Research Article - Biomedical Research (2017) Volume 28, Issue 8
Periprocedural cardiac troponin elevation: a potential indicator of enhanced ventricular threshold in permanent pacemaker recipients
Fatih Mehmet Uçar*, Gökay Taylan, Mustafa Adem Yilmaztepe and Meryem AktozDepartment of Cardiology, Trakya University Hospital, Edirne, Turkey
- *Corresponding Author:
- Fatih Mehmet Ucar
Department of Cardiology
Trakya University Hospital, Turkey
Accepted date: January 20, 2017
Abstract
Background and Aim: Permanent cardiac pacing is the most efficient treatment for patients with symptomatic bradycardia and high-degree atrioventricular (AV) block. For effective pacing, sensing and pacemaker battery longevity; ventricular pacing threshold (VPT) levels, lead impedance levels and Rwave amplitude levels must be desirable. We aimed to search the association between elevated serum troponin levels and VPT values in patients who has implanted single chamber permanent pacemaker.
Materials and Methods: We retrospectively analyzed a total of 109 patients (mean age: 78 ± 7.1 years, 53.6% male) who underwent single chamber permanent pacemaker implantation for indications such high-degree atrioventricular block and sick sinus syndrome. Hematological and biochemical parameters were measured prior to pacemaker implantation. Additionally troponin I and high-sensitivity C-reactive protein (hs-CRP) were sampled about 12 hours after pacemaker implantation.
Results: Over a median follow-up period of 17.3 months, 32 (29.3%) patients has positive troponin I levels after implantation. Troponin positive group has higher VPT values at the time of implantation (0.94 ± 0.33 vs. 0.71 ± 0.19, p<0.001) and at 30th day of implantation (0.69 ± 0.16 V vs. 0.91 ± 0.31 V, p<0.001). To identify in dependent risk factors for ventricular threshold values, a multivariate linear regression model was conducted and after implantation having positive troponin value (β=0.337, p=0.01) and troponin elevation ratio (β=0.365, p<0.001) were found to be independent risk factors for ventricular threshold.
Conclusion: Our results indicate that troponin I elevation after pacemaker implantation is associated with higher VPT values at the time of and the 30th day of implantation. For longevity of pacemaker batteries, low threshold values are preferable. To further extend the life of pacemakers, we recommend following patients more closely who has high troponin levels after permanent pace replacement.
Keywords
Cardiac pacemaker, Troponin, Ventricular pacing threshold
Introduction
Permanent cardiac pacing is the most efficient treatment for patients with symptomatic bradycardia and high-degree atrioventricular block. The pacing system is comprised of the pulse generator and the lead or leads that connect the pulse generator to the heart. Sensing, lead impedance, and pacing threshold are the basic pacing parameters and should be tested during PCM implantation. Pacing threshold is defined as the lowest voltage, which can produce 5 consecutive stimuli. Several factors such as electrolyte and metabolic abnormalities, antiarrhythmic drugs may increase pacing thresholds [1].
Cardiac troponins are routinely used for showing myocardial damage [2-4]. Originally, they were only intended for use in diagnosing acute coronary syndromes; however, there are many other clinical conditions that cause damage to cardiomyocytes and raising levels of troponin. An increase in troponin levels were showed after external cardioversion, intrenal shocks with ınternal cardiac defibrillators and radio frequency ablation [5-7]. The aim of this study was to examine the association between elevated serum troponin levels and ventricular pacing threshold (VPT) levels in patients who underwent single chamber PCM implantation.
Methods
Study design and population
We retrospectively analyzed a total of 109 patients who underwent permanent pacemaker implantation at our institution between January 2013 and December 2015 with the indications including high-degree atrioventricular block and sick sinus syndrome. Demographic/clinical data, echocardiographic data, and biochemical/haematological laboratory results were collected from digital/non-digital records.
Patients who had positive troponin levels before implantation, detected any size of pericardial effusion after implantation, dual chamber pacemakers, left ventricular ejection fraction <45%, hypo or hyperthyroidism, acute coronary syndrome or history of myocardial infarction, serum creatinine levels >1.5 mg/dL, passive fixation leads and patients using antiarrhythmic pills were excluded from the study.
