include a non-bonded surface in the composite [68]. Other methods also suggest that increasing the strength of the bond between the filler and matrix will not result in any improvements in the mechanical properties of particulate-reinforced composites in contrast to fiber-reinforced composites [69].
The interface between a solid surface and a cross-linked polymer can be strengthened by the addition of polymer brushes that can extend into the adjacent polymer layer. The properties of these brushes can be controlled to tailor shrinkage and stress in the polymeric network [70-72]. In the experimental model inclusion of Ultrahigh Molecular Weight Polyethylene (UHMWPE) fibers, allowing plastic deformation during stress development; and contraction stress in composites was reduced through stress relief [73]. In another research, flexible 16-functional alkene-terminated hyper branched oligomer covered 93% of the silica nanoparticle surface and the composite system with these functionalized silica nanoparticles demonstrated 30% reduction of shrinkage stress without decreasing the modulus. Furthermore, studies showed that the shrinkage stress of the composite system builds up at much later stages of the polymerization, as compared to the control system [74]. In a particular study, oligomers with different chain lengths and different levels of surface reactivity were applied to modify the surface of nano fillers. The result of the experimental composites showed that shrinkage stress reduced by as much as 30% compared with the traditionally used γ-MPS-based control, whilst preserving similar final conversion and modulus behaviour. The result also revealed that the higher the molecular weight of the brush, the lower the shrinkage stress, and the higher the reactive group concentration on the coupling agent backbone, had the effect of increasing the stress and stress transfer between the two constituent phases of the composites. All mechanisms of stress reduction were related to delayed network build-up due to a slow reaction at the interface. Hence, surface modification procedure can also affect other properties such as dynamic modulus development and fracture toughness [75].
In general, surface modification of the inorganic part of the composite filling materials possess a strong potential to improve dental composites in terms of the critical property of reducing the polymerization contraction and related stresses [76].
Polymer Grafted Particles
Grafting of synthetic polymers is a state-of-the-art approach which is used to modify the surfaces of inorganic particles, in other to enhance chemical functionality and alter the surface features of the original material. Such polymer grafted inorganic nanoparticles are considered to become more hydrophobic; show improved uniform dispersion and decreases polymerization shrinkage and stresses in the composite system. Although this is not a new technique in industrial composite technology, its application in the field of dental restorative composites is quite innovative.
Numerous studies have shown that particles with high grafting density of polymeric chains are compatible with matrix chains of the same architecture, as long as the polymeric matrix chains have lower molecular weight than the polymeric brush. A variety of strategies have been used to control particle dispersion and their three-dimensional arrangement by varying either the grafting density, grafted chain length or the matrix chain. The balance of energy in grafted particles results to an ordered super structure with improved mechanical properties [77].
On the other hand, composites containing particles grafted with chains similar to those comprising the polymer matrix, exhibit considerably higher moduli than composites containing bare particles.
This effect is known to arise from the additional distortion of the shear field in the polymer matrix resulting from grafted chains and from the slower relaxation time of the grafted chains, as compared to the matrix chains. Polymer chains can be covalently grafted on the surface of inorganic particles.
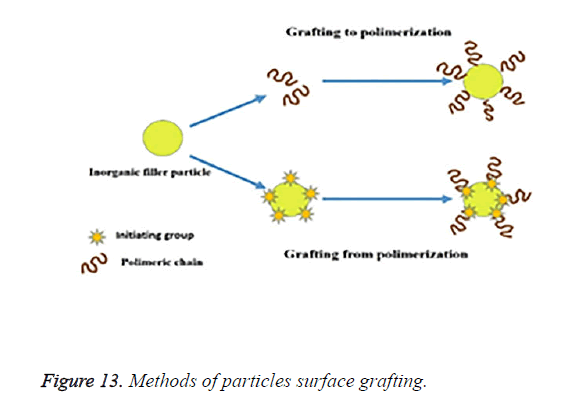
One approach to this is the “grafting-to” method, in which the end-functionalized polymers react with an appropriate and suitable surface. Although the grafting-to- method has the advantage of a simple and modular approach, steric repulsion between the already attached polymer chains and a chain diffusing to the surface; limits the available graft density. The other method is the “grafting-from” method in which polymer chains are grown from an initiator-terminated self-assembled monolayer. The diffusion of a relatively small monomer to the surface does not suffer from the same steric repulsion as a diffusing polymer chain. The schematic representations of the “grafting-to” and “grafting-from” methods are shown in Figure 13.
