Research Article - Journal of Parasitic Diseases: Diagnosis and Therapy (2016) Volume 1, Issue 1
Perfusion Study on Rat Small Intestine Exposed to Cholera Toxin to Observe absorption of Water and Electrolytes from a Liposome Based Ors
Rifat Faruqui, Hamida Khanum*, Pradip Kumar Bardhan and David Sack
Rifat Faruqui, International Centre for Diarrhoeal Disease Research, Bangladesh
Hamida Khanum, Department of Zoology, University of Dhaka, Dhaka-1000, Bangladesh
Pradip Kumar Bardhan, International Centre for Diarrhoeal Disease Research, Bangladesh Cheryl Mitchell, CRM Corporation, USA
David Sack, International Centre for Diarrhoeal Disease Research, Bangladesh
- *Corresponding Author:
- Hamida Khanum
Professor, Department of Zoology, University of Dhaka, Dhaka-1000, Bangladesh
Tel: +88 01712-039756
Fax: +880-2-8712583
E-mail: hamida_khanum@yahoo.com
Received Date: October 27, 2016; Accepted Date: October 31, 2016; Published Date: November 11, 2016
Abstract
Introduction: Oral Rehydration Therapy (ORT) is the standard clinical intervention for the treatment of dehydration due to diarrhoea. It does not reduce the volume and frequency of duration of diarrhoea. This has prompted searching for improving ORS. One way of improving ORT may be delivering ORS in liposomes, to stimulate faster and more efficient absorption of water and electrolytes from small intestine. Objectives: To increase the small intestinal absorption of water and electrolytes from ORS by perfusion study, incorporating ORS components into liposomes. And to compare the absorption rates of S-ORS, HS-ORS and Lipo-ORS in small intestine after intestinal secretion is stimulated by Cholera Toxin (CT). Methods: Thirty adult male Long Evans rats were selected for the present study. In this experiment, three types of Oral Rehydration Solutions (ORSs); standard ORS (S-ORS), hydrolyzed starch ORS (HS-ORS) and Liposomal ORS (Lipo-ORS) were used for in vivo perfusion, in the normal and CT stimulated small intestines of rats. The study describes the absorption of electrolytes (Na+, K+, Cl-) and water from these three types of ORSs and compared among these substances from each other. Results: By using an in vivo perfusion technique, liposome based ORS was associated with significantly greater Na+, K+, Cland water absorption compared to hydrolyzed starch ORS or standard ORS. It was observed that the difference among absorption rates of water and electrolytes of standard ORS or hydrolyzed starch ORS and liposome based ORS which was significant (P ≤ 0.01). Conclusion: It can be concluded that liposomes enhance net electrolytes absorption from a carbohydrate electrolytes solution in the CT stimulated small intestines than normal small intestine of rat. These findings underscore the further experiment, on rat model which would be 5-Fluorourasil treated mucosal injured intestinal diarrhea by perfusion technique with the help of liposome based electrolyte solution for rehydration.
Keywords
Perfusion study; Cholera Toxin (CT); rat and liposomal ORS
Introduction
Based upon the pathogenic mechanisms, the diarrhoeal organisms may be broadly divided into two groups, secretory and invasive. Vibrio cholera is the prototype pathogen causing secretory diarrhoea through liberation of an enterotoxin which stimulates intestinal secretion via intracellular accumulation of cyclic AMP (Field et al. 1972). Cholera is endemic in some parts of Asia, Africa and Hispanola (Ali et al. 2012., Barzilay et al. 2013). It is characterized by acute onset of vomiting, profuse watery diarrhoea and development of dehydration that might lead to death if not treated immediately. Sufficient loss of fluids and electrolytes leads to dehydration and thus the mainstay of treatment of patients with acute infections, secretory diarrhoea needs fluid replacement. The development of ORS for the treatment of dehydration due to diarrhoea is one of the most significant therapeutic advances in the history of medicine (Hirschhorn et al. 1968., Pierce et al. 1968., Fordtran et al. 1975). It is based upon the observations that even in a secreting small intestine; it is possible to achieve a positive gut balance of fluid and electrolytes by adding glucose to the salt solutions.
It is estimated that, ORS alone can successfully rehydrate 90% of patients with dehydration from acute diarrhoea who previously would have required intra-venous (i.v.) therapy (Hirschhorn et al. 1991). However, Oral Rehydration Therapy (ORT) with the present ORS formulation has certain limitations-ORT does not reduce the volume, frequency or the duration of diarrhoea (Mahalanabis et al. 1996). These limitations prompted the concept of developing an improved ORS (initially named ‘super ORS’) (Mahalanabis et al. 1986). Conceptually, an improved ORS should be able to exert some beneficial effects: (a) Reduce stool volume (by stimulating re-absorption of fluid secreted into the small intestine); (b) Shorten duration of diarrhoea (by reducing ideal effluent flow and stimulating colonic salvage); and (c) Reduce failure rate of ORT particularly in patients with high purging rate.
