Research Article - Journal of Fisheries Research (2022) Volume 6, Issue 3
Novel Xanthic phenotype of the silver carp, Hypothalamichthyes molitrix (Valenciennes, 1844) identification and characterization.
Pawar RT1*, Rankhambb SV2, Pathan TS3
1Department of Zoology, Sunderrao Solanke Mahavidyalaya, Majalgaon, Dist. Beed (MS), Maharashtra, India
2Department of Zoology, Late Ramesh Warpudkar ACS College, Sonpeth Dist. Parbhani (MS), Maharashtra, India
3Department of Zoology, Kalikadevi Arts, Commerce and Science College, Shirur Kasar, Dist. Beed (MS), Maharashtra, India
- *Corresponding Author:
- Pawar RT
Department of Zoology, Sunderrao Solanke Mahavidyalaya
Majalgaon, Dist. Beed (MS)
Maharashtra, India
E-mail: drrajtpawar@gmail.com
Received: 21-Apr-2022, Manuscript No. AAJFR-22-61408; Editor assigned: 25-Apr-2022, PreQC No. AAJFR-22-61408(PQ); Reviewed: 17-May-2022, QC No. AAJFR-22-61408; Published: 24-May-2022, DOI: 10.35841/aajfr-6.3.111
Citation: Pawar RT. Novel xanthic phenotype of the silver carp, Hypothalamichthyes molitrix (Valenciennes, 1844) identification and characterization. J Fish Res. 2022;6(3):111
Abstract
In this study Xanthic phenotype of the silver carp Hypophthalmichthys molitrix was identified and characterized. In the course of observation, we discovered eight brood fishes were infected with X. phenotype. The five malformed specimen’s complete body become yellow in shade and three specimens was malformed on body, head, eyes and fins that are having a faint yellowish and orange color. In the bizarre specimens, red to orange spots replacing the dark spots found in the ordinary specimens. At the same time as other deformities such as distorted lateral lines were seen in one of the abnormal specimen.
Keywords
Cyprinidae, Xanthism, Silver carp.
Introduction
Xanthism in fishes has a genetic basis that causes strange skin colouration of yellow to orange-gold. Xanthochromic specimens are rare found in nature. Certainly one of them Silver carp Hypophthalmichthys molitrix (Valenciennes) is an exotic carp belong to the Cyprinidae family which was brought in India during 1959 for enhancement in aquaculture sector and increasing yields. It was originally described as species of the genus Leuciscus and ultimately located in the genus Hypophthalmichthys [1]. This species is native to Eastern Asia and has been broadly introduced to Southern Asia, Europe and North America [2]. The xanthic phenotype has an impact at the survival of the individuals bearing this situation, where they are conspicuous to the predator more than those individuals with normal coloration [3]. This unfortunate occurrence of visibility makes the xanthic individuals within each species rare [4,5].
Yellow or xanthic (gold) coloration in fishes is attributed to xanthophores, which includes carotenoids [6,7]. The lipid soluble carotenoid pigments are dietary in starting place and stored in the middle of the pterinosomes in vesicles of different sizes. These vesicles possess an extremely thin membrane extending from or in direct contact with the endoplasmic reticulum [8]. Zeaxanthin and lutein carotenoids are responsible for the yellow coloration [9]. In addition to carotenoids, xanthophores also have yellow and colorless pteridines enclosed inside pterinosomes. The Xiphophorine fish’s content material xantho-erythrophore, is a single cell with both pteridine and carotenoid [10]. Its yellow compound contains carotenoids while pteridines are found within the red periphery.
Several groups of freshwater fish have been shown to have different cases of xanthism consisting members of the families Centrachidae, Cichlidae, Cyprinodontidae, Lepisosteidae, Percidae, Poeciliidae, Gadiformes [4,11-17]. Xanthism is most prevalent in order Cypriniformes [18-21]. Such deviations in pigmentation were the basis for the breeding of ornamental species, crucian carp (goldfish) Carassius auratus common carp (koi) Cyprinus carpio, ide (orfe), Leuciscus idus var, orfus, Tench Tinca tinca [22,23] . In natural populations, there also are recognized instances of catching golden individuals of roach Rutilus rutilus, silver carp Hypophthalmichthys molitrix, mud loach Misgurnus anguillicaudatus [18, 20-22]. The phenomena of xanthism have a genetic basis that explains the huge range presence across a wide taxonomic spectrum [16]. Xanthism has characterized by a partialyl or predominant yellow skin or colour of the integument that affects small parts of populations of species [25,26]. The purpose of our work is to investigate the xanthochromic specimens from the Maharashtra fish seed production centre (MFSPC) and discuss the reason for the paucity of xanthochromic reviews.
