Research Article - Current Pediatric Research (2023) Volume 27, Issue 6
Pathophysiology and management of secondary lactose intolerance in infants: Role of casein-based nutritional supplement.
Dhanasekhar Kesavelu1, Tanvi Paryani2*
1Consultant Pediatrician and Pediatric Gastroenterologist, SS Child Care, Chennai, Tamil Nadu, India
2Nutricia International Pvt. Ltd. Danone, Maharashtra, India
- Corresponding Author:
- Tanvi Paryani
Nutricia International Pvt. Ltd. Danone Maharashtra, India
E-mail: tanvi.paryani@danone.com
Received: 26 May, 2023, Manuscript No. AAJCP-23-100083; Editor assigned: 29 May, 2023, Pre QC No. AAJCP-23-100083(PQ); Reviewed: 12 June, 2023, QC No. AAJCP-23-100083; Revised: 19 June, 2023, Manuscript No. AAJCP-23-100083(R); Published: 29 June, 2023, DOI:10.35841/0971-9032.27.06.1902-1910.
Abstract
Diarrhea is a leading cause of malnutrition and is the third leading cause of death among children under five years. Secondary Lactose Malabsorption (SLM) induced by infectious gastroenteritis is more common and can be clinically significant, especially in infants for whom milk is the principal food. Compared to lactose-containing formulas, lactose-free supplementation can reduce the duration of diarrhea by an average of 18 hours. Also, several clinical studies have reported beneficial effects of on gut microflora, diarrhea, and immune function. This review discusses the role of casein-based nutritional supplements for infants with gastrointestinal disorders.
Keywords
Secondary lactose intolerance, Lactose malabsorption, Formula, Diarrhea, Casein, Lactose-free, Acute gastroenteritis
Abbreviations
AAP: American Academy of Pediatrics; AMP: Adenosine Monophosphate; BPP: Bacteroides-Porphyromonas Prevotella; CF: Control Formula; CMPA: Cow Milk Protein Allergy; CMP: Cytidine Monophosphate; ESPGHAN: European Society for Pediatrics Gastroenterology, Hepatology, and Nutrition; NF: Fortified with Nucleotides; GMP: Guanosine Monophosphate; Ig: Immunoglobulin; IMP-3: Inosine Monophosphate; LM: Lactose Malabsorption; MCT: Medium Chain Triglycerides; NF: Nucleotide Formula; NI: Nutritional Index; ORS: Oral Rehydration Solution; PUFA: Polyunsaturated Fatty Acids; RDA: Recommended Dietary Allowances; RTIs: Respiratory Tract Infections; SLM: Secondary Lactose Malabsorption; Ssc: Systemic sclerosis; UMP: Uridine 5'-Monophosphate; VDRs: Vitamin D Receptors; WHO: World Health Organization.
Introduction
Secondary lactose intolerance in infants-causes, symptoms, the burden in India
Lactose intolerance is a clinical syndrome characterized by gastrointestinal symptoms such as abdominal pain, abdominal discomfort, diarrhea, nausea, flatulence, and bloating followed by the consumption of lactose or lactose-containing foods. The amount of lactose required to evoke symptoms varies between the patients based on the quantity of lactose consumed, the severity of lactase deficiency, and the type of food in which the lactose is consumed [1]. For dairy to get digested quickly, the gut microbiota is known to change. The human body roughly inhabits 40 trillion bacteria, with the human colon containing approximately 99% of the microbiome. Fermentation products are necessary for colonic health and to generate extra calories from otherwise indigestible carbs. Lactose fermentation by saccharolytic bacteria can induce stomach discomfort in people with Lactose Malabsorption (LM) [2].
Lactose intolerance can occur in infants, children, and adolescents due to primary or secondary lactase insufficiency. Congenital lactose intolerance is extremely rare, the incidence is relatively unknown, and the cause remains elusive. Using of lactase-treated dairy products or oral lactase supplements, restriction of lactose-containing meals, or dairy elimination are all options for treatment. According to the American Academy of Paediatrics (AAP), dairy foods are a vital source of calcium for bone mineral health and other nutrients that help children and adolescents grow. Other calcium sources or calcium supplements must be provided if dairy products are eliminated from the diet [3].
Acute gastroenteritis is a leading cause of morbidity and mortality in children, accounting for 15% of all deaths in children globally. Diarrheal diseases continue to be a severe public health problem in developing countries with an almost double rate of incidence and each child suffers about three episodes each year. Diarrheal diseases constitute both macro and micro cost burdens [4].