Recently recommended reduced osmolarity ORS is the result of an intensive search for such an improved ORS. The ORS solution is similar to the original ORS but has a lower concentration of sodium (75 mmol rather than 90) and lower concentration of glucose (75 mmol rather than 111 mmol/L) yielding a solution with a total osmo-regulatory of 245 rather than 311. Though the new solution was associated with less vomiting, the duration of diarrhoea was not shortened, and there are still ORT failures in which patients initially rehydrated and place on ORT, become dehydrated again and require additional IV fluids. Thus, there is scope for further improvements of ORT.
One ingenious way may be delivering ORS in Liposomes to stimulate faster and more efficient absorption. The basic component of all clinically useful liposomes is the phospholipid molecule. Phospholipids are amphiphilic molecules composed of a polar hydrophilic head group and two hydrophobic fatty acid chains attached to a three-carbon glycerol backbone. When phospholipids are mixed with water, they spontaneously rearranged into concentric bilayer structures, termed liposomes or vesicles, separated by aqueous compartments (Gregoriadis et al. 1991). Incorporating ORS components into liposomes, as opposed to simply having salts and substrate in solution, have several potential advantages:
(a) It may add an additional mechanism of absorption to that already provided with glucose mediated transport,
(b) It may become especially important in patients who have severe purging or who have damaged intestinal epithelium (severe malnutrition, persistent diarrhoea),
(c) The solutions will have lower osmolarity and
(d) The solutions also will taste less salty. Such an ORS solution will also retain the potential advantage of all cereal based ORSs, i.e., slow release of substrate avoiding osmotic drag or load.
The aim of the present study is to determine the in vivo effect of absorption of water and electrolytes from liposome based glucose–containing solution over the whole length of rat small intestine. The whole length of rat small bowel was chosen to obtain results close to those in an intact animal and therefore relevant to the design of an improved ORS formulation (Patra et al. 1990). The present study has been reviewed and approved by the institutional Research Review Committee (RRC), Ethical Review Committee (ERC) and Animal Experimentation Ethics Committee (AEEC) of International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR).
Materials and Methods
Thirty adult male Long Evans rats were selected for the present experiment. The rat model was well established to study small intestinal absorption of water and electrolytes and had been extensively used to study “Oral Rehydration Solution” (ORS) (Rolston et al. 1987). In this study ORS was used as perfusion solution, prepared with analytical grade chemicals. The electrolyte concentrations of the three solutions are shown in Table 1. Polyethylene Glycol (PEG), Mol Wt 4,000 2 g/L was used as the unabsorbable marker. Three types of ORS were used as perfusion solution. These were Standard glucose based ORS (S-ORS), Hydrolyzed starch ORS (HS-ORS) and Hydrolyzed starch with Liposome based ORS (Lipo-ORS) (Table 2).
| Types of Experiment | Types of Group According to Used ORS | Type of ORS | No of Rats |
|---|---|---|---|
| Control Experiment | Control S-ORS group | S-ORS | 10 |
| Control HS-ORS group | HS-ORS | 10 | |
| Control Lipo-ORS group | Lipo-ORS | 10 | |
| CT-Treated Experiment | CT-treated S-ORS group | S-ORS | 10 |
| CT-treated HS-ORS group | HS-ORS | 10 | |
| CT-treated Lipo-ORS group | Lipo-ORS | 10 |
Table 1: Six Groups of Rats Were Treated With One of Three Oral Rehydration Solutions as Shown.
| Electrolytes | S-ORS (g) | HS-ORS (g) | Lipo-ORS (g) |
|---|---|---|---|
| Sodium (mmol) | 75 | 75 | 75 |
| Potassium (mmol) | 20 | 20 | 20 |
| Chloride (mmol) | 65 | 65 | 65 |
| Citrate (mmol) | 10 | 10 | 10 |
| Carbohydrate (g/L) | Glucose (13.6) | Hydrolyzed tapioca starch (25) | Hydrolyzed tapioca starch (25) |
| Osmolality | 245 | 210 (approx) | 125 (approx) |
Table 2: Composition of Solution Used to Perfuse the Rat Intestines.
A. Experimental Procedure
Sixty adult male rats were studied for the experiment. Rats were grouped into two experimental groups: (i) Control group; and (ii) CT (Cholera toxin)-treated group. Rats were further treated with one of three types of oral rehydration solution (Table 1). The abdomen was opened after a midline-incision (3-4 cm in length). The Intestine along with stomach and caecum was taken outside and placed on the lap-sheet. Thereafter, two incisions were performed into the intestine. The first was on the stomach 3-4 cm proximal to the duodeno-jejunal flexure and the second was into the ileum 3-4 cm before the ileocaecal junction for cannulation. The small intestine was then cannulated with two polyvinyl tubes (2 mm in diameter). A proximal cannula was introduced into the distal stomach through the incision and gently guided into the duodenum through the pylorus. The tip of the cannula was placed 2-3 cm distal to the pylorus, and the pylorus was tied externally to prevent backflow of the perfusate into the stomach. The distal cannula was inserted through the other incision and the ileum was tied just before the ileocaecal junction. The isolated and cannulated small intestine was gently rinsed to clear residual contents with the perfusion solution by gravity drainage. Prior to the final wash, the intestine was returned to the abdominal cavity and abdominal cavity was closed by suturing the incision, keeping the cannulas out of the abdomen.
