Research Article - Biomedical Research (2017) Volume 28, Issue 8
Nanoparticle methotrexate delivery system for the treatment of paediatric patients with inflammatory bowel disease
Gang Liu, Dun-Chen Li, Ping-Ping Li, Ran-Ran Li and Shu-Ying Chen*Department of Paediatrics, Affiliated Hospital of Taishan Medical University, Shandong 271000, PR China
- *Corresponding Author:
- Shu-Ying Chen
Taishan Medical University, 706 Taishan Street
Taian, Shandong 271000, PR China
Accepted date: April 28, 2016
Abstract
The purpose of this study to evaluate the efficacy and safety of methotrexate (MTX) nanoparticle in paediatric patients with inflammatory bowel disease (IBD). The paediatric patients with moderate to severe IBD were enrolled in randomized, open-label clinical study. All eligible presents randomized in two groups. One group is treated with MTX nanoparticle (15 mg/week) while other groups were received azathioprine (AZA) (2 mg/kg/day). The nanoparticle was synthesized according to the procedure previously described. The erythrocyte sedimentation rate, Creactive protein, aspartate aminotransferase, alanine transaminase and disease activity score were used to determine the remission. Total 28 patients were enrolled in the study and randomized into two arms. After 12 weeks of therapy, the mean PCDAI for control and treatment groups were 22.3 ± 2.14 and 16.8 ± 1.87, respectively. Similarly, the mean PUCAI for control and treatment groups were 24.3 ± 1.47 and 18.7 ± 1.92, respectively. At the end of 12 weeks, eight patients in the treatment and five patients in the control group achieved remission. Conclusion: As per our knowledge, our study is the first to evaluate the safety and efficacy of nanoparticle formulation in paediatric IBD patients. We conclude that the MTX nanoparticle safe and more effective as compared to the slandered formulations.
Keywords
Paediatric patient, Methotrexate nanoparticle, Inflammatory bowel disease, Azathioprine
Introduction
Inflammatory bowel disease (IBD) is a multi-factorial disorder characterised by inappropriate immune response which represents a group of inflammatory intestinal idiopathic and chronic diseases, which includes Crohn’s disease (CD) and Ulcerative colitis (UC) [1]. The IBD may present at any age; however, the peak period of presentation for UC and CD is the second and third decades of life, particularly in adolescence [2,3]. Over a recent decade, a growing incidence of IBD, mainly of CD, has been reported in early age [1-4].
The treatment strategies for IBD in children have dramatically changed over the past decade. The aim of therapy to control the chronic inflammatory process [5,6]. The role of immunomodulators and biologics like anti-tumour necrosis factor (TNF)-α are very important in the management of chronic inflammatory disorders, mainly the diseases, which are refractory to traditional medications [5-9]. However, the intolerance or unresponsiveness to immunomodulators like azathioprine (AZA) and 6-mercaptopurine (6MP) occurs in 30% to 50% of patients [10]. The patients unresponsive to an immunomodulator were treated with an alternative immunomodulator like methotrexate (MTX) or to introduce a biologic treatment (anti-TNF α) [11]. The MTX shown to be effective in induction and maintenance of remission in adult, as well as paediatrics patients with CD [12,13]; however, it did not show any advantage over placebo in UC [14]. Even though, few retrospective studies have demonstrated a clinical response in 60%-70% of patients with UC [15,16].
The effect of MTX in refractory inflammatory bowel disease (IBD) was first reported by Kozarek et al. [17]. Five controlled and several uncontrolled studies have demonstrated the effectiveness of MTX in inducing remission of steroid-dependent or chronic active CD [18-20], and as a steroid-sparing agent in refractory IBD. The reduction in the steroid use could also reduce the steroid-related adverse effects in the patients with chronic IBD; however high dose MTX are associated with some common side effects including nausea, vomiting, headache, abdominal pain, diarrhoea and skin rash. The active ingredient can be delivered to the site of inflammation to overcome this limitation. A site-directed targeting may result in higher drug concentrations at the site of inflammation, less unintended systemic drug absorption, and eventually less adverse effects.