Information including age, gender, diabetes mellitus, hypertension, hyperlipidemia, and smoking status were gathered. Hypertension was defined as blood pressure >140/90 mmHg on >2 occasions during office measurements or use of antihypertensive treatment. Diabetes mellitus was defined as fasting blood glucose >126 mg/dl or use of antidiabetics. Hyperlipidemia was considered to be present in patients with fasting total cholesterol ≥ 200 mg/dl or triglyceride ≥ 150 mg/dl.
The protocol was approved by the Central Ethical Committee and was conducted in accordance with. The Declaration of Helsinki. Written informed consent was obtained from each participant.
Pacemaker implantation and measurements
All procedures were performed under local anaesthesia in an electrophysiology laboratory by two experienced electrophysiologists. We implanted CapSureFix novus (Medtronic, Minneapolis, MN, USA) bipolar ventricular leads in all patients. All ventricular leads were steroid-eluting with an active fixation mechanism and were implanted into the right ventricular septum via a percutaneous subclavian vein puncture. At the time of implantation VPT values, lead impedance and R-wave amplitude were assessed. Pacing threshold was defined as the lowest voltage, which can produce 5 consecutive stimuli and was measured at pulse duration of 0.5 ms. After reaching a favourable measurement, the leads were implanted in the optimal position. All patients were reevaluated one month later after implantation.
Laboratory
Following a 12 hour fasting period, blood samples were collected before procedure. Additionally troponin I and highsensitivity C-reactive protein (hs-CRP) was determined by collecting a sample about 12 hours after pacemaker implantation. Cardiac troponin I (cTnI) was analyzed from lithium-heparinised plasma with AQT90 FLEX TnI immunoassay (Radiometer Medical ApS, Denmark).The limit of detection has been determined to be 0.0095 μg/L. The reportable range of the assay is 0.010-50 μg/L. The upper 99th percentile upper reference limit (URL) has been determined to be ≤ 0.023 μg/L. Hs-CRP concentration was determined by an immune turbidimetric test (Roche Diagnostics), Fasting blood glucose was analysed using the hexokinase method. Total and differential leukocyte counts were measured by an automated haematology analyzer (Abbott Cell-Dyn 3700; Abbott Laboratory, Abbott Park, Illinois, USA) and other blood parameters were measured using standard methods.
Echocardiography
All echocardiographic examinations were performed by a certified cardiologist experienced in this field using a Vivid-7 (GE Vingmed, Horten, Norway) device. LVEF was calculated from apical 4-chamber views before pacemaker implantation, according to the modified Simpson’s rule. After implantation echocardiography was performed all patients for the presence of pericardial effusion.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation or as median with interquartile range; Continuous data are presented as means ± SD. Differences in continuous variables between groups were determined by t test. A χ2 test or Fisher’s exact test was performed to compare the categorical variables. Correlation analysis was performed using Spearman’s test. Independent associations between VPT values and independent variables included in the multivariate regression model as covariates. Coefficients of standardized β regression along with their significance from the multivariate regression analysis were also reported. All statistical analyses were performed with SPSS software version 17.0 (SPSS Inc., Chicago, IL). A p value of 0.05 was considered statistically significant.
Results
The study population consisted of 109 patients [mean age, 78 ± 7,1 years; 59 males (53.6%)], of which 77 were in negative troponin levels after implantation [(mean age, 78,0 ± 7.3 years; 39 males (50.6%)] and 32 were in positive troponin levels [mean age, 80.7 ± 6.1 years; 20 males (62.5%)]. The baseline demographic and clinical characteristics, left ventricular ejection fraction and medications of the two groups were similar (Table 1).