Diarrhea is the passage of three or more loose or liquid stools per day. World Health Organization (WHO) states that "in low- income countries, children under three years old experience three episodes of diarrhea per year." Every episode deprives the child of essential nutrients for growth. As a result, diarrhea is a leading cause of malnutrition, and malnourished children are more at- risk of becoming un-well [5]. Diarrhea is the third leading cause of death among children under five years, accounting for 13% of all deaths in this age group. Mortality is estimated at around 300,000 children in India [6]. In India, approximately 78,000 children die due to rotavirus gastroenteritis annually, with about 59,000 in the under two year’s age group [7]. The three clinical types of diarrhea include acute watery diarrhea, dysentery, and persistent diarrhea [5].
Acute Gastroenteritis (GE) and secondary lactose intolerance
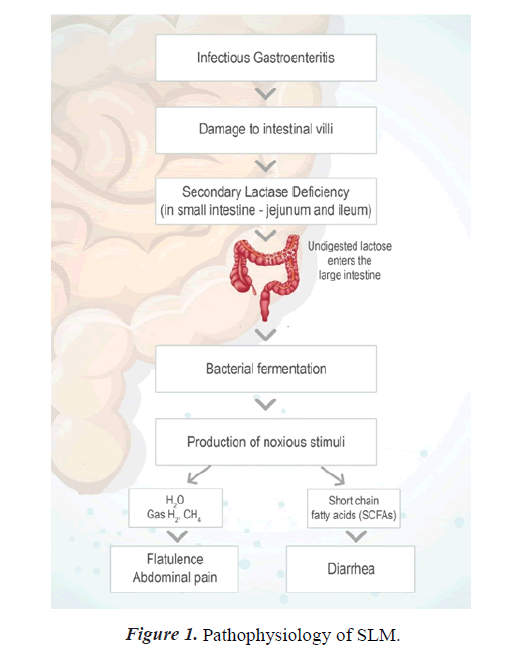
Secondary Lactose Malabsorption (SLM) is characterized by the onset of Lactose Malabsorption (LM) in those capable of digesting lactose [8,9]. Lactase is located at the tip of intestinal villi, making it vulnerable to intestinal damage, particularly since fresh immature enterocytes lack lactase [10]. As a result, SLM can exacerbate Gastrointestinal (GI) disorders such as infectious gastroenteritis, inflammatory bowel disease, celiac disease, and Systemic Sclerosis (SSc).
Secondary lactase insufficiency refers to lactase deficiency and associated lactose malabsorption caused by an underlying pathophysiologic disorder. Acute viral infection caused by rotavirus can damage the small intestine, resulting in the loss of lactase-containing epithelial cells from the villi's tips. Lactase deficit is expected in the young epithelial cells that replace them, resulting in secondary lactose shortage. Lactose intolerance is different from Cow Milk Protein Allergy (CMPA). The latter involves the immune system and causes varying degrees of injury to the intestinal mucosal surface [3].
SLM induced by infectious gastroenteritis is more common and can be clinically significant, especially in infants for whom milk is the principal food [11]. Infants suffering from rotavirus infection had higher frequency of SLM (p=0.002).
Pediatric diarrhea can last for several days, causing dehydration, electrolyte imbalance, and malnutrition in children under five. Children who are malnourished or have a compromised immune system are at a higher risk of diarrhea-related life-threatening consequences such as dehydration, metabolic acidosis, impaired consciousness, convulsions, circulatory shock, and prerenal azotemia [12]. In children under the age of 5, digestive tract infection caused by various viral, bacterial, or parasitic organisms is the most common cause of acute diarrhea. Lactose malabsorption can result from acute infectious diarrhea, which damages lactase-containing epithelial cells on the ends of the intestinal villi. The replacement of older epithelial cells with new ones often results in the cells being immature and lacking enough lactase, which worsens lactose malabsorption and extends the duration of diarrhea. [4]. Reintroducing lactose-containing formula or foods should be attempted after 2–4 weeks. Lactose restriction may be necessary for a new born with celiac disease or other minor intestine disorders until the underlying problem has been resolved or successfully treated [13]. Slight intestinal atrophy occurs in infants with severe malnutrition, resulting in secondary lactase insufficiency. Malnutrition is associated with lactose malabsorption and carbohydrate intolerance in lactose malabsorption has also been associated with poor growth [3].