B. Control Group
In control experiment, only the comparison of absorption rates of water and electrolytes were observed among three types of ORS (S-ORS, HS-ORS and Lipo-ORS) in normal small intestine of rats through perfusion.
C. Rat treated with Cholera Toxin (CT)
On the other hand in cholera toxin-treated experimental study, firstly a secretary intestine was established and thus absorption rates of water and electrolytes from S-ORS, HS-ORS and Lipo-ORS in secretary small intestine were observed. The secretory state was established by the instillation of chlolera toxin. CT (also known as choleragen and sometimes abbreviated to CTX, Ctx or CT) is protein complex secreted by the bacterium Vibrio cholerae. CT is responsible for the massive, watery diarrhea characteristic of cholera infection (Ryan et al. 2004., Faruque et al. 2008).
The secretory state was induced before perfusion by the instillation of 75 μg of pure chlolera toxin (dissolved in 5 mL of isotonic sodium chloride solution) via the distal cannula, and then both the cannulas were clamped (Elliot et al. 1991). The CT-saline solution was evenly distributed within the entire small intestine, and the abdomen closed. The CT-saline solution remained in the small intestine for 2 h to maximally stimulate intestinal secretion, after which the clamps on the cannulas were removed and the intestinal contents were allowed to drain out by gravity drainage and then intestinal perfusion was started. Each rat was perfused with only one of the three solutions: S-ORS, HS-ORS and Lipo-ORS as shown in Table 1. The perfusion was performed by attaching the proximal cannula to a constant infusion pump using a measuring burette as a reservoir. Each solution was infused at a constant rate of 0.5 mL/min. The distal cannula was extended to aid drainage of effluent by gravity. After 30 min of equilibration to achieve a steady state, the effluent was collected for 3 consecutive 15 min-collections (total 45 min) in Falcon tubes kept on ice. During the experiments the body temperature of the rats was maintained by controlling the ambient temperature using a spot lamp with thermostatic control and monitored by rectal thermometers.
After completing perfusion, aliquots of infusion and perfusion solutions were transferred from Falcon tubes to Eppendorf tubes by micro-pipette. The samples were stored at -50ºC for up to 48 h before analysis of net water and electrolyte movement. At the end of each experiment, the rats were sacrificed with overdose of pentobarbital. The perfused small intestinal segment was removed and stripped of excess mesentery (Figure 1). The segment wet weight was taken by Metler Toledo, College digital measuring scale (USA). After desiccation in an oven at 100°C for 18 h the dry weight of the perfused segment was also obtained by Metler Toledo, College digital measuring scale (USA).
D. Analytical Methods
Sodium (Na+), potassium (K+), chloride (Cl-) were measured by flame emission spectroscopy (Hunt et al. 1991), and PEG was measured by spectro-photometry Hyden et al. 1956a., Sidney et al. 1967).
E. Calculations
(Sladen et al. 1968): Net transport of water and electrolytes were calculated from the changes in the PEG concentration and the solute concentration (Gray et al., 1966., Levinson et al. 1966). The calculation for the net transport of water and ions was done as follows:
Net Transport = {F × ([S1]-[S2]) ([PEG1]/[PEG2])}/W
Where:
F is the flow rate;
S1 is the solute concentration in the perfusate;
S2is the solute concentration in the effluent;
PEG1 is the PEG concentration in the perfusion fluid;
PEG2 is the PEG concentration in the effluent;
W is the dry weight of the small intestinal segment used for perfusion.
F. Statistical Analysis
Results were provided by median (range) and values were expressed as μL.gm-1.min-1 for water and μmol.gm-1.min-1 for electrolytes while calculation performed on the dry weight of perfused segment. Positive results indicate net absorption and negative results indicate the net secretion into the lumen. Kruskal-Wallis Test was performed to test the statistical significance of the differences between the groups. Significant P value is ?0.05.
Results
The present study demonstrated that a liposome based ORS induced a significantly greater electrolytes (Na+, K+ and Cl-) and water absorption compared to standard-ORS and hydrolyzed starch-ORS solution. As expected rat intestines treated with CT initiated secretion of water and electrolytes and this model was used to simulate the secretory state of patients with severe cholera. The three types of ORSs were then evaluated to determine their absorptive characteristics.