Experimental work
All experiments were conducted at the Maharashtra Fish Seed Production Centre (MFSPC) Kesapuri camp, near Majalgaon Tahasil, Maharashtra, India. It is a well-known circular hatcheries and includes one breeding pond, three spawning tank, three nursery pond, six rearing pond and three stocking ponds. Its area is 12 acre, with pond size 150 × 100 × 6 and 180 cm high depth of 6 feet. At the MFSPC centre the seed production of three Indian major carps and two exotic carps are producing (Catla catla, Labeo rohita, Cirrhinus mrigala, Hypothalamichthyes molitric and Cyprinus carpio). Water source is having Majalgaon reservoir and wells (Figure 1). We were visited on four occasions (July and September -2018 as well as August and October -2019). Visual observations had been made within the uniformly clear stocking pond water. Specimens were collected through the use of gill net. Immediately after captured, a subset of individuals turned into positioned in a viewing apparatus a good way to recorded xanthic phenotype and colour traits of live fish. In addition body and fins were examined carefully for external parasites, malformations, amputations and any other morphological difference. The specimens were kept for frozen in the Maharashtra Fish Seed Production Centre, Maharashtra, India.
Results
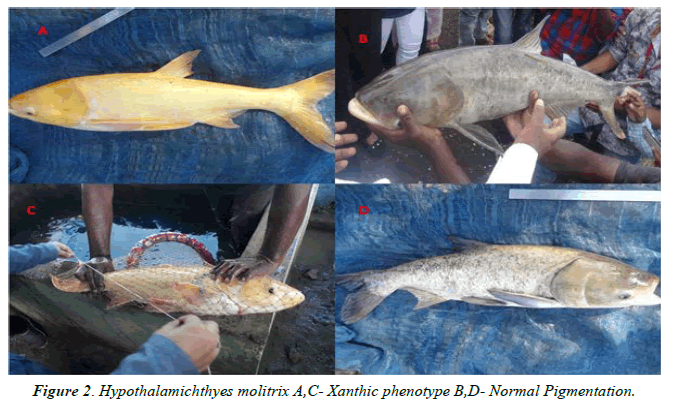
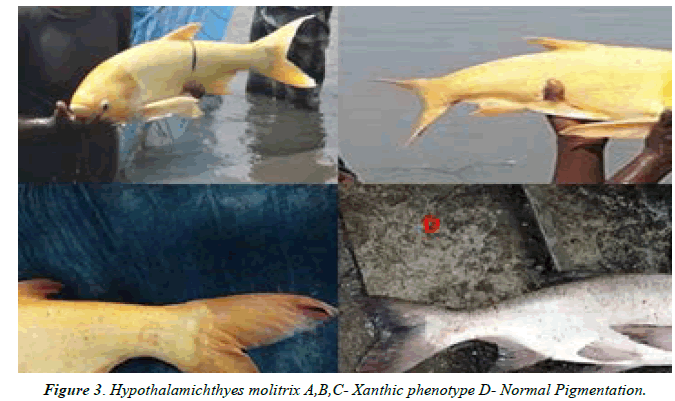
The Xanthic phenotype was observed from the eight specimens of Hypothalamichthyes molitrix on the basis of visual investigation in field visit. Among the eight specimen five were infected with xanthic phenotype as yellow colour is covering the entire body with faint orange colour on head, caudal fin except the eye, all different fins and within the typical specimens of Silver carp are olive green in colour on their dorsal side and silver on the ventral side (Figures 2 and 3). While three specimens were malformed with xanthic phenotype on body, head, eyes and fins which is having a faint yellowish and orange coloration (Figure 4). In the unusual specimens, red to orange spots replacing the dark spots discovered. Other deformities which include distorted lateral lines were noted in one of the abnormal specimens is given under based on the actual remarks and in comparison with that of the regular specimen. Xanthic specimens have proven a indistinct lateral line which is observed in the ascending part of the lateral line posterior to the top head and in the area dorsal to the anal fin.
It is known that the orange or yellow colour of the integument of the body of fish is due to the content of carotenoid pigments or pterins in xanthophore cells [23].
Discussion
In fishes, the xanthophores to facilitate showed the golden colours are referred to as the xanthophores. The presence of the melanic chromatophores are normally masks the outward display of the xanthophores, however with the whole disappearance of the preceding investigated from any region of the fish body, xanthophores became found out and the case recognized as xanthochromism. In such circumstances, the chromatophores contain E-carotene and canthaxanthin, and import a golden colouration [12]. It has been suggested to facilitate xanthophores may properly even be over-produced within the nonexistence of melanophores or other pigment cells [16]. The closest colour variants amongest cyprinids were previously determined in the common roach in a number of water bodies of the European part of Russia and in the Rutilus heckelii [20]. Other species of this family in natural populations, characterized by the presence of individuals with an aberrant xanthic colouration, in most instances, have a more intense orange colour and the cornea of the eye, bearing clusters of dark pigment spots [18, 22-24]. The complete disappearance of the melanic chromatophores in xanthism in each marine and freshwater fishes was observed in a number of fish species [5,21-27]. Unlike additional studies, the present xanthic phenotype has no longer surfaced on its body that maintain the original coloration.