A broad range of polymer chains with different length and structures have been investigated for surface modification of a variety of inorganic filler particles in composite materials. For example Polystyrene and polyacrylamide grafting on nanosized alumina particles showed remarkable improvement in the dispersibility of the nanoparticles [78]. Polystyrene grafted silica nanoparticles also showed an enhanced uniform dispersion of particles [79]. The grafting of poly (methyl methacrylate) on the surface of TiO2 particles, grafting of hyper branched polymers with pendant azo groups on silica nanoparticle, radical graft polymerization of vinyl monomers on the surface of polymethylsiloxane-coated titanium dioxide nanoparticles and grafting of polymethacrylic acid chains on the surfaces of ZnO nanoparticles to create better dispersion are examples of polymeric interfaces [80-86].
Montmorillonite is a very soft phyllosilicate group of minerals that typically form microscopic crystals, known as clay. Poly (acrylic acid) grafted montmorillonite is a novel filler for dental adhesives providing higher shear bond strength and dispersion of modified nanoparticles [87].
PMMA-grafted nano clay filler also enhance the flexural strength of fiber-reinforced dental composites. Grafted polymeric chains on the nano clay particles facilitate their distribution in the resin phase by making them polarized. This uniform dispersion in “Nano” scale prevents crack propagation resulting in a significant improvement in flexural strength properties [88].
Another particle that has been used as fillers in polymer-based materials is hydroxyapatite. It is used because of its chemical and crystallographic similarity to the composition of human teeth and bones. Bioactive dental resin composite containing poly (Bis-GMA) grafted hydroxyapatite whiskers and silica nanoparticles showed significantly improved mechanical properties and desirable bioactivity [77]. It is noteworthy that the appropriate selection of grafting polymer is crucial to prepare material with specifically prescribed properties.
The order of improvement of filler surface treatment is described chronologically in Table 1.
| Research | Coating | Description and results |
|---|---|---|
| Kurata et al. [35] | 3-Methacryloxy propyl triisocyanatosilane | High reactivity High adhesion High crosslinking High Hydrolytic stability |
| Nishiyama et al. [37] | Methacryloxy propyl vinyl Mercaptopryl (A silane with two silicone functional groups ) |
More stable at the interface than those with mono- or trisilicone functional groups |
| Soderholm et al. [15] | Gamma-Methacryloxypropyltrimethoxysilane (MPS) molecules | Number of available OH-on the filler surface determine the amount of silane needed for filler treatment |
| Umemoto et al. [43] | Mixed salines, 3-methacryloxypropylsilytriisocyanate and 3-mercaptopropyltrimethoxysilane | Higher adhesion |
| Craig et al. [38] | Compared fluoroalkyl-, aminoalkyl-, phenyl-, vinyl-, bis silyl ethane- and 3-methacryloxypropyltrimethoxysilane | Vinyltriethoxysilane increases the hydrolytic stability of the composite |
| Hussain et al, [89] | Titanate coupling agent | Better dispersion of filler particles and strong adhesion/bonding |
| Liu et al. [50] | MPS at concentrations of 1% and 5% | The higher efficiency of monolayer MPS compare to loosely adsorbed multi-layer silane |
| McDonough, et al. [90] | 3-Methacryloxypropyltrimethoxysilane (MPTMS) and 10-methacryloxydecyltrimethoxysilane (MDTMS) | More hydrophobic silane coupling agent MDTMS promotes resistance to degradation |
| Nihei et al. [91] | Mixture of 3-methacryloyloxypropyltrimethoxysilane and one of the following hydrophobic fluoroalkyltrimethoxysilanes: trifluoropropyl-, nonafluorohexyl-, tridecafluorooctyl-, heptadecafluorodecyl-, and henicosafluorododecyl-trimethoxysilane. | Higher tensile strength of mixture of 3-MPTMS andfluoroalkyltrimethoxysilane, |
| Yoshida et al. [92] | Pre-silanization decontamination | Decontamination of filler promote resistant to degradation |
| Halvorson et al. [49] | γ-methacryloxypropyltrimethoxysilane | Reduced conversion and impair the mobility of the reactants. |
| Debnath et al. [69] | Compare γ-methacryloxypropyltrimethoxysilane and a rubber-epoxy coating |