A. Comparative Analysis of Absorption Rates of Water and Electrolytes from Three Types of ORSs
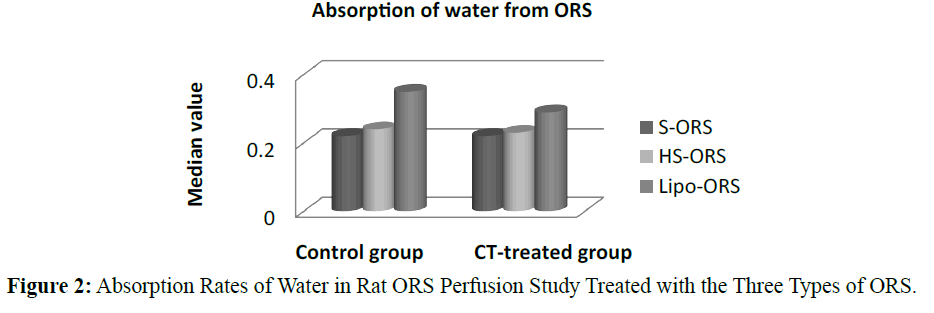
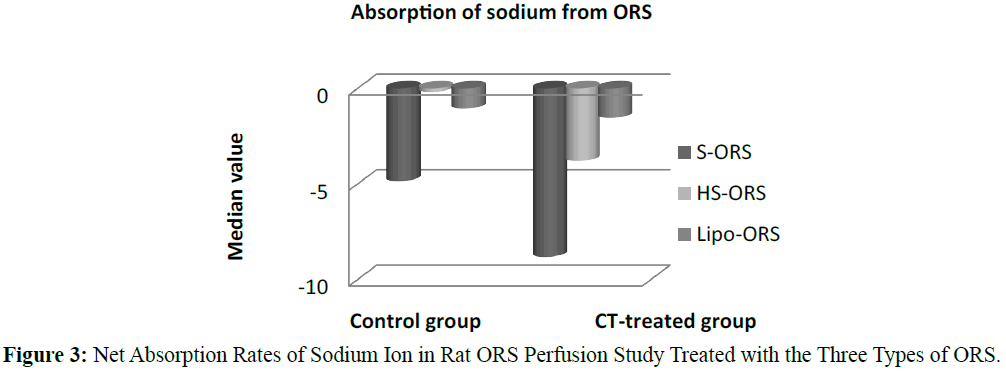
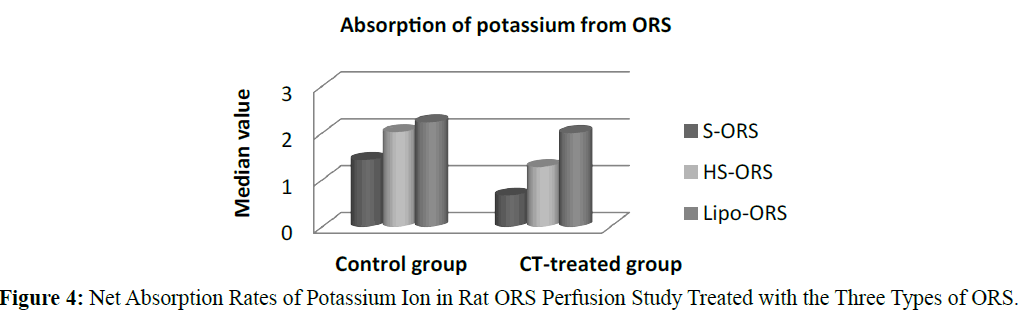
In the control unstipulated rats as well as the CT treated rats, the liposome based ORS solution induced a greater absorption of water and electrolyte compared to other two ORSs. In the control (no cholera toxin) rats, the highest absorption of water, K+ and Cl- were observed in the rats perfused with Lipo-ORS. In the CT treated rats highest absorption of water and K+ as well as lowest secretion of Na+ and Cl- were observed. One of the interesting findings of this study was that the median values of net Na+ transport were negative in all groups, suggesting net intestinal secretion; however the Na+ secretion was observed lowest among the Lipo- ORS group. In contrast, the median values of net K+ transport were positive in all groups, suggestive net intestinal absorption; again, the highest K+ absorption was noted in the Lipo-ORS group. In case of chloride ion absorption the difference among three ORSs was significant in both groups (Table 3). Net transport of water and electrolytes were derived from the changes in the PEG concentration and the solute concentration (Gray et al. 1966., Levinson et al. 1966). Net sodium, potassium, chloride and water absorption were higher from the Lipo-ORS compared to the other two solutions, S-ORS and HS-ORS in the CT-treated studies (Table 3).
| Name of the group | Control group | CT treated group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No of rats | 10 | 10 | 10 | 10 | 10 | 10 | |||
| Dry Weight of segment (g) | 1.34 | 1.44 | 1.27 | 1.68 | 1.65 | 1.63 | |||
| Types ofORSSample concentration (µl.cm-1.min-1 (or µl.g-1.min-1 & µmol.cm-1.min-1 or µmol.g-1.min-1) |
S-ORS | HS-ORS | Lipo-ORS | K-W Test Sig level (P) |
S-ORS | HS-ORS | Lipo-ORS | K-W Test Sig level (P) |
|
| H2O | Median | 0.22 | 0.24 | 0.35 | 0.07 | 0.22 | 0.23 | 0.29 | ≤0.01* |
| Na+ | Median | -4.85 | -0.18 | -1.05 | 0.14 | -8.81 | -3.78 | -1.53 | ≤0.01* |
| K+ | Median | 1.43 | 2.02 | 2.23 | 0.26 | 0.67 | 1.28 | 2.00 | ≤0.01* |
| Cl- | Median | -1.79 | 2.09 | 3.45 | 0.01 | -3.28 | 0.05 | -0.00 | ≤0.01* |
Table 3: Comparative Analysis, Among the Absorption Rates of Water and Electrolytes from Three Types of Orss in Perfusion Study on Whole Small Intestine of Rat.