| Troponin (-) (n=77) | Troponin (+) (n=32) | p | |
|---|---|---|---|
| Male (%,n) | 50.6% (39) | 60.6% (20) | 0.25 |
| Age | 78.0 ± 7.3 | 80.8 ± 6.8 | 0.12 |
| HT (%,n) | 70.1% (54) | 68.7% (22) | 0.88 |
| DM (%,n) | 20.7% (16) | 15.6% (5) | 0.53 |
| Hyperlipidemia (%,n) | 27.2% (21) | 37.5% (12) | 0.29 |
| History of CAD (%,n) | 24.6% (19) | 18.7% (6) | 0.50 |
| Atrial Fibrillation (%,n) | 17.9% (14) | 28.1% (9) | 0.26 |
| Ejection fraction (%) | 58.83 ± 4.6 | 58.0 ± 4.5 | 0.37 |
| Systolic Blood Pressure- Pre implantation (mmhg) | 151 ± 10 | 152 ± 8 | 0.90 |
| Systolic Blood Pressure- Post implantation (mmhg) | 130 ± 8 | 132 ± 5 | 0.46 |
| Medications ACE/ARB (%,n) |
53.2% (41) | 56.2% (18) | 0.77 |
| Beta-bloker (%,n) | 23.3% (18) | 40.6% (13) | 0.07 |
| Diüretik (%,n) | 12.9% (10) | 9.3% (3) | 0.59 |
| Statin (%,n) | 25.9% (20) | 31.2% (10) | 0.57 |
| Acetylsalicylic acid (%,n) | 44.1% (34) | 40.6% (13) | 0.73 |
Table 1. Baseline demographic and clinical characteristics of the patients.
Laboratory measurements were also similar in both groups, except that hs-CRP elevation ratio (after implantation/before implantation) levels were higher in troponin positive group (3.17 ± 2.98 vs. 6.01 ± 5.39, p=0.01) (Table 2).
| Troponin (-) (n=77) | Troponin (+) (n=32) | p | |
|---|---|---|---|
| Glucose (mg/dL) | 107.47 ± 37 | 105.1 ± 27.06 | 0.99 |
| Creatinine (mg/dL) | 0.99 ± 0.26 | 0.98 ± 0.31 | 0.73 |
| Na (mEq/L) | 138.9 ± 4.15 | 138.8 ± 3.8 | 0.73 |
| K (mEq/L) | 4.2 ± 0.5 | 4.4 ± 0.9 | 0.13 |
| AST (mg/dL) | 22 ± 8.8 | 25.2 ± 7.0 | 0.06 |
| ALT (mg/dL) | 16.4 ± 10.2 | 20.1 ± 10.6 | 0.06 |
| LDL (mg/dL) | 99.2 ± 32.3 | 105 ± 36.4 | 0.42 |
| HDL (mg/dL) | 44.6 ± 10.6 | 45.7 ± 15.3 | 0.69 |
| TSH (ng/dl) | 2.4 ± 4.1 | 2.0 ± 1.6 | 0.73 |
| WBC, × 109/L | 7.5 ± 2.2 | 9.5 ± 10.08 | 0.45 |
| Neutrophil, × 109/L | 4.7 ± 1.8 | 5.2 ± 2.5 | 0.4 |
| Lymphocytes, × 109/L | 1,7 ± 0,7 | 1,8 ± 1,4 | 0.92 |
| Platelet, × 103/L | 216.5 ± 61.6 | 197.5 ± 58.6 | 0.14 |
| Pre implantation hs-CRP (mg/dL) | 1.28 ± 1.60 | 1.32 ± 1.54 | 0.73 |
| Post implantation hs-CRP (mg/dL) | 2.70 ± 2.65 | 4.12 ± 3.13 | 0.06 |
| hs-CRP elevation ratio (after implantation/before implantation) | 3.17 ± 2.98 | 6.01 ± 5.39 | 0.01 |
Table 2. Baseline laboratory measurements of patients.
Pacing parameters of ventricular leads at the time of implantation and at day 30 are presented in Table 3. Mean R wave amplitudes and impedance values were similar between the two groups, except that the mean threshold values were significantly higher in the troponin positive group at implantation (0.72 ± 0.19 V vs. 0.94 ± 0.33 V, p<0.001) and at 30th day of implantation (0.69 ± 0.16 V vs. 0.92 ± 0.31 V, p<0.001). The scope time of procedure was similar between groups (7.3 ± 3.9 min. vs. 7.1 ± 4.2 min, p=0.67).
| Troponin (-) (n=77) | Troponin (+) (n=32) | p | |
|---|---|---|---|
| At implantation Threshold (V) |
0.72 ± 0.19 | 0.94 ± 0.33 | <0.001 |
| Impedance (Ώ) | 752.8 ± 192.2 | 770.3 ± 253.6 | 0.7 |
| R wave(mV) | 11.1 ± 4.96 | 11.1 ± 4.95 | 0.89 |
| Scope Time of Procedure (min) | 7.3 ± 3.9 | 7.1 ± 4.2 | 0.67 |
| At 30th day Threshold (V) |
0.69 ± 0.16 | 0.92 ± 0.31 | <0.001 |
| Impedance (Ώ) | 756.4 ± 184.8 | 784.2 ± 245.8 | 0.52 |
Table 3. Pacemaker parameters of the groups at the time of implantation and 30 days later.