Increasing the strength of the bond between filler and matrix will not result in improvements in the mechanical properties of the composites |
| Wilson et al. [56] | Mixture of 3-methacryloxypropyltrimethoxysilane (MPTMS) and octyl trimethoxysilane (OTMS) |
Dual silanized nanoparticles improve workability of composite pastes with higher filler loadings that lead to higher modulus composites with lower polymerization shrinkage |
| Bose et al. [20] | Tetra isopropyl titanate (TPT) | Improved tensile strength |
| Matinila et al. [44] | 3-Methacryloyloxypropyltrimethoxysilane, N-(3-(trimethoxysilyl) propyl) ethylenediamine and (3-(triethoxysilyl) propyl) urea blended with 1,2-bis-(triethoxysilyl) ethane solutions |
Silanization with a blend of a functional silane and a cross-linker silane might improve the hydrolytic stability of a siloxane film |
| Wilson et al. [42] | Methacryloxypropyltrimethoxysilane styrylethyltrimethoxysilane |
Dual silanization improve the handling characteristics and the mechanical properties of dental restorative nano composites |
| Karabela et al. [29] | Compared 3-((1,3(2) dimethacryloyloxypropyl)-2(3)-oxycarbonylamido]propyltriethoxysilane, which is a urethane dimethacrylate silane (UDMS) with 3-methacryloxypropyltrimethoxysilane (MPS), and octyltrimethoxysilane (OTMS) | UDMS with the hydrophilic urethane group showed the highest amount of sorbed water OTMS, which does not contain a methacrylate moiety, cannot react with the dimethacrylate monomers showed the highest solubility both in water and ethanol/water, MPS-composite with the 3-methacryloxypropyltrimethoxysilane sorbed the lowest amount of water/ethanol |
| Nihei et al. [39] | The novel silane coupling agent containing hydrophobic phenyl group 3-(3-methoxy-4-methacryloyloxyphenyl) propyltrimethoxysilane (p-MBS) | p-MBS significantly lower wear compared with the composites with 3-MPTM treated fillers excellent affinity with the base resin highly hydrophobic layer on the filler surface |
| Xia, Yang et al. [54] | allyltriethoxysilane (ATES) on TiO2 nanoparticles (Z100, 3M ESPE). |
better dispersion, smaller clusters improved micro hardness and flexural strength |
| Sideridou et al. [51] | 5 different amounts of gamma-MPS (1.0, 2.5, 5.0, 7.5 and 10 %weight) relative to silica | The amount of silane affect the orientation of the silane and mechanical properties of composites |
| Sabzi et al. [93] | Aminopropyltrimethoxysilane (APS) on TiO2 nanoparticles | Improved mechanical properties of the urethane polymers |
| Li, Gui-Juan et al. [94] | Isopropyl tri (dioctyl phosphate) titanate | The surface property of TiO2 changed from hydrophilic to hydrophobic improved dispersion of filler particles |
| Hajian et al. [95] | titanate as a coupling agent on nano scale particles such as TiO2 and ZnO | The impact strength, elastic modulus and toughness of the samples were enhanced |
| Tham et al. [96] | 2% titanate on Hydroxyapatite | Higher flexural modulus and strength |
| Laleh Solhi et al. [87] | Graft polymerization of acrylic acid onto nano clay platelets to be utilized as reinforcing fillers | Modified particles fillers improve mechanical properties |
| Sheng Ye et al. [74] | Hyper branched oligomer functionalized glass filler | Reduce the shrinkage stress in UV curable resin composite without sacrificing mechanical properties |
| Mortazavi et al. [88] | PMMA-grafted nano clay filler of fiber-reinforced dental composites. | Facilitate filler distribution Enhance the flexural strength |
| Liu F et al. [77] | Poly (Bis-GMA) grafted hydroxyapatite whiskers and silica nanoparticles | Showed significantly improved mechanical properties and bioactivity |
| Shah et al. [75] | Modify the surface of nano fillers used oligomers with different chain lengths | Reduced shrinkage stress |
| Liu et al. [45] | Methyltrimethoxysilane (C1) Octyltriethoxysilane (C8) Octadecyltrimethoxysilane (C18) on aluminium oxide nanoparticles |
Multi-layers of silsesquioxane coating in the C1-coated nanoparticles and monolayer coating in C8- and C18-coated nanoparticles Improved particle dispersion in C8-coated nanoparticles The highest interfacial adhesion in C18-coated nanoparticles |
Table 1. Chronological improvement of filler surface treatment.