Now a days, the targeted deliveries of drugs to specific anatomical sites by nanoparticle have turned into be promising tools for the management of chronic inflammatory diseases. The nanoparticles are designed to control drug release after oral administration; the development of such nanoparticles may reduce the dosage frequency as well as adverse effects of the drug. Furthermore, the nanoparticles may protect encapsulated drugs from mucosal metabolism; consequently, an increased intracellular drug concentration was achieved [21].
Alginic acid, and sodium and potassium alginates have emerge as one of the most widely used mucoadhesive biomaterials because of their good biocompatibility, biodegradation [22], sol-gel transition properties, and chemical versatility that make it possible for further modifications [23]. From a regulatory prospective, the US Food and Drug Administration (US-FDA) recognizes alginates as a “Generally Referred As Safe” (GRAS) material, a designation that applies to substances accepted as safe for alimentary use [24]. GRAS excipients are listed in the Code of Federal Regulations Title 21 (21 CFR) parts 182 and 184 [25]. Due to very good biocompatibility, compatibility for oral use and USFDA approval the alginate is extensively used in the development of nanomedicines.
In the present study our aim to evaluate the safety and efficacy of MTX-alginate nanoparticles in paediatric patients with inflammatory bowel disease.
Experimental
Materials
Methotrexate was obtained from BBI (USA). Sodium Alginate with molecular weight 12000–40000 and poly-L-lysine were purchased from Sigma - Aldrich, Germany. Calcium chloride (CaCl2) and sodium chloride (NaCl) were supplied from Merck Chemicals Co. All the reagents used in this research were obtained as an analytical grade.
Patient selection and study design
A randomized, open-label, pilot clinical study was conducted at hospital of Taishan medical university. The study protocol was approved by the local ethics committee of Taishan medical university. Children and adolescents of age between 8 to 17 years with moderate to severe IBD were recruited in the study. The patients with Paediatric ulcerative colitis activity index (PUCAI) score >34 or Paediatric Crohn’s Disease Activity Index (PCDAI) >30 were included in this study. The patients with ileostomy or colostomy, toxic megacolon, prior sensitivity or allergy to methotrexate and who received anti-tumour necrosis factor in the previous 8 weeks were excluded from the study. The written informed consent was obtained from all the parents, and assent was obtained from all the participants. The study was conducted according to the International Conference on Harmonization (ICH) Good Clinical Practices (GCP) and in compliance with the Declaration of Helsinki 1975.
All eligible patients were randomized in to two groups. The patients of the treatment group received MTX nanoparticle and control group consisted of patients receiving azathioprine. The patients in the treatment group received the oral MTX nanoparticles equivalent to 15 mg of MTX per week for 12 weeks. The patients in the control group received 2 mg/kg/day of oral AZA for 12 weeks. The daily folic acid supplement was prescribed to all patients in the treatment group. Corticosteroids were used in 64.2% treatment and by 71.4% in the control group. After the 4 weeks of study, if the patient’s condition was remained stable the steroid tempering was started by 5 mg/week. During the study, all patients were refrained from other immunosuppressants, antibiotics and aminosalicylates.
Preparation of MTX nanoparticle
The MTX nanoparticle was synthesized by the method previously reported by Rajaonarivony et al. [26]. Briefly, 4 mL of 18 mM calcium chloride was added in to 76 ml of 0.06% sodium alginate solution that contained MTX under stirring condition. Then, 16 ml of 0.05% w/v of a poly-L-lysine aqueous solution was added. Afterwards, the solution was stirred for 2 hours and kept at room temperature overnight. Subsequently, this solution was centrifuged at 18000 rpm for 30 minutes and washed three times with distilled water to acquire the nanoparticles.
Characterization of nanoparticles
Particle size measurement: The mean particle size was determined by Photon correlation spectroscopy (PCS) (NANOPHOX, Sympatec GmbH, Germany). The MTX loaded nanoparticle formulation was diluted with deionized water up to a pertinent scattering intensity. The data analysis was performed by the cumulative method, considering spherical particles. The results were evaluated as the effective diameter, and the poly-dispersity index (PDI) was calculated as the relative width of the particle size distribution.