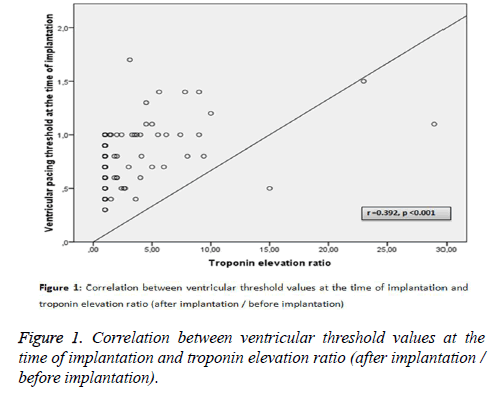
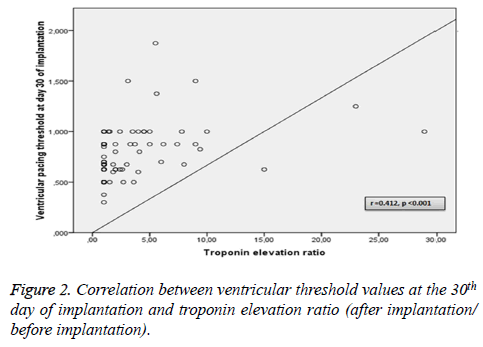
Among the above-mentioned parameters, ventricular threshold values at the time of implantation and at day 30 of implantation were found to correlate with troponin elevation ratio (after implantation/before implantation) levels (r=0.392, p<0.001 vs. r=0.412, p<0.001, respectively) (Figures 1 and 2).
To identify independent risk factors for ventricular threshold values, a multivariate linear regression model was conducted that included the following covariates: age, sex, diabetes mellitus, history of cardiovascular disease, hs-CRP, troponin values and ejection fraction. After implantation having positive troponin value (β=0.337, p=0.01) and troponin elevation ratio (β=0.365, p<0.001) were found to be independent risk factors for ventricular threshold.
Discussion
To our best knowledge, this is the first study to investigate the relationship between serum troponin levels and VPT values in patients who underwent single chamber PCM implantation. Our results indicated that troponin elevation after PCM implantation is associated with high VPT values at the time of and at the 30th day of implantation. Additionally, after implantation having positive troponin value and troponin elevation ratio (after implantation troponin value/before implantation troponin value) were found to be independent risk factors for VPT values.
Several factors such as hyperkalemia, acid-base imbalance, hypothermia, hypothyroidism myocardial ischemia, hyper and hipoglycemia, acid-base imbalance, severe hypoxia, antiarrhythmic drugs may increase pacing thresholds [1,8]. Balli et al. revealed that VPT values at the time of and at the 30th day of implantation were increased in patients with high serum uric acid (UA) levels [9]. This is explained that high serum UA levels might affect the pacing threshold by causing subclinical endomyocardial changes such as inflammation and fibrosis. Spear et al. showed in an animal study that fibrosis in myocardial tissue causes increased threshold of myocardial excitability [10].
Troponin is currently defined as a quantitative marker that can accurately indicate the presence of injury and the extent of damage to myocardial cells [11-13]. However, there are many other clinical conditions that cause damage to cardiomyocytes and raising levels of troponin [5,7]. Yoshida et al. showed that greater elevation of the troponin level was associated with favourable outcomes after radiofrequency catheter ablation of atrial fibrillation [6]. High myocardial damage is associated with greater troponin elevation and stop atrial undesirable activity in atrium. In our study we detected that in patients with positive troponin values, an increasing level of tissue damage occurs during active fixation. And we consider that fibrosis occurring in this area leads to fall of the threshold values.