Conclusion
The successful application of appropriate coupling agents on the surface of filler particles can affect many physicochemical features of dental composites including hydrolytic stability, degree of conversion, filler distribution, polymerization shrinkage, and contraction stresses. The optimum condition of the features mentioned above in a single composite can lead to a promising material that reduces the occurrence of restoration fracture and secondary caries and ensures a long term service of dental restorative materials.
1-Interfaces in dental composites have a critical role in filler/ matrix adhesion. They serve as links between the resin phase and the fillers, but the utility of the interface is more than just a bond between the two phases. Surface modification can be considered as a versatile option to optimize material properties.
2-The present silane coupling agents are not hydrolytically stable and can provide only the minimum requirements of stable interface. Therefore, the development of novel surface conditioning methods and coupling agents are required to resolve bond durability problems.
3-The effect of interface quality on particle dispersion and interaction with polymeric matrix has a very determined effect on the overall mechanical properties of the composite. The fracture resistance of composites containing aggregated particles decreases with increasing number of aggregates. A proper coupling agent on the surface of particles can induce repulsive interaction that helps disperse particles which in turn improves the hardness and flexural strength of the composites.
4-Although reactive functional groups are needed for free radical polymerization in the composite interphase, high concentrations of those groups are not necessary. The presence of reactive groups at the interface, limits the mobility and penetration of methacrylate resin and as a result; the degree of conversion and final mechanical properties will be reduced.
5-Surface interactions control the stress relaxation characteristics of materials. When more reactive groups are present at the filler interface, lower stress recovery will occur due to a reduced mobility at the interfacial interactions with the bulk polymer. Stress reduction in dental composites can be enhanced by merely changing the interface between the fillers and polymer matrices.
6-Grafting of polymeric molecules to the surface of particles will provide a chance to design the fillers for desired properties through the proper selection of the grafting moiety.
The above mentioned intervention plays a pivotal role in manufacturing future dental restorative composites with increased durability and service life.
References
- Iurii Sergeevich Lipatov YSL. Polymer reinforcement. Chem Tec Publ 1995; 406.
- Nielsen MS. Interface structure and strength in model dental resin composites 2010.
- Kenneth J, Anusavice S, Rawls P. Science of Dental Materials. Elsevier (12th edn.) 2013; 592.
- Blitz, Little. Fundamental and applied aspects of chemically modified surfaces. Elsevier (1st edn.) 1999.
- Almeida GS, Poskus LT, Guimaraes JGA, Silva EM. The Effect of mouthrinses on salivary sorption, solubility and surface degradation of a nanofilled and a hybrid resin composite. Operative dentistry 2010; 35: 105-111.
- Ray SSOM. Polymer/layered silicate nano composites-A review from preparation to processing. Prog Polym Sci 2008; 28: 1539-1641.
- Jeon IY, Baek JB. Nano composites derived from polymers and inorganic nanoparticles. Materials 2010; 3: 3654.
- Sarita Kangoa SKB, Annamaria CB, James ND. Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites-A review Prog Poly Sci 2013; 38: 1232-1261.
- Mitra SB, Wu D, Holmes BN. An application of nanotechnology in advanced dental materials. J Am Dent Assoc 2003; 134: 1382-1390.
- Ferracane JL. Resin composite-state of the art. Dent Mater 2011; 27: 29-38.
- Matinlinna JP, Lassila LV, Ozcan M, Yli-Urpo A, Vallittu PK. An introduction to silanes and their clinical applications in dentistry. Int J Prosthodont 2004; 17: 155-164.
- Ferracane JL, Marker VA. Solvent degradation and reduced fracture toughness in aged composites. J Dent Res 1992; 71: 13-19.
- Ferdous SF, Sarker MF, Adnan A. Role of nanoparticle dispersion and filler-matrix interface on the matrix dominated failure of rigid C60-PE nanocomposites: A molecular dynamics simulation study. Polymer 2013; 54: 2565-2576.
- Fekete E, Pukanszky B, Toth A, Bertoti I. Surface modification and characterization of particulate mineral fillers. J Collo Interf Sci 1990; 135: 200-208.
- Soderholm KJ, Shang SW. Molecular orientation of silane at the surface of colloidal silica. J Dent Res 1993; 72: 1050-1054.
- Monte SJ. Titanate, zirconate and aluminate coupling agents 1993.
- Cheng HCK, Tsoi JKH, Zwahlen RA, Matinlinna JP. Effects of silica-coating and a zirconate coupling agent on shear bond strength of flowable resin-zirconia bonding. Int J Adhesi Adhes 2014; 50: 11-16.
- Wong JDC, Kei Lung CY, Tsoi JKH, Matinlinna JP. Effects of a zirconate coupling agent incorporated into an experimental resin composite on its compressive strength and bonding to zirconia. J Mech Behav Biomed Mat 2014; 29: 171-176.
- Kemal I, Whittle A, Burford R, Vodenitcharova T, Hoffman M. Toughening of unmodified polyvinylchloride through the addition of nano particulate calcium carbonate and titanate coupling agent. J Appl Poly Sci 2013; 127: 2339-2353.
- Bose S, Mahanwar PA. Effect of titanate coupling agent on the mechanical, thermal, dielectric, rheological, and morphological properties of filled nylon 6. J Appl Polym Sci 2006; 99: 266-272.
- Mittal KL. Silanes and other coupling agents 2009.
- Plueddemann EP. Silane coupling agents. Springer (2nd edn.) 1991.
- Antonucci JM, Dickens SH, Fowler BO, Xu HH, McDonough WG. Chemistry of Silanes: Interfaces in Dental Polymers and Composites. J Res Natl Inst Stand Technol 2005; 110: 541-558.
- Uyanik M. Synthesis and characterization of TiO2 nanostars. Saarland Uni Saarbrucken 2008.
- Lung CY, Matinlinna JP. Aspects of silane coupling agents and surface conditioning in dentistry: an overview. Dent Mater 2012; 28: 467-477.
- Shah MB1, Ferracane JL, Kruzic JJ. Mechanistic aspects of fatigue crack growth behaviour in resin based dental restorative composites. Dent Mater 2009; 25: 909-916.
- Curtis AR. The influence of nano cluster reinforcement on the mechanical properties of a resin-based composite material. Uni Birmingham 2009.
- Takeshige F, Kawakami Y, Hayashi M, Ebisu S. Fatigue behaviour of resin composites in aqueous environments. Dent Mater 2007; 23: 893-899.
- Karabela MM, Sideridou ID. Effect of the structure of silane coupling agent on sorption characteristics of solvents by dental resin-nano composites. Dental Materials. 2008; 24: 1631-1639.
- Ferracane HXB, Condon JR. In vitro aging of dental composites in water-Effect of degree of conversion, filler volume, and filler/ matrix coupling. J Biomed Mater Res 1998; 42: 465-472.
- Ortengren U, Wellendorf H, Karlsson S, Ruyter IE. Water sorption and solubility of dental composites and identification of monomers released in an aqueous environment. J Oral Rehabil 2001; 28: 1106-1115.
- Soderholm KJ, Zigan M, Ragan M, Fischlschweiger W, Bergman M. Hydrolytic degradation of dental composites. J Dent Res 1984; 63: 1248-1254.
- Bean TA, Zhuang WC, Tong PY, Eick JD, Yourtee DM. Effect of esterase on methacrylates and methacrylate polymers in an enzyme simulator for bio-durability and biocompatibility testing. J Biomed Mater Res 1994; 28: 59-63.
- Freund M, Munksgaard EC. Enzymatic degradation of BISGMA/TEGDMA-polymers causing decreased micro hardness and greater wear in vitro. Scand J Dent Res 1990; 98: 351-355.
- Kurata S, Yamazaki N. Effect of isocyanatosilane coupling agents on silica surface. Shika Zairyo Kikai 1988; 7: 729-735.
- Matinlinna JP, Lassila LV, Vallittu PK. The effect of five silane coupling agents on the bond strength of a luting cement to a silica-coated titanium. Dent Mater 2007; 23: 1173-1180.
- Nishiyama N, Ishizaki T, Horie K, Tomari M, Someya M. Novel poly functional silanes for improved hydrolytic stability at the polymer-silica interface. J Biomed Mater Res 1991; 25: 213-221.
- Craig RG, Dootz ER. Effect of mixed silanes on the hydrolytic stability of composites. J Oral Rehabil 1996; 23: 751-756.
- Nihei T, Dabanoglu A, Teranaka T, Kurata S, Ohashi K, Kondo Y. Three-body-wear resistance of the experimental compo