Determination of zeta potential
The MTX nanoparticle formulation’s zeta potential was determined by the NanoSight (NS500, Malvern Instruments Ltd, UK). To obtain the zeta potential, the MTX nanoparticles sample was prepared by diluting nanoparticle in double-distilled water and kept in an electrophoretic cell.
Fourier transform infra-red spectroscopy (FTIR)
FTIR transmission spectra were determined using a FTIR-8400S spectrophotometer (Shimadzu, Japan). The sample for analysis was prepared by mixing of pure drug and nanoparticle samples (1% of the potassium bromide amount) with potassium bromide powder. Potassium bromide disc was prepared by a hydraulic compression at 10000 psi. The potassium bromide disc was scanned over 400-4000 cm-1 wave number regions.
Determination of drug encapsulation efficiency
The drug encapsulation efficiency of nanoparticle formulation was obtained by separation of the MTX nanoparticles from the aqueous medium holding free MTX. In the present study, a separation was achieved by dialysis bag method [27]. The nanoparticle was first taken into the dialysis bag (Spectra/Por® 3,500 MWCO, Spectrum Medical Industries, CA, USA), which allows dialyzing the free drug till the complete release of the encapsulated drug. At the time of the dialysis, the medium 0.9% NaCl was changed in every half an hour, to determine the accurate amount of drug. The formulation was separated when there was not any detection of free drug concentration. To the 2 ml of sample solution, Triton X 100 (0.1 ml) was added which results in a clear solution. Then the percentage of encapsulation efficiency (E%) of MTX of nanoparticle was measured by UV spectrophotometry (UV-2600, Shimadzu Co. Ltd. China) at 303 nm and calculated as follows:
E%=100 × (Drug concentrations before the dialysis-Drug concentrations after the dialysis)/Drug concentrations before the dialysis.
In vitro release studies
The MTX nanoparticle formulation’s in-vitro release was obtained by dialysis bag method in phosphate buffer saline solution with pH 7.4. The optimized MTX nanoparticles (equivalent to 2.5 mg of drug) were filled in a dialysis bag and kept in 50 mL of dissolute on a medium, placed in shaker incubator that was persistently rotated at 100 rpm at 37°C. A small amount of solution was withdrawn for the analysis. The amount of MTX released from nanoparticles was determined according to the UV absorbance intensity at 303 nm. The values were evaluated according to the standard curve of the Methotrexate. All of the experimental procedure was performed three times. The cumulative release percentage (CR %) of MTX at each time point was calculated using the following equation:
CR%=(The amount of drug released at the time t/Initial amount of drug encapsulated in the nanoparticle)×100
Outcome measures
The primary outcome was to determine the feasibility and safety of administering methotrexate nanoparticle in children by recording any probable adverse reactions. The secondary outcome was calculation of the efficacy of MTX nanoparticle on the disease activity. The clinical response was interpreted as a decrease in10-point on PCDAI or PUCAI score as compared to the pre-treatment scores, and a score of <10 defined as inactive disease or complete remission. Patients could be withdrawn from the study in case of severe infection, persistent thrombocytopenia or leukopenia, imminent surgery, unacceptable adverse reaction, deterioration of disease symptoms, noncompliance or on patient’s request.
Follow-up
Patients were followed up at 2, 4, 8 and 12 weeks after randomization and afterwards, in every 4 weeks for subsequent 6 months. At each follow-up visits, the patients were evaluated according to the PCDAI or PUCAI score. At each visit, the patients underwent complete blood count, haemoglobin, C-reactive protein (CRP), aspartate aminotransferase (AST), alanine transaminase (ALT) and erythrocyte sedimentation rate (ESR).
Statistical analysis
All pre- and post-treatment data were analysed by using Student's paired t test. All data are presented as mean values ± standard error. A P value of <0.05 was considered as statically significant. All count variables were calculated by the chi-square test. The adverse events were reported quantitatively as well as qualitatively by using CTCAE grade. SPSS 9.0 program was used in the statistical analysis.