Troponin elevation after pacemaker implantation was revealed in recent studies [14,15]. Pulmonary embolism is an undesirable complication of pacemaker implantation with troponin elevation [16]. In our study, patients were in apparently good clinical condition without evidence of respiratory or hemodynamic distress after pacemaker implantation. The other troponin elevation factor is mechanical effect of leads. Active leads penetrate into myocardium and causes myocardial damage. Transvenous insertion of endocardial pacemaker leads is followed by a sequence of cardiac histopathological changes, starting with acute inflammation and leading eventually to the formation of fibrous connective tissue scar [17-19].
Troponin values do not reach positive values after each implantation [20]. Significant predictors of peak troponin levels are maximum electrode diameter and the number of implanted electrodes [21]. Thicker endocardial leads, both atrial and ventricular lead implantation are related with high troponin levels [22]. We include only single pacemaker implantation to our study and used the same pacemaker types and same ventricular lead models.
Leads contain steroid for suppress inflammation. Although there are no large randomized studies on this subject, several small studies have suggested that steroid elution improves the ventricular thresholds of passive-fixation electrodes [23-25]. There is no data for active fixation leads. It can be speculated that leads do not have enough steroid to suppress inflammation or some patients have steroid resistance. More comprehensive studies are needed to confirm this hypothesis.
There are several limitations of the present study. Sample size was small and enrolment was retrospective. Ventricular Rwave amplitude was noted at baseline but most of patients were pacemaker dependent and at 30th day of implantation Rwave amplitude cannot be noted. Many other clinical conditions that cause troponin elevation such as pulmonary embolism and pericarditis/myocarditis did not adequately excluded; however during hospitalization follow up there were not any clinical evidence of these disorders. Due to those limitations, more comprehensive studies are needed to confirm our findings.
Conclusion
Our results indicate that troponin I elevation after pacemaker implantation is associated with higher ventricular threshold values at the time of implantation and the 30th day of implantation. For longevity of pacemaker batteries low threshold values are preferable. After permanent pace replacement, we recommend to follow more closely those patients having high troponin levels at implantation.
References
- Dohrmann ML, Goldschlager NF. Myocardial stimulation threshold in patients with cardiac pacemakers: effect of physiologic variables, pharmacologic agents, and lead electrodes. Cardiol Clin 1985; 3: 527-537.
- Adams JE, Bodor GS, Davila-Roman VG, Delmez JA, Apple FS, Ladenson JH, Jaffe AS. Cardiac troponin I. A marker with high specificity for cardiac injury. Circul 1993; 88: 101-106.
- Cummins B, Auckland ML, Cummins P. Cardiac-specific troponin-I radioimmunoassay in the diagnosis of acute myocardial infarction. Am heart J 1987; 113: 1333-1344.
- Katus HA, Looser S, Hallermayer K, Remppis A, Scheffold T, et al. Development and in vitro characterization of a new immunoassay of cardiac troponin T. Clin Chem 1992; 38: 386-393.
- Alaiti MA, Maroo A, Edel TB. Troponin levels after cardiac electrophysiology procedures: review of the literature. Pacing and clinical electrophysiology. PACE 2009; 32: 800-810.
- Yoshida K, Yui Y, Kimata A, Koda N, Kato J, Baba M, Misaki M, Abe D, Tokunaga C, Akishima S, Sekiguchi Y, Tada H, Aonuma K, Takeyasu N. Troponin elevation after radiofrequency catheter ablation of atrial fibrillation: relevance to AF substrate, procedural outcomes, and reverse structural remodeling. Heart rhyth 2014; 11: 1336-1342.
- Miranda CH, Schmidt A, Pazin-Filho A. Prognostic evaluation of the troponin I elevation after multiple spontaneous shocks of the implantable cardioverter/defibrillator. Am J Emerg Med 2014; 32: 1085-1088.
- Atlee JL. Cardiac pacing and electroversion. Cardiac anesthesia (4thed) WB Saunders, Philadelphia, 1999: 959-989.
- Ballı M, Çetin M, Taşolar H, Tekin K, Çağlıyan ÇE, et al. Increased ventricular pacing threshold levels in patients with high serum uric acid levels. J Cardiol 2014; 64: 207-210.