Importance of nutrition in children with secondary lactose intolerance (0-2 years)
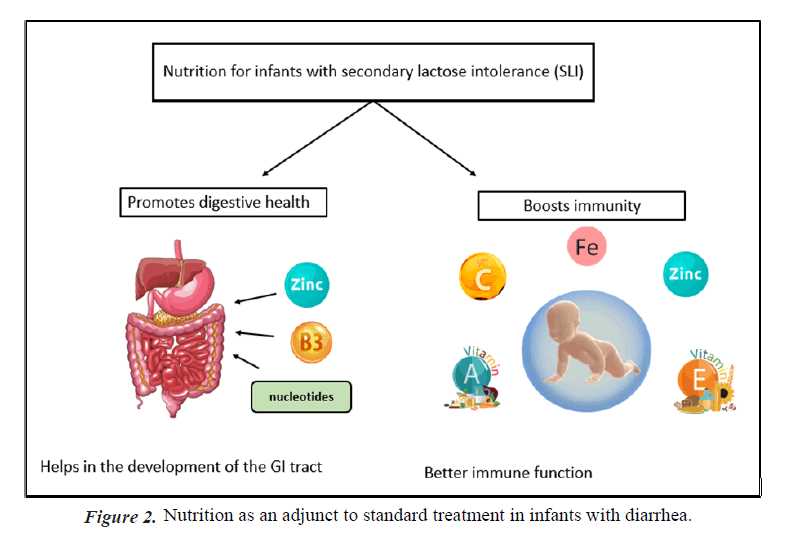
Importance of nutritional solutions for reducing Acute GE symptoms: Adequate nutrition and hydration is seen an important adjunct to therapy in treatment of diarrhea, since antibiotics and other medications play only a limited impact," according to the centres for disease control and prevention [14]. According to the Indian Academy of Pediatrics children suffering from diarrhea who continue breast feeding tend to recover more quickly and regain weight better when they recover than children whose diet is restricted [15]. Breast- feeding in infants with acute gastroenteritis is widely practiced. Many responding physicians follow the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) recommendation to continue breastfeeding during acute diarrhea [16]. Treating acute diarrhea with a combination of oral rehydration and prompt nutritional support has been shown to be effective (Figures 1 and 2) [17].
Lactose avoidance for young children with acute diarrhoea
Breast-feeding should be continued for infants diagnosed with lactose intolerance. Continuation of breastfeeding is widely practiced. A brief trial of lactose-free formula in formula- fed infants may be indicated for viral gastroenteritis. Lactose restriction reduces the duration of gastrointestinal symptoms in children with prolonged diarrhea following acute gastroenteritis [18].
Compared to lactose-containing formulas, the use of lactose-free supplements has been shown to shorten the average duration of diarrhea by 18 hours. Lactose-free supplementation may also help to prevent treatment failures, such as vomiting, weight loss, or the need for additional fluids [4].
Lactose-free supplementation: The preferred form of nutrition solutions for infants
Supplementing with a lactose-free option may lower the risk of treatment failure, such as on-going or worsening diarrhea or vomiting, or the requirement for additional rehydration therapy, or continuing weight loss by about 50% (RR 0.52). There is no evidence that diluting lactose-containing milk has an impact on the duration of diarrhea when compared to undiluted milk or milk-based products. Thus, young children with acute diarrhea who are not predominantly breastfed when switched to a lactose-free diet may have an earlier resolution of acute diarrhea and reduced treatment failure [18]. As the carbohydrate and the lactose free alternatives have been discussed so far it is important to understand the protein source as well. The protein present in breast milk provides essential amino acids necessary for the growth and replacement of protein losses via urine, faeces, and the skin. Breast milk has two types of protein, whey, and casein. In the first two weeks of lactation, it is found that the concentration of whey protein is very high compared to casein. The ratio of the same observed is as high as 80:20, which then drops to 65:35 by the second week and then stays constant at about 60:40 for 6 months. Furthermore, the ratio drops down to 50:50 in the late lactation stages [19].
Literature Review
Casein protein
Casein is a milk-specific protein, representing about 80% of the total protein in bovine milk. Casein is a mixture of three proteins called alpha, beta, and gamma caseins. The insoluble part of casein comprises alphas (s1 and s2) and beta caseins, while k-casein makes up the soluble fraction of casein protein. From the point of view of the stability and properties of the casein micelle, k-casein is its most crucial component. In milk, the caseins are large colloidal particles, 50 nm–600 nm in diameter, called ‘casein micelles’. In mammary glands, casein micelles enable milk secretion with a high concentration of calcium phosphate in a ‘‘soluble’’ form. The casein micelles are designed to be solidified by chymosin in the neonate’s stomach, a proteinase intended for this function [20].
Casein based therapeutic nutritional solutions
pH lowering and gastric emptying: A study was conducted to compare the resistance of purified beta-casein and beta- lactoglobulin in the stomach of an infant model in which casein got digested swiftly after 10 minutes and lactoglobulin remained stable and was only hydrolyzed in the small intestine phase. Purified caseins from raw and processed milk, on the other hand, disappeared in the new born gastrointestinal stage within 20-40 minutes [21].
Digestibility rate–casein compared to other lactose-free options (Soy/Whey)
A higher percentage of caseins in dairy infant formulations led to a higher degradation of protein as the casein in the stimulated duodenal phase degraded easily. Infant formulations that were soy based exhibited the least in vitro protein hydrolysis when compared to dairy formulation intimating that the hydrophobic β-sheet structures of soy protein is responsible for the protein aggregation and the possible effect of heat treatment on soy protein structure during processing [21].
The in vitro study using digestibility assay by pH drop method showed that the digestibility rate is highest for formulations with a higher proportion of caseins (whey to casein=2:8) and least for soy protein formulation. Formulations with whey to casein ratio of 2:8 show a maximum pH drop, while soy formulation created the tiniest depth. Thus, the pH drop method suggests rapid digestion of the formulation with a higher proportion of caseins. So, caseins are easily digested in the duodenal phase compared to whey and soy protein [21].
Casein coagulation and micelle formation-slower release of amino acids leading to better digestion
The biological functionality of casein strongly associates with this protein’s phosphorylation which transports the bioavailable calcium, phosphate, and other minerals from the mother to the neonate. The casein micelles, i.e., association colloids are formed by the mineralization of casein in the mammary glands. K-casein, found rarely in the casein micelle core plays an important role by providing steric stabilization to the casein micelle.
The gastric curd is formed due to the solidifying of the caseins, whey proteins compared to casein empties into the intestine at a faster rate. Gastric coagulation ensures the transit of the protein through the stomach in a controlled manner and thus resulting in a sustained release of amino acids into the blood following intestinal digestion and absorption. This controlled release of protein into the intestine helps avoid overloading of the digestive capacity of the intestine which is normally observed in the population with a reduced digestive or absorption capacity. There are notable differences in the extent of gastric coagulation such as the importance of the firmness of the casein curd through gastric coagulation has become known from a large body of work carried out in the first half of the 20th Century, focused on so-called 'soft curd milk' and 'hard curd milk.’
It is significant to note that work regarding the formation of hard casein curd of the coagulum under gastric conditions observed the propensity of caseins to thicken and the type of coagulum formed was significant in the digestive process. Soft curd milk led to less digestive difficulties compared to when hard curd milk was consumed. It was observed that the rate of gastric digestion and stomach clearance is related to curd the hardness of gastric curds formed. A study on gastric emptying in infants observed that preterm infants experienced faster gastric emptying rate of 30 mL portions of human milk compared to the 30 mL portions of infant formula, with an average of 24.8 mL of human milk emptied after one hour indicating that human milk forms softer clots compared to infant formulation in the stomach [22].
The pepsin induced hydrolysis of protein that maintains the steric stabilization can cause the coagulation of casein milk and milk fat globules. Delayed gastric emptying of casein and fat is due to gastric coagulation [22]. Casein exists in milk in the form of a micelle, which is a sizeable colloidal particle. An attractive property of the casein micelle is its ability to form a gel or clot in the stomach. The ability to form this clot makes it very efficient in nutrient supply. The lump can provide a sustained slow release of amino acids into the bloodstream, sometimes lasting for several hours. Slow-release of amino acids provides better nitrogen retention and utilization by the body [23].
Nucleotides
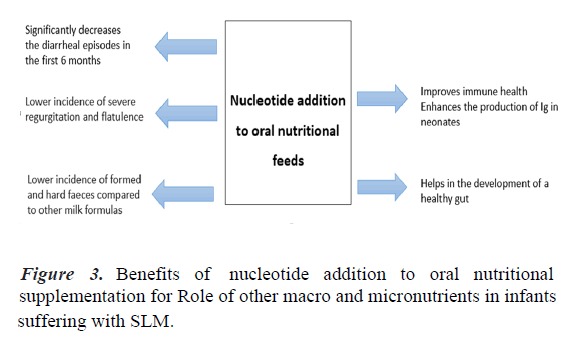
Importance of nucleotides in infant growth and development: Nucleotides present in human milk are ubiquitous compounds crucial for the repair of the gastrointestinal tract and the development of the neonatal immune function. During diarrhea, an intake of nucleotides might spare infants the metabolic cost of de novo synthesis or salvage and optimize their physiological function [24]. Numerous clinical studies have reported beneficial effects of added nucleotides to oral nutritional feed on gut microflora, diarrhea, and immune function [25].
In one study, nucleotide supplemented milk decreased the risk of diarrheal episodes during the first six months of life in healthy infants [24]. Another study showed that incorporating nucleotides to the oral nutritional feed improved the diversity of gut microbiota composition in formula-fed infants. Since this effect could contribute to previously described benefits for the gastrointestinal tract and immune function, these findings have important implications for optimizing the diet of formulafed infants [26]. Dietary nucleotides appear to exert actions on immature human neonate lymphocytes, enhancing the in vivo production of Immunoglobulins (Ig) which may have a role in the defence capacity of neonates [27]. Healthy term infants from 8 to 28 weeks of life are less likely to experience diarrhea. They have higher serum immunoglobulin A, concentrations with fortified nucleotide formula compared with a formula without added nucleotides [28]. A study showed that lower incidence of formed and hard faeces compared to other milk formulas (34.7% vs. 44.8%, p>0.001), and likewise a lower incidence of severe regurgitation (9.7 vs. 18.1%, p>0.001) and wind (62.7% vs. 72.5%, p>0.001) [29]. Thus, milk formula enriched with nucleotides has beneficial effects on the consistency of faeces, flatulence, and regurgitation.