Results
Clinical characteristics of patients
The baseline characteristics of patients in each arm were shown in Table 1. Thirty-three patients was screened for the study; however, five patients were excluded due to prior history of colostomy (1 patient), exposure to infliximab (1 patient) and too mild disease (3 patients). Total 28 patients (17 male and 11 female) were enrolled in the study and randomized into two arms. Fourteen patients were included in each arm. There were 21 patients with CD and 7 patients with UC with a mean age at the time of enrolment 13.15 years. Corticosteroids were used in 64.2% treatment and by 71.4% in the control group.
| Characteristic | Control group | Treatment group |
|---|---|---|
| No. of patients | 14 | 14 |
| Male (female) | 9 (5) | 8 (6) |
| Age, (years), mean ± S.E.M (Range, years) | 12.7 ± 1.6 (9.1-16.8) | 13.6 ± 1.4 (8.6-16) |
| BMI ( range), kg/m2 |
19.6 ± 1.5 (15.4-25.6) | 18.2 ± 1.2 (14.4-25.2) |
| Type of disease CD UC |
10 4 |
11 3 |
| PCDAI PUCAI |
48.6 ± 2.2 49.1 ± 2.3 |
47.3 ± 1.9 49.2 ± 1.7 |
| AST | 37.4 ± 2.9 | 39.3 ± 2.3 |
| ALT | 19.3± 1.7 | 15.7± 1.3 |
| CRP | 3.8 ± 2.1 | 4.1 ± 1.7 |
Table 1: Baseline characteristics of patients in each arm.
Characterization of MTX nanoparticle
The characteristics of MTX nanoparticles were summarised in table II. The particle sizes of the nanoparticles are the most important characteristics formulation. The mean particle size of the nanoparticle formulation was 164.4 nm with PDI of 0.218. The low value of PDI showed a uniform particle size distribution. The encapsulation efficacy was found to be 97.8%. The zeta potential was found to be -32.6 mV that indicates a good stability of the formulation. This was attributed to the decrease in electrostatic repulsion between the particles and their stabilization due to the formation of a coat over their surfaces.
| Characteristic | Value (Mean ± standard error) |
| Entrapment efficiency (%) | 97.8 ± 4.2 |
| Particle size (nm) | 164.4 ± 6.9 |
| Zeta-potential (mV) | -32.6 ± 3.7 |
Table 2: The characteristics of MTX nanoparticle.
FTIR analysis
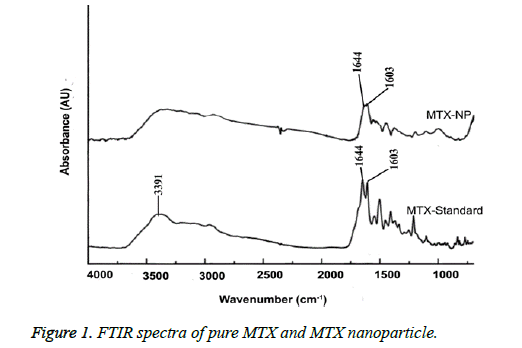
The FTIR spectra of MTX and MTX alginate nanoparticle was showed in Figure 1. In these spectra a significant peak at 3391 cm-1 was obtained due to -NH stretching and characteristic MTX peaks of 1644 cm-1 and 1603 cm-1. In the spectrum of MTX alginate nanoparticle formulation, significant peaks at 3437 cm-1, 1644 cm-1 and 1603 cm-1 were obtained. Some additional peaks were present due to presence of polymer. This indicates that there were no interactions between the polymer and drug in this nanoparticle formulation.
In-vitro release
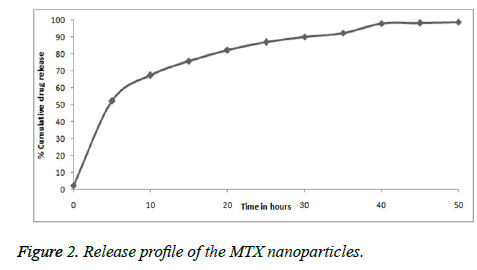
The Figure 2 shows in-vitro release of the MTX nanoparticle. The in-vitro releases of MTX were obtained by the percentage of MTX release with respect to an amount of MTX loaded in nanoparticles. The MTX nanoparticles showed initial burst release with about 32.7% followed by a sustained release up to 48 hours. The early burst release was possibly because of the drug precipitation from the nanoparticles or absorbed drug over the nanoparticles surface. Due to slow diffusion of the drug from polymer matrix, a prolonged release occurred.