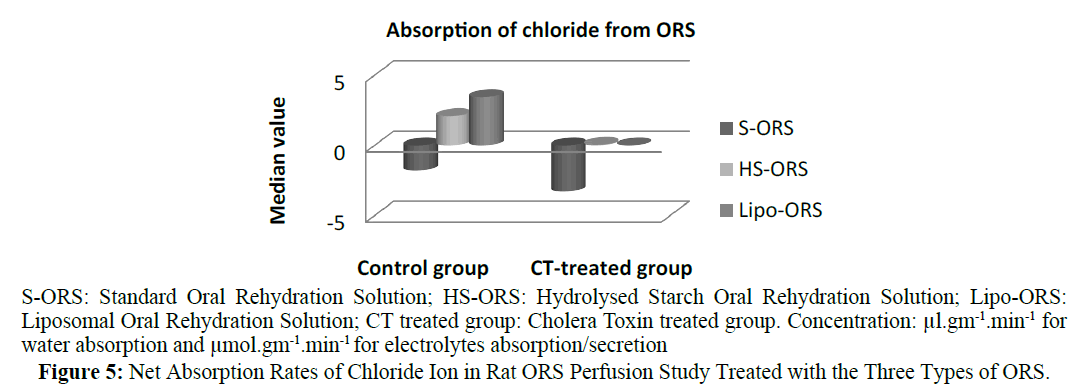
In case of water absorption, for both in the control and the experimental studies absorption rates from Lipo-ORS were the highest 0.347 μL.cm-1.min-1 and 0.289 μL.cm-1.min-1 respectively (Table 3) but the differences between three ORSs were statistically significant only in the CT treated group (p ≤ 0.01), not in the control group (Table 3 and Figure 2). On the other hand, secretion of sodium ion was observed in all experiments. In control group net secretion of sodium was lower in Lipo-ORS group than S-ORS group. In CT exposed experiments, net secretion of sodium was lowest in the Lipo-ORS group. The differences between three ORSs were statistically significant in the CT treated group only (p ≤ 0.01) (Table 3 and Figure 3). Moreover, in case of potassium ion, for both in control and CT treated groups absorption occurred from three ORS. In control group absorption of K+ from Lipo-ORS was highest 2.23 μmol.cm-1.min-1 (Table 3) although the difference was not statistically significant. In CT treated group absorption rates of K+ was highest 2.00 μmol.cm-1.min-1 than the other two ORSs and the difference among three ORS was statistically significant P ≤ 0.001 (Table 3 and Figure 4). In control group absorption rate of Cl- was highest from Lipo-ORS and the difference between three ORS was statistically significant (P ≤ 0.01). Whereas in the CT treated group, Cl- secretion in the HS-ORS and Lipo-ORS groups were significantly lower than that of in the S-ORS group (p ≤ 0.01) (Table 3 and Figure 5).
B. Comparative Analysis between the Absorption Rates of Water and Electrolytes from S-ORS and Lipo-ORS in Perfusion Study
The absorption rate of water was higher in Lipo-ORS than S-ORS, in both control and CT treated groups, whereas the difference was only significant only in CT-treated group (p≤0.02) (Table 4). The secretion rate of Na+ was lower in Lipo-ORS group than S-ORS group and the difference was significant in both groups (p≤0.04 and p≤0.00) (Table 4). In control group, the absorption rate of K+ was higher in Lipo-ORS than in S-ORS and the difference was not significant, whereas the difference between absorption rate of K+ Lipo-ORS and S-ORS was significant, in CT treated group ((p≤0.00) (Table 4). In case of net absorption of Cl-, the difference was highly significant between Lipo-ORS and S-ORS, in Control group. The secretion rate of Cl- was lower in Lipo-ORS than S-ORS and the difference between absorption rate of K+ of two types of ORS was also highly significant, in CT treated group (p≤0.02 and p≤0.00) (Table 4).
| Name of the group | Control group | CT treated group | |||||
|---|---|---|---|---|---|---|---|
| No of rats | 10 | 10 | 10 | 10 | |||
| Dry Weight of segment (gm) | 1.34 g | 1.27 g | 1.68 g | 1.63 g | |||
| Types of ORS Sample concentration µl.cm-1.min-1 or µl.g-1.min-1 & µmol.cm-1.min-1 or µmol.g-1.min-1 |
S-ORS | Lipo-ORS | P (M-W) |
S-ORS | Lipo-ORS | P (M-W) |
|
| H2O | Median | 0.22 | 0.35 | 0.08 | 0.22 | 0.29 | 0.02* |
| Na+ | Median | -4.85 | -1.05 | 0.04* | -8.81 | -1.53 | 0.00* |
| K+ | Median | 1.43 | 2.23 | 0.85 | 0.67 | 2.00 | 0.00* |
| Cl- | Median | -1.79 | 3.45 | 0.02* | -3.28 | -0.00 | 0.00* |
Table 4: Comparative Analysis Between the Absorption Rates of Water And Electrolytes from S-ORS and Lipo-ORS in Perfusion Study on Whole Small Intestine of Rat Using Dry Weight of Perfused Segment.
C. Comparative analysis between the absorption rates of water and electrolytes from HS-ORS and Lipo-ORS in perfusion study
The absorption rate of water was higher in Lipo-ORS than S-ORS, in both control and CT treated groups, whereas the difference was only significant only in CT-treated group (p ≤ 0.01) (Table 5). The secretion rate of Na+ was higher from Lipo-ORS than S-ORS and the difference was not significant in control group. The secretion rate of Na+ was lower from Lipo-ORS than S-ORS and the difference was highly significant in CT treated group (p ≤ 0.04) (Table 5). The absorption rate of K+ was higher in Lipo-ORS than in S-ORS and the difference was not significant in both groups (Table 5). In case of net absorption of Cl-, the difference was not significant between Lipo-ORS and S-ORS, in Control group. The difference between net absorption rate of Cl- of two types of ORS was highly significant, in CT treated group (p ≤ 0.01) (Table 5).
| Name of the group | Control group | CT treated group | |||||
|---|---|---|---|---|---|---|---|
| No of rats | 10 | 10 | 10 | 10 | |||
| Dry Weight of segment (g) | 1.44 g | 1.27 g | 1.63 g | 1.63 g | |||
| Types of ORS Sample concentration µl.cm-1.min-1 or µl.gm-1.min-1 & µmol.cm-1.min-1 or µmol.gm-1.min-1 |
HS-ORS | Lipo-ORS | P (M-W) |
HS-ORS | Lipo-ORS | P (M-W) |
|
| H2O | Median | 0.24 | 0.35 | 0.12 | 0.23 | 0.29 | 0.01* |
| Na+ | Median | -0.18 | -1.05 | 0.53 | -3.78 | -1.53 | 0.04* |
| K+ | Median | 2.02 | 2.23 | 0.53 | 1.28 | 2.00 | 0.28 |
| Cl- | Median | 2.09 | 3.45 | 0.68 | 0.05 | -0.00 | 0.01* |
Table 5: Comparative Analysis Between the Absorption Rates of Water and Electrolytes From HS-ORS and Lipo-ORS in Perfusion Study on Whole Small Intestine of Rat Using Dry Weight of Perfused Segment.
Discussion
The use of Oral Rehydration Solution (ORS) has revolutionized the management of acute diarrhoea. The implementation of World Health Organization ORS (WHO-ORS) has resulted in decreased mortality associated with acute diarrhoeal illnesses in children, although in general stool volume and diarrhoea durations were not reduced (WHO et al. 2002). Several strategies were used to develop and test for improved ORS. Though the new hypo-osmolar ORS has advantages over the previously used standard ORS, the duration of diarrhoea was still not shortened, and there were failures with ORT (Alam et al. 1999). Thus there was scope for further improvements of ORT.
Various modifications to the standard ORS have been derived. These modification have included hypo-osmolar or hyperosmolar solutions, use of rice-based ORS, and the use of amino acids, including glycine, alanine, and glutamine (Rhoads et al. 1990). Some of these variations have been successful, some have not, and others are still under investigation. ORS has also been used to decrease intravenous (IV) fluid requirements in patients with short bowel syndrome (SBS) who require parenteral nutrition (Atia, 2009., Buchman, 2009).
Animal models have been extensively used to develop modifications in the formulation of ORS. Rolston et al. (1989) has investigated the effect of bicarbonate, acetate and citrate on water and sodium transport in normal and also in secreting (Cholera toxin-treated) rat small intestine using a single-pass perfusion technique. In normal jejunum bicarbonate and acetate produced net absorption and citrate produced net secretion of both water and sodium. In the secreting jejunum, however bicarbonate had no effect on water and sodium secretion, whereas acetate and citrate actually enhanced the secretory state for both water and sodium. Those observations suggest that those anions to ORSs may have no beneficial effect with regard to the promotion of water and sodium absorption in the secreting intestine during acute diarrhoeal states and could actually be deleterious (Rolston et al. 1989).
(Patra et al. 1990) studied that the effect of citrate on sodium, potassium, chloride and water absorption in the presence of glucose from the whole rat small intestine by an in vivo marker perfusion technique. The perfusion solutions contained glucose and were similar in their electrolyte composition to the currently recommended oral rehydration solution for the treatment and prevention of diarrhoeal dehydration. Significantly more sodium and water absorption occurred from the citrate-containing solution than from the one without citrate (Patra et al. 1990).
Clinical studies showed improved absorption with alternative types of ORS. In a non-randomized open trial, children receiving an oral rehydration salt solution with a lower concentration of glucose and was hyptonic had reduced frequency of diarrhoeal stools and could be discharged sooner than other children who received the standard ORS which was isotonic (Rautanen et al. 1993). A comparative study performed between hypo-osmolar ORS with sucrose replacing glucose (Na+ 60, K+ 15, Cl- 60, and citrate 5, sucrose 58 mmol/L-1, calculated osmolality 198 mOsm kg-1) and mildly hyperosmolar glucose ORS (WHO) in 46 children aged 6-30 months with acute diarrhoea and dehydration. In the hypo-osmolar sucrose ORS group (n=18) faecal output was 30% less during the initial 24 and 48 h compared with controls (Faruque et al. 1996).
In a randomized controlled clinical trial, (Dutta et al. 2000) found that children, aged 2-10 years with severe cholera who were treated with a rice-based hypo-osmolar ORS had reduced (p<0.05) stool output, ORS consumption and diarrhea duration than patients who received either WHO-ORS or glucose-based hypo-osmolar ORS (Dutta et al. 2000). In another randomized controlled trial, Ramakrishna (2008) tested a hypotonic ORS in which the carbohydrate was an amylase resistant starch in adults with acute dehydrating diarrhoea. Compared to hypo-osmolar (HO-ORS) ORS, amylase resistant starch-ORS reduced diarrhoea duration by 55% and significantly reduced fecal weight after the first 12 h of ORS therapy in adults with cholera like diarrhoea (Ramakrishna et al. 2008).
Various liposome-based medications are already used in diverse clinical situations. Many pharmacological agents of varying solubility and size (anti-tumour and antimicrobial agents, enzymes, peptides, hormones, vaccines and genetic materials) have already been encapsulated in either the aqueous or the lipid phase of the liposomes (Gregoriadis et al. 1991). Proteins and other non-lipid molecules can be incorporated into the lipid membranes. Drug ligands (e.g. antibodies) can also be linked with the outer bilayer. In fact, liposomes can be designed to satisfy particular needs in a variety of applications ranging from biochemical and immunological assay kits and diagnostic reagents to therapeutic preparations for enteral and parenteral uses as well as vaccines (Gregoriadis et al. 1988., Lopez-Berestein, 1989., Fidler, 1989., Baemner et al. 2002., Wu et al. 2002., Dima et al. 2001., Brouwers et al. 2000).
Conclusion
In the present investigation liposomes incorporated into ORS for intestinal perfusion to stimulate absorption of water and electrolytes resulted in significantly enhanced absorption of water, K+, Cl- and reduced secretion of Na+ when compared with other ORSs. The improved lipsome based ORS (Lipo-ORS) may have potentials in reducing stool volume, duration of diarrhoea and failure rate of ORT in patients with high purging rate. Future RCTS are therefore warranted to evaluate its clinical efficacy in infectious diarrhea.
References
- Alam, N.H., Mazumder, R.N., Fuchs, G.J.(1999). Efficacy and safety of oral rehydration solution with reduced osmolarity in adults with cholera: a randomized double-blind clinical trial. Lancet, 354 (9175), 296-299.
- Ali, M., Lopez, A.L., You, Y.A., Kim, Y.E., Sah, B.,Maskery, B. (2012).The global burden of cholera.Bull World Health Organ, 90(3), 209-218A.
- Atia, A.N., Buchman, A.L. (2009). Oral rehydration solutions in non-cholera diarrhea: a review.Am J Gastroenterol,104(10), 2596-2604.
- Baemner, A.J., Schlesinger, N.A., Slutzki, N.S., Romano, J., Lee, E.M.,Montagna, R.A. (2002). Biosensor for dengue virus detection: sensitive, rapid and serotype specific. Anal Chem,74 (6),1442-1448.
- Barzilay, E.J., Schaad, N., Magloire, R., Mung, K.S., Boncy, J.,Dahourou, G.A. (2013). Cholera surveillance during the Haiti epidemic--the first 2 years.N Engl J Med,368(7), 599-609.
- Brouwers, A.H., Jong, D.J., Dams, E.T., Oyen, W.J., Boerman, O.C., …Laverman,P. (2000). Tc-99m-PEG-Liposomes for the evaluation of colitis inCrohn’s disease.J Drug Target,8,225-233.
- Dima, V.F., Lonescu, M.D., Palade, R.,Balotescu, C., Becheanu, G., Dima, S.V. (2001). Stimulation of mucosal immune response following oral administration of enterotoxigenicEscherichia coli fimbriae (CFA/1) entrapped in liposomes in conjunction with inactivated whole-cell Vibrio cholerae vaccine. Roum Arch MicrobiolImmunol,60(1), 27-54.
- Dutta, D., Bhattacharya, M.K., Deb, A.K.,Sarkar, D., Chatterjee, A., Biswas, A.B., … Bhattacharya, S.K. (2000). Evolution of oral hypo-osmolar glucose-based and rice-based oral rehydration solutions in the treatment of cholera in children.ActaPaediatr, 89(7), 787-790.
- Elliot, E., Watson, A., Walker-Smith, J., Farthing, M.J.G. (1991). Search for the ideal rehydration solution: studies in a model of secretory diarrhoea. Gut, 32, 1314-1320.
- Faruque, A.S.G., Mahalanabis, D. (1996). Reduced osmolarity oral rehydration salt in cholera.Scand J Infect Dis, 28,87-90
- Faruque, S.M., Nair, G.B. (2008). Vibrio cholerae: Genomics and Molecular Biology. Caister Academic Press.
- Field, M., Fromm, D., al-Awqati, Q., Greenough, W.B. (1972). Effect of cholera enterotoxin on ion transport across isolated ileal mucosa. J Clin Invest, 51(4), 796-804.
- Fordtran, J. S. (1975). Stimulation of active and passive sodium absorption by sugars in the human jejunum.J Clin Invest,55,728-37.
- Gray, G.M., Ingelfinger, F.J. (1966). Intestinal absorption of sucrose in man: Interrelation of hydrolysis and monosaccharide product absorption. J Clin Invest,45,388-398.
- Gregoriadis, G. (1988). Liposomes as drug carriers: recent trends and progress. Wiley, Chichester.
- Gregoriadis, G. (1991). Overview of liposomes. J AntimicrobChemother, 28, 39-48
- Hirschhorn, N., Greenough, W.B.(1991). Progress in oral rehydration therapy.ScientAmer, 264,50-56.
- Hirschhorn, N., Kinzie, J.L., Sachar, D.B., Northrup, R.S., Taylor, J.O., Ahmad, S.Z. (1968). Decrease in net stool output in cholera during intestinal perfusion with glucose-containing solutions. N Engl J Med,279(4),176-81.
- Hunt, J. B., Elliott, E. J. and Farthing, M. J. G. (1991). Comparison of rat and human intestinal perfusion models for assessing efficacy of oral rehydration solutions. Aliment PharmacolTher, 5(1),49-59.
- Hyden, S. (1956a). A turbidimetric method for the determination of higher polyethylene glycols in biological materials. K Lantbr-Hogsk Annlr, 22, 139-145.
- Levinson, R.A., Schedl, H.P.(1966). Absorption of sodium, Chloride, water and simple sugars in rat small intestine. Am J Physiol,211,939-942.
- Lopez-Berestein, G., Fidler, I. J. (1989). Liposomes in the therapy of infectious diseases and cancer. Alan Liss, New York.
- Mahalanabis, D. (1996). Current status of oral rehydration as a strategy for the control of diarrhoeal diseases. Indian J Med Res, 104,115-24.
- Mahalanabis, D., Merson, M.H.(1986). Development of an improved formulation of oral Rehydration solution (ORS) with antidiarrhoeal and nutritional properties: a ‘Super ORS’. In Holmgren J, Lindberg A and Molby R (eds.) Development of vaccines and drugs against diarrhoea; 11th Nobel Conference, Stockholm, 1985. Lund Student Literature 240-256.
- Patra, F.C., Rahman, A.S.M.H., Wahed, M.A., Al-Mahmud, K.A. (1990). Enhanced sodium absorption by by citrate: an in vivo perfution study of rat small intestine. J Ped Gastroenterol Nutr,11,385-388.
- Pierce, N.F., Banwell, J.G., Rupak, D.M.(1968). Effect of intragastric glucose-electrolyte infusion upon water and electrolyte balance in Asiatic cholera. Gastroenterology, 55, 333-343.
- Ramakrishna, B.S., Subramanian, V., Mohan, V., Sebastian, B. K., Young, G.P., Farthing, M.J., Binder, H.J. (2008). A randomized controlled trial of glucose versus amylase resistant starch hypo-osmolar oral rehydration solution for adult acute dehydrating diarrhoea. PLos One 3(2),e1587.
- Rautanen, T., El-Radhi, S., Vesikari, T.(1993). Clinical experience with a hypotonic oral rehydration solution in acute diarrhoea. Acta Paediatr, 82, 52-54.
- Rhoads, J. M., Keku, E.O., Bennett, L.E., Quinn, J., Lecce, J. G. (1990). Development of L-glutamine-stimulated electroneutral sodium absorption in piglet jejunum. Am J Physiol, 259, G99-G107.
- Rolston, D.D.K., Borodo, M.M., Kelly, M.J., Dawson, A.M., Farthing, M.J.G. (1987). Efficacy of oral rehydration solutions in a rat model of secretory diarrhea. Pediatr Gastroenterol Hepatol Nutr, 6, 624-630.
- Rolston, D.D.K., Kelly, M.J., Borodo, M.M., Dawson, A.M.,Farthing, M.J.G. (1989). Effect of bicarbonate, acetate and citrate on water and sodium movement in normal and cholera toxin-treated rat small intestine. Scand J Gastroenterol Suppl,24, 1-8.
- Ryan, K.J., Ray, C.G. (2004). Sherris Medical Microbiology(4th ed.). McGraw Hill.375.
- Sidney, J. M., Captain, M.C., Don, W.Pl., Captain,M.C. (1967). An improved turbidimetric analysisof polyethylene glycomutilizing an emulsifier. Gastroenterology, 53(2), 250-256.
- Sladen, G.E., Dawson, A. M. (1968). An evaluation of perfusion techniques in the study of water and electrolyte absorption in man: the problem of endogenous secretions. Gut, 9, 530-535.
- WHO. (2002). New formulation of Oral Rehydration Salts (ORS) with reduced osmolarity. Unicef.
- Wu, J., Nantz, M.H., Zern, M.A. (2002). Targeting hepatocytes for drug and gene delivery: emerging novel approaches and applications. Front Bio sci,7, 717-725.