This means that a mutation in the gene for xanthophores in all body areas has been taken place that leads to a lost in the black pigments reported some mutation in about 7 genes would lead to faint or dark yellow colour [28-30]. Mutations may be affect the nervous system and causes a reversible colour change, which leads to permanent colour patterns. According to a number of researchers, variations in xanthic colouration, in which an individual has no or practically no normally coloured body parts, are caused by a recessive mutation of the xanthophore genes [21,28] . According to Jorge San Gil-Leon reported Xanthism in Costa Rican Cichlid fish (Cichliformes: Cichlidae), and ontogenetic variation in Parachromis dovii [31-35]. He has concluded eight species of Costa Rican fish show xanthism, and a genetic (heritable) origin is presumed; this was corroborated in the captive breeding exercise. In present study the xanthic pigmentation was found in eight specimens of H. molitrix appeared too permanent and is almost certainly the effect of a single gene recessive mutation [36- 40]. Because the occurring of this xanthic pigmentation of H. molitrix was not reported in different areas of the globe, this variation appears to have occurred locally and subsequently spread throughout the relatively isolated gene pool.
Conclusion
Later on no record of xanthic phenotype for any fish species was reported from India and in particular for the silver carp H. molitrix. Therefore, the present study is important as it is report for the increasing appearance of the xanthic phenotype in reared H. molitrix specimens. In the future, the study of the mechanisms of the emergence and inheritance of such colour variants in bream can serve as a basis for breeding decorative forms of this species, by analogy with trout, rudd, carp, ide, or other species.
References
- Oshima M. Contributions to the study of fresh water fishes of the island of Formosa. Ann Carnegie Mus. 1919;12:169-328.
- Kolar CS, Chapman DC, Courtenay WR, et al. Bigheaded Carps: A Biological Synopsis and Environmental Risk Assessment. Amer Fisheries Society: Bethesda, MD, USA. 2007;13(2):419-21.
- Endler JA. Natural selection on color patterns in Poecilia reticulata. Evol.1980;34:76-91.
- Turner BJ, Liu RK. Xanthic variants in a natural population of the Salt Creek Pupfish, Cyprinodon salinus. Southwest Nat. 1977;22:538-40.
- Pattengill-Semmens CV. Occurrence of a unique color morph in the smooth trunkfish (Lactophrys triqueter L.) at the Flower Garden Banks and Stetson Bank, northwest Gulf of Mexico. Bull Mar Sci. 1999;65:587-91.
- Fujii R. Chromatophores and pigments. Fish Physiology. Academic Press, New York, USA. 1969; 3:307-53.
- Fujii R. Cytophysiology of fish chromatophores. Inter Rev Cytol: A Sur of Cell Bio.1993;143:191-255.
- Obika M. Formation of pterinosomes and carotenoid granules in xanthophores of the teleost Oryzias latipes as revealed by the rapid freezing and freeze-substitution method. Cell Tissue Res. 1993;271:81-6.
- Goodrich HB. A study of the development of Mendelian characters in Oryzias latipes. J Exp Biol. 1927;49:261-87.
- Valenti RJ. A Qualitative and Quantitative Study of Red and Yellow Pigmentary Polymorphism in Xiphophorus. Ph.D. dissertation, New York Univ, USA. 1973.
- Allen ER, Neill WT, Xanthic largemouth bass (Micropterus) from Florida. Copeia. 1953;2:116–117.
- Webber R, Barlow GW, Brush AH, Pigments of a color polymorphism in a cichlid fish. Comp Biochem Physiol B. 1973;44 B:1127–35
- Mcilwain TD, Waller R. A xanthochroic gar, Lepisosteus oculatus, from Mississippi.Trans Am Fish Soc. 1972;101:362.
- Tyler D. Xanthochroistic gar in Oklahoma. Southwest Nat. 1990;35:225
- Denoncourt RF. Sexual dimorphism and geographic variation in the bronze darter, Percina palmaris (Pisces: Percidae). Copeia. 1976;12(1):54-9
- Angus RA, Blanchard PD, Genetic basis of the gold phenotype in sailfin mollies. J Heredity. 1991;82(5):425-8.