Response evaluation
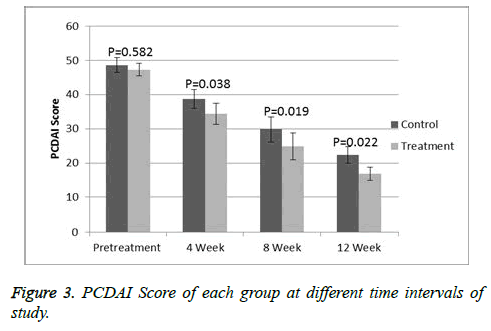
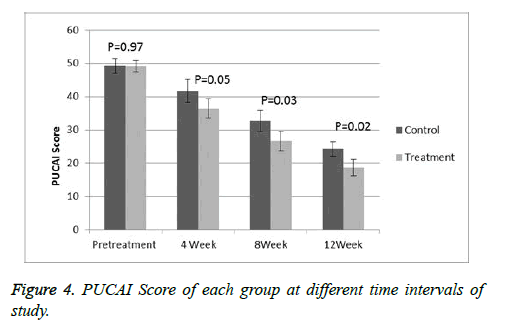
At the start of the study, all the patients had a moderate to severe disease with a mean PCDAI of 48 (range 33-59) and PUCAI of 49.1 (range 35-61). On the comparison of the both groups at baseline then there is no significance difference was observed. A significant deference was observed (p<0.05) when comparing PCDAI (Figure 3) or PUCAI (Figure 4) scores after 4, 8 and 12 weeks of treatment. At 12 weeks, the mean PCDAI for control and treatment groups were 22.3 ± 2.14 and 16.8 ± 1.87, respectively with a p value of 0.022. Similarly, the mean PUCAI for control and treatment groups were 24.3 ± 1.47 and 18.7 ± 1.92, respectively with a p value of 0.02. The table III shows laboratory tests of control and treatment groups at different time intervals. At 8 and 12 weeks of study, a significant statistical difference in the mean CRP and ESR values between the control and treatment groups was noted. At the beginning of the study, 9 treatment group patients in the treatment group and 10 control group patients were receiving steroids. A significant tampering in steroid dose was noted in both groups with total 12 patients (8 in MTX group and 5 in AZA group) were steroid free. At the end of 12 weeks, total 13 out of 28 patients achieved remission, 8 from treatment group and 5 in the control group.
| Tests | At 4 Weeks | At 8 Weeks | At 12 weeks | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Treatment | P value | Control | Treatment | P value | Control | Treatment | P value | |
| CRP (mg/L) | 2.9 ± 0.11 | 2.5 ± 0.12 | 0.031* | 2.4 ± 0.21 | 2.01 ± 0.17 | 0.013* | 1.8 ± 0.19 | 1.2 ± 0.23 | 0.023* |
| ESR (mm/hr) | 34.4 ± 1.53 | 32.6 ± 1.72 | 0.35 | 26.6 ± 1.93 | 21.4 ± 1.36 | 0.012* | 19.7 ± 2.13 | 13.9. ± 1.87 | 0.0413* |
| AST (U/L) | 33.9 ± 2.37 | 31.6 ± 1.97 | 0.40 | 31.4 ± 2.17 | 28.9 ± 2.76 | 0.36 | 29.7 ± 1.85 | 24.3 ± 2.13 | 0.035* |
| ALT (U/L) | 16.3 ± 1.47 | 20.6 ± 1.54 | 0.02* | 19.7 ± 1.77 | 21.9 ± 1.95 | 0.49 | 20.6 ± 1.69 | 24.3 ± 1.37 | 0.1 |
| *P<0.05 | |||||||||
Table 3: The laboratory tests of treatment and control groups at different time intervals of study.
Toxicity
The adverse events observed during the study were shown in Table 4. No deaths were reported in both groups. The commonly reported adverse events thrombocytopenia, leukopenia, nausea and vomiting were noted in azathioprine groups and thrombocytopenia, leukopenia and liver enzyme elevation in methotrexate groups. No serious adverse events were reported in both groups except one patient developed grade 3 leukopenia related to azathioprine which recovered after the discontinuation of the drug. No permanent discontinuation of treatments was required in treatment groups.
| Side Effect | Control group | Treatment group |
| Thrombocytopenia | 4 | 1 |
| Leukopenia | 2* | 1 |
| Nausea | 4 | 0 |
| Vomiting | 5 | 0 |
| Elevated lever enzyme | 0 | 2 |
| *One patient developed grade 3 leukopenia | ||
Table 4: The side effects reported in the study.
Discussion
In recent times, many studies of nanoparticle formulation in cell culture and small-animal models especially in the rodent model have been performed; however, only a few numbers of studies for evaluating nanoparticle formulation are being conducted in human till now. The nanoparticle application in IBD patients was first studied by Schmidt et al. [28]. The present study, methotrexate nanoparticle was evaluated in children with moderate to severe active IBD for safety and efficacy. We compared MTX nanoparticle with AZA. At baseline, the disease activity score (PCDAI or PUCAI), ESR, CRP and monthly steroid dose were almost similar in both groups (p>0.05).
One previous study showed the evidence that the low-dose weekly oral methotrexate may be safe and effective as a steroid sparing agent in IBD patients those have not responded to other therapies, particularly in Crohn's disease [17]. In the current study, that’s why we used a low dose of MTX was used in this study.
The numbers of controlled studies were conducted on the effect of MTX in IBD patients. Oren et al presented a double-blind, randomized, placebo-controlled study of the MTX in the chronic active ulcerative colitis. This study results demonstrated that there is no advantage of MTX over placebo in patients with moderate to severe UC [14]. Although, our study showed a significant advantage of MTX nanoparticle when compared the PUCAI scores between treatment and control groups, as well as with the baseline values.
Other study conducted by Ardizzone et al. showed that the MTX is useful in inducing remission in patients with chronic active CD; however, its efficacy is almost equal to AZA [29]. Although, the current study showed that the efficacy of MTX nanoparticle is significantly higher than AZA. The increased effect of MTX nanoparticle may be due to the property of nanoparticle. Many published reports revealed that the nanoparticle formulation have an increased therapeutic efficacy and low toxicities in comparison to the standard drugs for example, by the use rolipram, 5-ASA, tacrolimus and heparin [20,29-31]. The nanoparticle also hind the encapsulated drugs from P-glycoprotein, which is the mucosal enzyme that metabolized the drugs, resulting in an increased intracellular concentration of the drugs [8].
In current study, the disease activity scores were significantly less in the treatment group as compared to control group. A significant number of patients were achieved remissions in both groups; however, more number of patients in MTX group was achieved complete remission. It is quite clear that; methotrexate nanoparticle provides significant advantages over conventional therapy. In addition, unlike other methotrexate trials low incident and less severe adverse event were reported in our study without any discontinuation of the drug because of toxicities.
The favourable results in our study may be due to targeted and sustained drug delivery by nanoparticle formulation. The accumulation of nanoparticle in ulcerated lesions in IBD patients were already reported by Schmidt et al. The increased efficacy and low toxicity in our study may be due to this increased accumulation effect. Additionally, preferential uptake of nanoparticle into the inflamed tissue has been confirmed in many animal and cell culture studies [31-34]. These pieces of evidence are suggestive of the increased efficacy of MTX in our study. We speculated that the increased efficacy and low toxicity of MTX as compared to previous study may be because of the capability of nanoparticles to hide the drug from the systemic absorption, increased accumulation effect at inflamed tissue and the preferential nanoparticle uptake into the inflamed tissue.
Our study had some limitations such as a small number of participants, and an open label nature that have the potentials for biasness. Although, the random assignment of the patients in both groups was reduce the chances of potential biasness. So the randomized double-blinded clinical trial is recommended to confirm these results.
Conclusion
To our knowledge, the current study is the first to evaluate the drug loaded nanoparticle formulation in paediatric IBD patients. Many studied showed that the MTX is a good alternative in patients with AZA/6MP intolerant or resistant patients. Because of the high cost of biologics, the MTX should be considered before the induction of biological therapy. However, the systemic adverse reaction of MTX is the major limitation of MTX therapy. These limitations can be overcome by using nanoparticle drug delivery system. Due to the selectively targeting of inflamed tissue the increased efficacy and low toxicity as compared with the conventional formulation were noted. In the current study, we conclude that MTX nanoparticle safe and more effective in paediatric patients as compared to the slandered formulations.
Acknowledgements
The authors acknowledge Hospital of Taishan Medical University, Shandong, China for providing support for this work.
Contribution of Authors
We declare that this work was done by the author(s) named in this article and all liabilities pertaining to claims relating to the content of this article will be borne by the authors. Shu-Ying Chen, Gang Liu and Dun-Chen Li contributed in the study deign. Gang Liu, Dun-Chen Li and Ran-Ran Li contributed in the patient treatment and follow-up of patients. Ping-Ping Li prepared the nanoparticles. Ping-Ping Li and Shu-Ying Chen carried out analysis of data. Shu-Ying Chen and Dun-Chen Li contributed in the writing of manuscript. All authors read and reviewed the manuscript.
References
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012; 142: 46-54.
- Binder V. Epidemiology of IBD during the twentieth century: an integrated view. Best Pract Res Clin Gastroenterol 2004; 18: 463-479.
- Griffiths AM. Specificities of inflammatory bowel disease in childhood. Best Pract Res Clin Gastroenterol 2004; 18: 509-523.
- Pfefferkorn M, Burke G, Griffiths A, Markowitz J, Rosh J, Mack D, Otley A, Kugathasan S, Evans J, Bousvaros A. Growth abnormalities persist in newly diagnosed children with crohn disease despite current treatment paradigms. J Pediatr Gastroenterol Nutr 2009; 48: 168-174.
- Aloi M, Nuti F, Stronati L, Cucchiara S. Advances in the medical management of paediatric IBD. Nat Rev Gastroenterol Hepatol 2014; 11: 99-108.
- Walters TD, Kim MO, Denson LA, Griffiths AM, Dubinsky M, Markowitz J, Baldassano R, Crandall W, Rosh J, Pfefferkorn M. Increased effectiveness of early therapy with anti-tumor necrosis factor-a vs. an immunomodulator in children with Crohn’s disease. Gastroenterology 2014; 146: 383-391.
- Eisenstein EM, Berkun Y. Diagnosis and classification of juvenile idiopathic arthritis. J Autoimmun. 2014; 48-49.
- Ruperto N, Martini A. Emerging drugs to treat juvenile idiopathic arthritis. Expert Opin Emerg Drugs. 2011; 16: 493-505.
- Martini A, Lovell DJ. Juvenile idiopathic arthritis: state of the art and future perspectives. Ann Rheum Dis. 2010; 69: 1260-1263.
- Teml A, Schaeffeler E, Herrlinger KR, Klotz U, Schwab M. Thiopurine treatment in inflammatory bowel disease: clinical pharmacology and implication of pharmacogenetically guided dosing. Clin Pharmacokinet 2007; 46: 187-208.
- Hyams J, Crandall W, Kugathasan S, Griffiths A, Olson A, Johanns J, Liu G, Travers S, Heuschkel R, Markowitz J. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology 2007; 132: 863-873.
- Feagan BG, Fedorak RN, Irvine EJ, Wild G, Sutherland L, Steinhart AH, Greenberg GR, Koval J, Wong CJ, Hopkins M. A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. N Engl J Med 2000; 342: 1627-1632.
- Uhlen S, Bellbouab R, Narebski K, Goulet O, Schmitz J, Cézard JP, Turck D, Ruemmele FM. Efficacy of methotraxate in pediatric Crohn’s disease: a French multicenter study. Inflamm Bowel Dis 2006; 12: 1053-1057.
- Oren R, Moshkowitz M, Odes S, Becker S, Keter D, Pomeranz I, Shirin H, Reisfeld I, Broide E, Lavy A. Methotrexate in chronic active Crohn’s disease: a double-blind, randomized, Israeli multicenter trial. Am J Gastroenterol 1997; 92: 2203-2209.
- Cummings JR, Herrlinger KR, Travis SP, Gorard DA, McIntyre AS, Jewell DP. Oral methotrexate in ulcerative colitis. Aliment Pharmacol Ther 2005; 21: 385-389.
- Wahed M, Louis-Auguste JR, Baxter LM, Limdi JK, McCartney SA, Lindsay JO, Bloom SL. Efficacy of methotrexate in Crohn’s disease and ulcerative colitis patients unresponsive or intolerant to azathioprine/mercaptopurine. Aliment PharmacolTher 2009; 30: 614-620.
- Baron TH, Truss CD, Elson CO. Low-dose oral methotrexate in refractory inflammatory bowel disease. Dig Dis Sci 1993; 38: 1851-1856.
- Feagan BG, Rochon J, Fedorak RN, Irvine EJ, Wild G, Sutherland L, Steinhart HA, Greenberg GR, Gillies R, Hopkins M. Methotrexate for the treatment of Crohn’s disease. New Engl J Med 1995; 332: 292-297.
- Egan LJ, Sandborn WJ, Tremaine WJ, Leighton JA, Mays DC, Pike MG, Zinsmeister AR, Lipsky JJ. A randomized dose–response and pharmacokinetic of methotrexate for refractory inflammatory Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther 1999; 13: 1597-1604.
- Lamprecht A, Schafer U, Lehr CM. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm Res 2001; 18: 788-793.
- Cho K, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008 14: 1310-1316.
- Pawar SN, Edgar KJ. Alginate derivatization: a review of chemistry, properties and applications. Biomaterials 2012; 33: 3279-3305.
- Swain S, Behera A, Beg S, Patra CN, Dinda SC, Sruti J, Rao ME. Modified alginate beads for mucoadhesive drug delivery system: an updated review of patents Recent Pat Drug Deliv Formul. 2012; 6: 259-277.
- Chang D, Chang RK. Review of current issues in pharmaceutical excipients. Pharmaceutical Technology 2007; 31: 56-66.
- FDA. Sodium Alginate, CFR-code of federal regulations title Food and Drug Administration 2013.
- Rajaonarivony M, Vouthier C, Couarrze G, Puisieux F, Couvreur P. Development of a new drug carrier made from alginate, J Pharm Sci 1993; 82: 912‐917.
- Azmin MN, Florence AT, Handjani-Vila RM, Stuart JFB, Vanlerberghe G, Whittaker JS, Hardey JS. The effect of non-ionic surfactant vesicle (niosome) entrapment on the absorption and distribution of methotrexate in mice. J. Pharm. Pharmacol. 1985; 37: 237-242.
- Schmidt C, Lautenschlaeger C, Collnot EM, Schumann M, Bojarski C, Schulzke JD, Lehr CM, Stallmach A. Nano- and micro scaled particles for drug targeting to inflamed intestinal mucosa-A first in vivo study in human patients. J Control Release 2013; 165: 139-145.
- Ardizzone S, Bollani S, Manzionna G, Imbesi V, Colombo E, Bianchi Porro G. Comparison between methotrexate and azathioprine in the treatment of chronic active Crohn’s disease: a randomised, investigator-blind study. Dig Liver Dis. 2003; 35: 619-627.
- Laroui H, Dalmasso G, Nguyen HT, Yan Y, Sitaraman SV, Merlin D. Drug-loaded nanoparticles targeted to the colon with polysaccharide hydrogel reduce colitis in a mouse model. Gastroenterology. 2010; 138: 843-853.
- Pertuit D, Moulari B, Betz T, Nadaradjane A, Neumann D, Ismaïli L, Refouvelet B, Pellequer Y, Lamprecht A. 5-amino salicylic acid bound nanoparticles for the therapy of inflammatory bowel disease. J Control Release. 2007; 123: 211-218.
- Moulari B, Pertuit D, Pellequer Y, Lamprecht A. The targeting of surface modified silica nanoparticles to inflamed tissue in experimental colitis. Biomaterials. 2008; 29: 4554-4560.
- Lamprecht A, Yamamoto H, Takeuchi H, Kawashima Y. A pH-sensitive microsphere system for the colon delivery of tacrolimus containing nanoparticles. J Control Release. 2005; 104: 337-346.
- Leonard F, Collnot EM, Lehr CM. A three-dimensional coculture of enterocytes, monocytes and dendritic cells to model inflamed intestinal mucosa in vitro. Mol. Pharm. 2010; 7: 2103-2119.