Oral nutritional feed incorporated with nucleotides reduces the risk of a diarrheal episode
Both nucleotide-supplemented formula and mixed breastfeeding and nucleotide-supplemented formula-fed infants show this favourable impact. Though nucleotides are significantly more abundant in human milk than in formula milk, nucleotides boost infant's immunological defences via boosting cellmediated immunity. Infants who are given formula that has been Fortified with Nucleotides (NF) at levels comparable to the free nucleotides found in human milk (10 mg/L–29 mg/L) are shown to have improved immunological function and lesser incidence of diarrhea [30,31].
Nucleotides help in improving the immune function and influence the gut health positively
Dietary nucleotides are immunomodulatory and improve immunity, evident in preclinical and in vitro studies. The mechanism is unprecise, but it is hypothesized that lymphoid cells require an exogenous supply of nucleotides for optimal metabolism and function. Dietary nucleotides enhance lymphocyte proliferation, thereby improving immunity. The effects of nutritional nucleotides are also seen in the gut including the differentiation and growth of the immature neonatal heart. Dietary nucleotides reduce the risk of invasive bacterial proliferation in the developing gastrointestinal tract and systemic infection.
Nucleotide enhances mononuclear function, increases the antibody response, and increases IgM and IgA plasma levels, including immunity. Stimulated production of long chain polyunsaturated fatty acids and increased serum lipoprotein concentration [25].
Incorporating nucleotides to nutritional feed improves the composition of the gut microbiota in formula-fed infants. Because this effect could contribute to previously described benefits of added nucleotides for the gastrointestinal tract and immune function, these findings have important implications for optimizing the diet of formula-fed infants [26]. Numerous clinical studies have reported beneficial effects of added nucleotides to oral nutritional feed on gut microflora, diarrhea, and immune function. One study has reported better catch- up growth in term infants with severe intrauterine growth retardation (Figure 3) [25].
Vitamins and minerals
Zinc supplementation is critical for treating diarrheal episodes in children. Recent research indicates that taking zinc and using new oral rehydration solutions with low osmolarity can shorten the length and lessen the impact of diarrhea episodes for a period of up to three months. [32]. The mechanism of action of zinc in the management of diarrhea is not entirely understood. It is also most likely to improve the absorption of fluids from the intestine, helping with the clearance of organisms and supporting regeneration and mucosal integrity. It is expected to have an immunity-related mechanism [33]. Diarrhoea can lead to zinc deficiency due to reduced dietary intake and increased stool loss. Zinc contributes to the early regeneration of intestinal mucosa, the restoration of enteric enzymes, and the enhancement of humoral and cellular immunity, all of which help with water and electrolyte absorption [34]. Supplementing with zinc was found to shorten the length and lessen the intensity of episodes of diarrhea, as well as decrease the frequency of follow-up infections, for a period of 2 to 3 months. Generally, zinc supplements (zinc sulfate, zinc acetate, or zinc gluconate) are accepted by children and adults [35].
Interventions to prevent diarrhea, including safe drinking water, improved sanitation, and handwashing with soap, can reduce disease risk. Diarrhea should be treated with Oral Rehydration Solution (ORS), a solution of clean water, sugar, and salt. In addition, a 10-14 day supplemental treatment course of dispersible 20 mg zinc tablets shortens diarrhea duration and improves outcomes (Table 1) [5].
| Study design | Country | N | Clinical condition | Mean age | Intervention | Dose and duration of treatment | Outcome measures | Results | Ref |
|---|---|---|---|---|---|---|---|---|---|
| RCT | United Kingdom | 116 | Healthy infant | 20.4 week | Nucleotide-supplemented, breastfed, control formula | 31 mg/L birth until age 20 weak | The ratio of Bacteroides-Porphyromonas-Prevotella group (BPP) to Bifidobacterium species | The ratio of BPP to Bifidobacterium spp. rRNA to the nucleotide-supplemented formula was lower than control formula (mean difference: -118%; -34%; P=0.007) | [26] |
| RCT | Italy | 3315 | Healthy infants | - | Nucleotide-supplemented formula, formula without nucleotide supplementation, mixed breastfeeding and nucleotide-supplemented formula, mixed breastfeeding, and formula without nucleotide supplementation | 1st month up to the end of the 3rd month of life | Growth-length, weight, and mass index | The incidence of diarrhea was significantly lower in the nucleotide-supplemented formula group than in the formula without nucleotide supplementation group (RR=0.567) | [24] |
| RCT | Spain | 27 | Preterm infants | 32.8 ± 1.8 weeks gestation | Milk formula, formula supplemented with CMP, AMP, UMP, GMP | First three months of life | Lymphocyte subsets and plasma IgG, IgM, and IgA levels | Infants fed the nucleotide formula exhibited higher plasma levels of IgM and IgA | [27] |
| DB, RCT | Taiwan | 336 | Healthy infants | 1 to 7 day-old | Infant formula Fortified with Nucleotide (NF), Control Formula (CF) | 12 months | Incidence of diarrhea, RTIs, serum immunoglobulin concentrations, and response to hepatitis B vaccine. | 1. In NF-reduced risk of diarrhea from 8 to 48 weeks of age and a significantly lower risk of 25.4% (P=0.05) between 8 and 28 weeks. | [28] |
| 2. increased risk of upper RTIs | |||||||||
| Prospective, observational study | Italy | 5009 | Healthy infants | Aged between new-born and four months old | Formula enriched with nucleotides | 14 days | Number and consistency of feces, frequency of regurgitation, and wind | Lower incidence of formed and hard feces compared to other milk formulas (34.7% vs. 44.8%, p>0.001), and likewise a lower incidence of severe regurgitation (9.7 vs. 18.1%, p>0.001) and wind (62.7% vs. 72.5%, p>0.001). | [29] |
| RCT | Chile | 284 | Infants | 0-1 year | Nucleotide-enriched formula and formula without nucleotide | One year | Episodes of diarrhea | Infants fed with nucleotide-enriched formula showed significantly fewer episodes of diarrhea and the total number of days with the disease (p<0.05 and p<0.0000, respectively). | [45] |
| DB, RCT | Brazil | 81 | Male infants | >1 year and <1 year | Nucleotide-enriched dietary supplement and non-supplemented formula | - | Stool output, diarrhea episodes, body weight | Stool output and duration of diarrhea were not significantly different between both groups | [46] |
| Observational cohort study | Spain | 3243 | Infant | - | Nucleotide supplements | Until six months of age | Duration and severity of each episode of diarrhea, height and weight, Nutritional Index (NI) | 1. The incidence of diarrhea was significantly lower (p<0.001) in the nucleotide supplemented group (11.1%) vs. the unsupplemented group (17.4%). 2.The maximum duration of diarrhea (p<0.05) and the severity of episodes of diarrhea (p<0.001) were significantly lower in the nucleotide supplemented group |
[47] |
| Note: Randomized Controlled Trial (RCT); Data Base (DB). | |||||||||
Table 1. Clinical trial summary of supplemented nucleotides.
Vitamins play several important physiological roles in maintaining gut microbiota and excluding opportunistic microbes leads to vitamin A deficiency even weeks to months after the diarrheal episode. Vitamin A deficiency leads to transient immune compromise and ineffective mucosal repair, thus perpetuating. Therefore, vitamin A is beneficial in children with diarrhea [36]. Vitamin D Receptors (VDRs) can be located on immune cells such as T cells, B cells, and dendritic cells. Still, they are also highly expressed in intestinal enterocytes. Vitamin D receptors function as transcription factors, promoting the secretion of antimicrobial peptides and tight junction proteins that preserve the integrity of the intestinal epithelial barrier. Vitamin D plays a significant role in innate and adaptive immunity by promoting the toll-like receptor signaling of macrophages in response to pathogens and inhibiting the proliferation of T cells and secretion of inflammatory cytokines [37]. Thus, vitamin D is useful in boosting immunity and help fight infection in children with diarrhea.
Discussion
Ensuring adequate calcium intake throughout the lifespan is essential to building and maintaining bone. Lactose intolerance may predispose individuals to low calcium intake as many lactose-free, calcium-rich food sources are limited. In immune-compromised children, calcium administration might effectively “halt” viral and parasitic diarrhea. Furthermore, calcium supplementation can reduce the severity and duration of bacterial diarrhea caused by Escherichia coli [38].
Relatively low intakes of phosphorus, as occur with human milk, may confer an advantage to the infant by the low residual phosphorus in the lower bowel. Low intestinal phosphorus reduces the faecal pH, thereby reducing the proliferation of potentially pathogenic microorganisms. Thus phosphorous provides an immune protective effect [39].
Multivitamins and minerals should be given to all children twice the RDA for 2-4 weeks. Iron supplements should never be started in the acute phase of diarrhea. Iron supplementation could induce diarrhea by causing intestinal damage through oxidative stress or initiating bacterial dysbiosis and gut inflammation. Iron reduces the quantity of bifid bacteria, causing a change in the gut microbial balance away from beneficial strains that provide a barrier towards a profile that is potentially more harmful. [34,40,41].
Carbohydrate malabsorption of both monosaccharide and disaccharide contribute to post-enteritis problems in these infants [42]. High consumption of commercial sugar alcohols can result in gastrointestinal upset, including excessive gas, cramping, and diarrhea [43].
Medium Chain Triglycerides (MCTs) are an easy and quick source of energy. MCTs also help better absorb fats in infants due to the immaturity of their digestive tract and high energy demand. The proportion of MCT is undoubtedly crucial for potential deficiencies [44]. Lipids provide about 50% of an infant's daily calorific intake. Most lipids (98%) are triglycerides while the rest consist of diglycerides, monoglycerides, free fatty acids, cholesterol, and phospholipids. Long-chain Polyunsaturated Fatty Acids (PUFAs) are essential for developing an infant's central nervous system and retinas [45-47].
Conclusion
Adequate nutrition for diarrhea children is a crucial adjunct to therapy since antibiotics and other medications play only a “limited impact.” Lactose-free oral nutritional supplement consumption during acute gastroenteritis would significantly reduce the duration and severity of diarrhea in infants. A casein- based therapeutic nutritional solution with essential micro and macronutrients would benefit infants with secondary lactose intolerance. Fats are essential for reducing transit and gastric time via hormonal mechanisms. Fat intake of less than 3 g/kg/d may contribute to a toddler's diarrhea, especially in excessive free fluid and carbohydrate intake (e.g., large amounts of fruit juice).
Statement and Declaration
Funding
The Article Processing Charges (APC) was paid by Nutricia International Pvt. Ltd. (Danone India).
Conflict of interests
Tanvi Paryani is an employee of Nutricia International Pvt. Ltd. (Danone India).
Author contribution
All authors contributed to the content of the manuscript. All authors read and approved the final manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Ethics approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Acknowledgment
The authors would like to thank Dr. Amit Khandeparkar of Nutricia International Pvt. Ltd. (Danone India) and Ms. Kruti Shah for reviewing the manuscript. The authors also acknowledge Intellimed Healthcare Solutions LLP for medical writing support.
Data availability
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
Consent to participate
N/A
Consent to publish
N/A
References
- Yadav S, Rawat AKR, Singh HP, et al. Study of lactose intolerance in children below 24 months. J Evol Med Dent Sci 2013; 24(2): 3704–9.
- Misselwitz B, Butter M, Verbeke K, et al. Update on lactose malabsorption and intolerance: Pathogenesis, diagnosis and clinical management. Gut 2019; 68(11): 2080–91.
- Heyman MB. Lactose intolerance in infants, children, and adolescents. Pediatrics 2006; 118(3): 1279–86.
- Nabulsi M, Yazbeck N, Charafeddine F. Lactose-free milk for infants with acute gastroenteritis in a developing country: Study protocol for a randomized controlled trial. Trials 2015; 16: 46.
- WHO. Diarrhoeal disease. 2017.
- Lakshminarayanan S, Jayalakshmy R. Diarrheal diseases among children in India: Current scenario and future perspectives. J Nat Sci Biol Med 2015; 6(1): 24-8.
- Girish Kumar CP, Giri S, Chawla-Sarkar M, et al. Epidemiology of rotavirus diarrhea among children less than 5 years hospitalized with acute gastroenteritis prior to rotavirus vaccine introduction in India. Vaccine 2020; 38(51): 8154–60.
- Deng Y, Misselwitz B, Dai N, et al. Lactose intolerance in adults: biological mechanism and dietary management. Nutrients 2015; 7(9): 8020–35.
- Misselwitz B, Pohl D, Frühauf H, et al. Lactose malabsorption and intolerance: Pathogenesis, diagnosis and treatment. United Eur Gastroenterol J 2013; 1(3): 151–9.
- Grenov B, Briend A, Sangild PT, et al. Undernourished children and milk lactose. Food Nutr Bull 2016; 37(1): 85–99.
- Davidson GP, Goodwin D, Robb TA. Incidence and duration of lactose malabsorption in children hospitalized with acute enteritis: Study in a well-nourished urban population. J Pediatr 1984; 105(4): 587–90.
- Koletzko S, Osterrieder S. Acute infectious diarrhea in children. Dtsch Ärztebl Int 2009; 106(33): 539–48.
- Heine RG, AlRefaee F, Bachina P, et al. Lactose intolerance and gastrointestinal cow’s milk allergy in infants and children–common misconceptions revisited. World Allergy Organ J 2017; 10(1): 41.
- Duggan C, Santosham M, Glass RI. The management of acute diarrhea in children: Oral rehydration, maintenance, and nutritional therapy. Centers for disease control and prevention. MMWR Recomm Rep 1992; 41(RR-16): 1–20.
- Parekh B, Basavaraja G, Ugra D. Under auspices of IAP action plan 2020–2021.
- Szajewska H, Hoekstra JH, Sandhu B, et al. Management of acute gastroenteritis in europe and the impact of the new recommendations: A multicenter study. The working group on acute diarrhoea of the European society for paediatric gastroenterology, hepatology, and nutrition. J Pediatr Gastroenterol Nutr 2000; 30(5): 522–7.
- King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: Oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep 2003; 52(RR-16): 1–16.
- MacGillivray S, Fahey T, McGuire W. Lactose avoidance for young children with acute diarrhoea. Cochrane Database Syst Rev 2013; 2013(10): CD005433.
- Haschke F, Haiden N, Thakkar SK. Nutritive and bioactive proteins in breastmilk. Ann Nutr Metab 2016; 69(Suppl 2): 17–26.
- Fox PF, Brodkorb A. The casein micelle: Historical aspects, current concepts and significance. Int Dairy J 2008; 18(7): 677–84.
- Nguyen TTP, Bhandari B, Cichero J, et al. Gastrointestinal digestion of dairy and soy proteins in infant formulas: An in vitro study. Food Res Int Ott Ont 2015; 76(Pt 3): 348–58.
- Huppertz T, Chia LW. Milk protein coagulation under gastric conditions: A review. Int Dairy J 2021; 113: 104882.
- Hoffman JR, Falvo MJ. Protein–Which is best? J Sports Sci Med 2004; 3(3): 118–30.
- Merolla R, Gruppo Pediatri Sperimentatori. Evaluation of the effects of a nucleotide-enriched formula on the incidence of diarrhea. Italian multicenter national study. Minerva Pediatr 2000; 52(12): 699–711.
- Yu VYH. Scientific rationale and benefits of nucleotide supplementation of infant formula. J Paediatr Child Health 2002; 38(6): 543–9.
- Singhal A, Macfarlane G, Macfarlane S, et al. Dietary nucleotides and fecal microbiota in formula-fed infants: A randomized controlled trial. Am J Clin Nutr 2008; 87(6): 1785–92.
- Navarro J, Maldonado J, Narbona E, et al. Influence of dietary nucleotides on plasma immunoglobulin levels and lymphocyte subsets of preterm infants. BioFactors 1999; 10(1): 67–76.
- Yau KIT, Huang CB, Chen W, et al. Effect of nucleotides on diarrhea and immune responses in healthy term infants in Taiwan. J Pediatr Gastroenterol Nutr 2003; 36(1): 37–43.
- Alvaro G. Evaluation of the effect of a new milk formula with added nucleotides on some gastrointestinal functions. Results of a nation-wide study of 5009 infants. Pediatric Study Group. Minerva Pediatr 1998; 50(7–8): 347–58. [Crossref]
- Carver JD, Pimentel B, Cox WI, et al. Dietary nucleotide effects upon immune function in infants. Pediatrics. 1991; 88(2): 359–63.
- Brunser O, Espinoza J, Araya M, et al. Effect of dietary nucleotide supplementation on diarrhoeal disease in infants. Acta Paediatr 1994; 83(2): 188–91.
- Chaitali B, Vijay T. Role of zinc in pediatric diarrhea. Indian J Pharmacol 2011; 43(3): 232-5.
- Goldman RD. Zinc supplementation for acute gastroenteritis. Can Fam Physician 2013; 59(4): 363–4.
- Satish T, Akash B. Nutritional support in diarrhea. 2009.
- Study of lactose intolerance in children below 24 months.
- Suraj G. Recent Advances in Pediatrics: Pediatric gastroenterology hepatology and nutrition. Jayapee Brothers Medical Publisher. Special Volume 23.
- Drall KM, Field CJ, Haqq AM, et al. Vitamin D supplementation in pregnancy and early infancy in relation to gut microbiota composition and C. difficile colonization: Implications for viral respiratory infections. Gut Microbes 2022; 12(1): 1799734.
- Cheng SX, Bai HX, Gonzalez-Peralta R, et al. Calcium ameliorates diarrhea in immune compromised children. J Pediatr Gastroenterol Nutr 2013; 56(6): 641–4.
- Institute of medicine (US) standing committee on the scientific evaluation of dietary reference intakes. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin d, and fluoride. National Academies Press (US) 1997;
- Jaeggi T, Kortman GAM, Moretti D, et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 2015; 64(5): 731–42.
- Ghanchi A, James PT, Cerami C. Guts, germs, and iron: A Systematic review on iron supplementation, iron fortification, and diarrhea in children aged 4–59 months. Curr Dev Nutr 2019; 3(3): nzz005.
- Stern M. Secondary carbohydrate and protein intolerances following gastroenteritis. Monatsschrift Kinderheilkd 1989; 137(9): 585–9.
- Łoś-Rycharska E, Kieraszewicz Z, Czerwionka-Szaflarska M. Medium Chain Triglycerides (MCT) formulas in paediatric and allergological practice. Prz Gastroenterol 2016; 11(4): 226–31.
- Nunes L, Martins E, Perron