- Spear JF, Michelson EL, Moore EN. Cellular electrophysiologic characteristics of chronically infarcted myocardium in dogs susceptible to sustained ventricular tachyarrhythmias. J Am Coll Cardiol 1983; 1: 1099-1110.
- de Lemos JA. Increasingly sensitive assays for cardiac troponins: a review. JAMA 2013; 309: 2262-2269.
- Kociol RD, Pang PS, Gheorghiade M, Fonarow GC, O'Connor CM, Felker GM. Troponin elevation in heart failure prevalence, mechanisms, and clinical implications. J Am Coll Cardiol 2010; 56: 1071-1078.
- Newby LK, Jesse RL, Babb JD, Newby LK, Jesse RL, Babb JD, Christenson RH, De Fer TM, Diamond GA, Fesmire FM, Geraci SA, Gersh BJ, Larsen GC, Kaul S, McKay CR, Philippides GJ, Weintraub WS. ACCF 2012 expert consensus document on practical clinical considerations in the interpretation of troponin elevations: a report of the American College of Cardiology Foundation task force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2012; 60: 2427-2463.
- Boos CJ, Gough S, Wheather M, Medbak S, More R. Effects of transvenous pacing on cardiac troponin release. Pacing Clin Electrophysiol 2004; 27: 1264-1268.
- Martignani C, Diemberger I, Biffi M, Ziacchi M, Saporito D, Valzania C, Bertini M, Domenichini G, Branzi A, Boriani G. Troponin I rise after pacemaker implantation at the time of "universal definition of myocardial infarction". Am J Cardiol 2009; 103: 1061-1065.
- van Rooden CJ, Molhoek SG, Rosendaal FR, Schalij MJ, Meinders AE, Huisman MV. Incidence and risk factors of early venous thrombosis associated with permanent pacemaker leads. Journal of cardiovascular electrophysiology 2004; 15: 1258-1262.
- Beyersdorf F, Schneider M, Kreuzer J, Falk S, Zegelman M. Studies of the tissue reaction induced by transvenous pacemaker electrodes. I. Microscopic examination of the extent of connective tissue around the electrode tip in the human right ventricle. Pacing Clin Electrophysiol 1988; 11: 1753-1759.
- Epstein AE, Kay GN, Plumb VJ, Dailey SM, Anderson PG. Gross and microscopic pathological changes associated with nonthoracotomy implantable defibrillator leads. Circulation 1998; 98: 1517-1524.
- Ford SE, Manley PN. Indwelling cardiac catheters. An autopsy study of associated endocardial lesions. Arch Pathol Lab Med 1982; 106: 314-317.
- Hurst TM, Hinrichs M, Breidenbach C, Katz N, Waldecker B. Detection of myocardial injury during transvenous implantation of automatic cardioverter-defibrillators. J Am Coll Cardiol 1999; 34: 402-408.
- Nikolaou NI, Spanodimos SG, Tsaglis EP, Antonatos DG, Patsilinakos SP, Fournarakis GM, Tsigas DL. Biochemical evidence of cardiac damage following transvenous implantation of a permanent antibradycardia pacemaker lead. PACE 2005; 28: 1174-1181.
- Nikolaou NI, Christou AH, Spanodimos SG, Antonatos DG, Korkonikitas PI, Patsilinakos SP. Marked troponin elevation after implantation of a permanent antibradycardia pacemaker. Hellenic J Cardiol 2011; 52: 489-492.
- Gillis AM, Rothschild JM, Hillier K, Fudge W, Kieser TM, Maitland A. A randomized comparison of a bipolar steroid-eluting electrode and a bipolar microporous platinum electrode: implications for long-term programming. PACE 1993; 16: 964-970.
- Mond H, Stokes K, Helland J, Grigg L, Kertes P, Pate B, Hunt D. The porous titanium steroid eluting electrode: a double blind study assessing the stimulation threshold effects of steroid. PACE 1988; 11: 214-219.
- Wish M, Swartz J, Cohen A, Cohen R, Fletcher R. Steroid-tipped leads versus porous platinum permanent pacemaker leads: a controlled study. PACE 1990; 13: 1887-1890.