- Quigley DTG, Lord R, MacGabhann D, et al. First records of xanthochromism in three-bearded rockling Gaidropsarus vulgaris (Cloquet 1824) and pollack Pollachius pollachius (Linnaeus 1758). J App Ichthyol. 2017;33:1208-10.
- Kobayasi H. On the colour variations of the mud loach, Misgurnus anguillicaudatus (Cantor). J Facul of Sci. Hokkaido University. Series 6, Zool. 1957;13:63–66.
- Vekhov DA. Population of silver crucian carp Carassius auratus (Cypriniformes, Cyprinidae) with golden individuals in the pond of the city of Volgograd. J Ichthyol. 2008;48:326-35.
- Podushka SB. Colour aberrations of the roach. 2013;25.
- Pawar RT, Jawad LA, First report of a xanthic phenotype of the silver carp, Hypophthalmichthys molitrix (Valenciennes, 1844) (Teleostei: Cyprinidae) from Maharashtra Fish Seed Production Centre, India. J Internat Aquacul. 2017;3:101–105.
- Koopmans JH, Van Emmerik WAM. Knowledge paper bindweed, Leuciscus idus (Linnaeus, 1758). Sport Fishing Netherlands Bilthoven. 2004;50.
- Yarzhombek AA, Zhukova KA. Red and orange skin colour of fish. Trudy VNIRO. 2018;170:124–129.
- Kvasnicka P, Flajshans M, Rab P, et al. Inheritance studies of blue and golden varieties of tench. (Pisces: Tinca tinca L.). J Heredity. 1998;89(6):553-6.
- Smith CL. A revision of the American groupers: Epinephelus and allied genera.Bull Am Mus Nat Hist.1971;146: 67-242
- Béarez P, Trevi-o H, Huamani I, A case of partial xanthism in Aplodactylus punctatus (Teleostei: Aplodactylidae) from southern Peru. Rev Peru Biol. 2006;13(1):113-5.
- Palacios-Salgado DS, Rojas-Herrera AA. Scientific Note Partial xanthism in a specimen of Acapulco major, Stegastes acapulcoensis (Teleostei: Pomacentridae), from the Tropical Eastern Pacific. Pan-Am J Aquat Sci 2012;7:175-7.
- Lister JA, Robertson CP, Lepage T, et al. Nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development. 1999;126(17): 3757-67.
- Watanabe M, Kondo S. Is pigment patterning in fish skin determined by the Turing mechanism? Trends Genet. 2015;31:88-96.
- Odenthal J. Rossnagel K. Haffter P. et al. Mutations affecting xanthophore pigmentation in the zebrafish, Danio rerio. Development. 1996;123(1):391-98
- Jorge SGL, Arturo A. Xanthism in Costa Rican cichlid fishes (Cichliformes: Cichlidae), and ontogenetic variation in Parachromis dovii. J UNED Research (e-ISSN 1659-441X), 2021;13(1):3093.
- Dunham RA, Childers WF, Genetics and implications of the golden color morph in green sunfish. Prog Fish Cult. 1980;42:160–3
- Bagnara JT. Developmental aspects of vertebrate chromatophores. Am. Zool. 1983;23(3):465-78.
- Bagnara Béarez P, Trevi-o H, Huamani I, A case of partial xanthism in Aplodactylus punctatus (Teleostei: Aplodactylidae) from southern Peru. Rev Peru Biol. 2006;13(1):113-5.
- Daniel G, Maria CF, Yaron T, The occurrence of two xanthochromic fish, Epiniphelus marginatus (Serranidae) and Diplodus vulgaris (Sparidae) (Osteichthyes) in the eastern Mediterranean. Zool Middle East. 2019;65:3,215-220,
- Kottelat M, Freyhof J. Handbook of European freshwater fishes. Kottelat, Cornol and Freyhof, Berlin. 2007; 646.
- Popov PA. Distribution of cyprinid fish in the reservoirs of the Siberian subarctic region. Contemporary Problems of Ecology (2015). 8:65-71.
- Singh AK, Lakra WS. Risk and benefit assessment of alien fish species of the aquaculture and aquarium trade into India. Rev Aquac. 2011;3:3-18
- Valencia-Méndez O. Domínguez-Domínguez O, López-Pérez A, et al. Partial albinism in the Revillagigedo sea chub Kyphosus sectatrix (Perciformes: Kyphosidae) from Clarion Island, Mexico. Revista Mexicana De Biodiversidad. 2018;89:572–576.
- Zuev IV, Vyshegorodtsev AA, Chuprov SM, et al. Modern composition and distribution of alien fish species in the water bodies of the Krasnoyarsk Territory. Russ J Biol Invasions. 2016;7:324–332.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref