Review Article - Materials Science and Nanotechnology (2021) Volume 5, Issue 1
Nanobiosensors impacting in medicinal electronics
A. Mohamed Sikkander*
Department of Chemistry, Velammal Engineering College, Chennai, India
- Corresponding Author:
- A. Mohamed Sikkander
Velammal Engineering College, India
E-mail: ams240868@gmail.com
Accepted date: January 25, 2021
Citation: Sikkander AM. Nanobiosensors impacting in medicinal electronics. Mater Sci Nanotechnol. 2021;5(1):1-5.
Abstract
Biosensing has been one of the foremost recent topics attracting scientific minds in view of the actual incontrovertible fact that long back. It’s so as biological entity are incredibly complex and are directly connected with the existence of a healthy environment. The intend of biosensors also has witnessed notable changes within the recent past.The use of assorted nanomaterials ranging from nanoparticles, nanotubes, nanorods, and nanowires has enabled more rapidly detection and its reproducibility during how better way. The only one of its kind properties of nanomaterials like far above the ground electrical conductivity, better shock bearing ability, and thus the susceptible responses like piezoelectric and pliable color based detection mechanisms are only the results of parishioners of nanomaterial properties.
Keywords
Assorted nanomaterials, Neuromorphic engineering, Implantable electrochemical biosensors, Glucose Biosensor, Biotelemetry, Glioma
Introduction
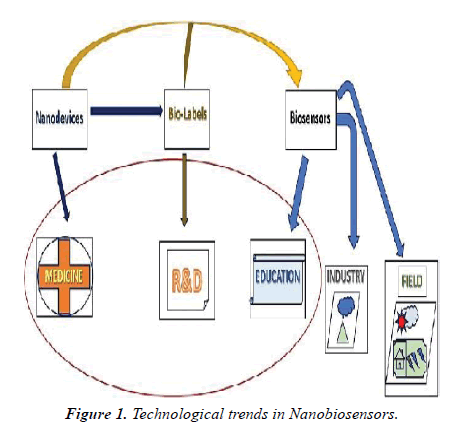
This paper places of interest the plentiful kinds of biosensors supported differing kinds of nanomaterials and their developmental and implicational aspects in medicinal electronics. In the medical sector is on the brink of endure deep changes by exploiting the normal strengths of the semiconductor industry – miniaturization and integration. The predictable electronics have found many applications in biomedicine medical monitoring of important signals, biophysical studies of excitable tissues, implantable electrodes for brain stimulation, pacemakers, and limb stimulation the utilization of nanomaterials and nanoscale applications will fetch an additional push towards implanted electronics within the physical body [1]. A man-made retina for color vision; nanomaterial-based breath analyzer as exploratory tools; Nano generators to power self-sustained Biosystems and implants; future bio-nanotechnology might even use computer chips inside living cells. Much nanotechnology work goes on within the world of brain research. A little further out, and truly beyond the scope of drugs are efforts to form artificial brains something that's called neuromorphic engineering: a replacement interdisciplinary discipline that has nanotechnologies and whose objective is to style artificial neural systems with physical architectures almost like biological nervous systems [2]. Implantable biosensors the physiology of disease tissue is often markedly dissimilar thereto of healthy tissue, with diseases like cancer or DM resulting in quantifiable changes within the body. Methodology which may make available continuous data on the number of a target analyte, enabling trends and changes in concentrations over time to be analysed, with none need for intrusion from the patient or clinician, would be very valuable. (Figure 1).
Advances within the fields of electronics and microfabrication techniques have caused increased interest within the use of implantable medical devices in meticulousness medicine. Biosensors are analytical devices containing a biological detector that transforms a biological respond into electrical signals [3]. Biosensors have a variety of applications, from environmental monitoring and food safety, to security and defence; however, the utilization of biosensors for medical diagnostics represents the foremost imperative driver for biosensor development and application today.
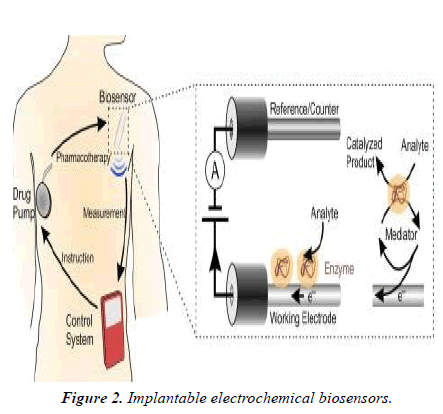
Implantable electrochemical biosensors
The bio-recognition element of the sensor identifies a target analyte, while a transducer converts the output from the molecular detection into an electrical signal. Different molecular recognition elements are often employed, including enzymes, nucleic acids, antibodies, proteins and peptides [4] (Figure 2).
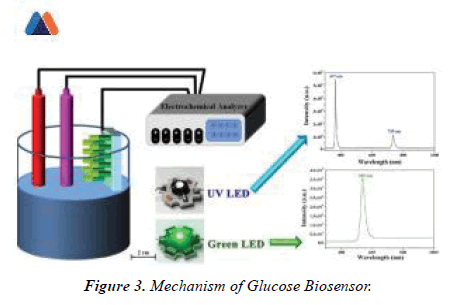
Clark is credited with developing the primary biosensor in 1962; this ‘enzyme electrode’ was an idea built on his earlier invention the Clark oxygen electrode. Having enzymes because the molecular recognition element depends on the catalytic conversion of an enzymatic substrate to a product. Enzymes have highly specific binding pockets; enzyme electrodes have high selectivity against their chosen analyte. Clark’s paper described the electrochemical detection of O2 or CO2 by immobilised enzymes. In one example, the enzyme glucose oxidase (GOx) was entrapped on a platinum O2 electrode over a semi-permeable dialysis membrane, with the amount of O2 consumed by the electrode acting as an indirect measure of glucose levels. Electrochemical biosensors have the potential to supply the sensitive and rapid detection of an honest range of biomarkers; their relative fabrication simplicity, amenability to miniaturization, in conjunction with the reduced cost of instrumentation, has also furthered interest in their development [5].Biosensors and metabolic diseases one of the primary major successful applications of implantable biosensors was within the world of metabolic diseases, specifically DM. Despite advances in insulin therapy delivery and thus the event of more physiological insulin preparations, the avoidance of hypoglycemic episodes still remains a challenge. Blood sugar measurements are traditionally performed with the blood from pin-prick samples and aerometric home-use glucose sensors. (Figure 3)
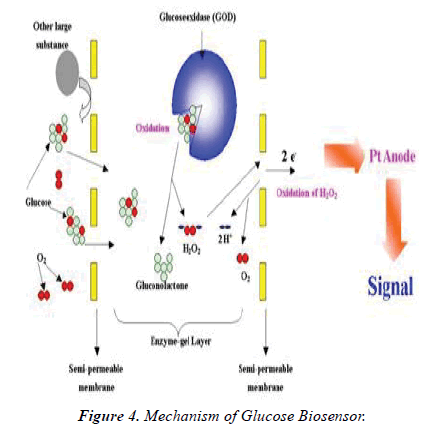
Sampling is typically aching and time consuming, causing emotional anguish and poor patient compliance, noncompliance among diabetic patients has been estimated within the range of 50–80%. Generally used monitoring methods to manage diabetic dogs and cats are classified as direct or indirect. Blood sugar measurements are the mainstay monitoring method utilized in clinical practice; this might be problematic due to large day-to-day variations in blood sugar levels in dogs and stress hyperglycaemia in cats. Owners predominately believe home-use urine tests to watch glucose levels; however, these urine tests only reflect the glucose levels over an outsized timeframe, and transient hypoglycaemia could even be masked by hyperglycaemic periods. Even animals whose diabetes is in check and knowledge several ours of euglycaemia will likely be glycosuric for several hours a day (Figure 4).
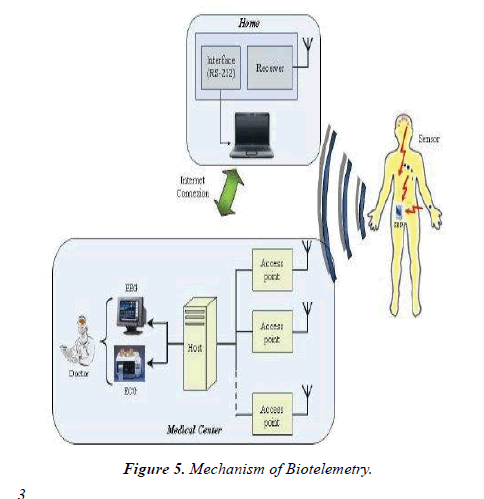
The Somogyi effect is additionally seen in both dogs and cats, whereby sustained rebound hyperglycaemia occurs after an acute decrease in blood sugar levels; insulin levels therefore shouldn't be increased supported persistent hyperglycaemia. To beat sort of the problems outlined with blood sugar measurements in dogs and cats, glycosylated proteins like glycosylated haemoglobin and fructosamine concentrations are often measured to provide a quantitative indirect assessment of diabetes diagnosis and management. the event of continuous glucose monitoring (CGM) over the last 15 years aims to supply real-time interstitial glucose readings, alerting patients to impending hypo- and hyperglycaemic episodes. Although CGM systems for veterinary patients aren't in mainstream use, they need been successfully utilized in clinics. CGM systems are often connected with subcutaneous insulin infusion systems, creating sensor augmented pumps with the automated administration of insulin, acting as a man-made pancreas. Continuous glucose-monitoring systems using long-term (> 90 days) fully implantable sensors have now been delivered to the market (Eversense, Senseonics Inc.). This technique uses an entrenched glucose sensor, a removable wearable smart transmitter and a mobile app to display real-time glucose measurements. Enduring implantable CGM systems are beneficial to patients because the quantity of times the sensor must get replaced is decreased, thus reducing warm-up procedures and thus the danger of sensor damage from each implantation [6]. These sorts of amperometric enzyme-based biosensors have also been utilized within the world of experimental neuroscience. Regulatory disturbances in brain energy metabolism (particularly in reference to glucose, lactate and pyruvate levels) can have a negative impact on cognitive learning and memory, and is assumed to be involved in multiple neurological diseases/disorders. This biosensor was implanted into the medial prefrontal cortex of anaesthetized rats, where researchers showed that the biosensor was ready to continuously and simultaneously monitor these three metabolism-related biomarkers during a specific brain region with temporal and spatial accuracy. Biotelemetry Biotelemetry (the remote measurement of an activity, function or condition) utilizes implantable technology as how of obtaining data in an experimental setting in conscious, unrestrained animals (Figure 5).
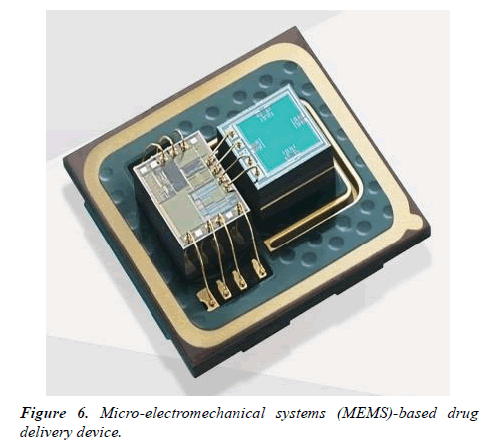
Electromyogram (EMG), electroencephalogram (EEG), electrocardiogram (ECG), pulse, sign, blood heat, activity and time data are often collected using biotelemetry techniques [7]. It’s thought that information obtained from conscious, unrestrained animals is probably going to be more like that seen in humans, in contrast to classically derived data from anaesthetized or restrained animals. Wireless radio telemetry has been utilized during a selection of laboratory animals, including mice as small as 20 g and fish, with transmitters commonly implanted either intraperitoneally, subcutaneously or between abdominal layers [8]. Biotelemetry has been shown to be useful within the characterization of varied animal models of human diseases, including epilepsy, sleep disorders, neurodegenerative and neuropsychiatric disorders. This system also features a task within the investigation of the pharmacokinetic, pharmacodynamics and toxicological properties of medicine, helping to work out their safety margins and efficacy. For instance, EEG recordings are often wont to assess a medicine effect on the central systema nervosum, including the detection of seizure activity, whereas ECG, pulse and sign are often wont to investigate the results of cardiac drugs. Biosensors and drug delivery contrary types of biosensors feature drug delivery systems are urbanized with the power to deliver drugs in response to biosensor readings. Micro-electromechanical systems (MEMS)-based drug delivery devices are example, with BioMEMS defined as a category of MEMS which incorporate biological entities or have a biological application (Figure 6).
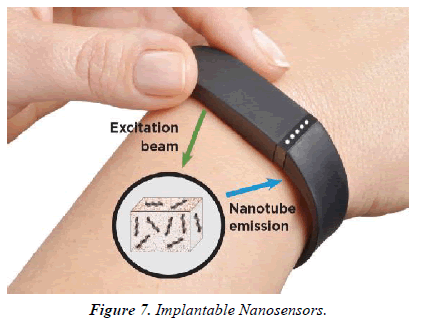
The utilization of techniques created for the industry has enabled the assembly of the micro-reservoirs, micro pumps, valves and sensors needed to form miniaturized devices. These devices have the power to sense, monitor, mix, and pump and control the flow of small amounts of fluid, a number of which are commercially available. Smart polymers, which are produced to travel through structural alterations when subjected to changes to external stimuli like temperature or pH also, can be used as biosensors. They need the power to deliver drugs when needed. One such example is that the attachment of both glucose oxidase and insulin within a hydrogel that's aware of changes in pH, enabling this smart polymer to act both as a sensor of glucose concentration and as a drug delivery vehicle for insulin. These sorts of biosensor-drug delivery systems can reduce the danger of overdosing/underdosing a patient whilst allowing the patient to receive the drug at a selected time point. Implantable drug delivery systems have also great potential to be used in diseases during which treatment regimens are difficult to implement. Osteoporosis affects bone density resulting in weakened/ fragile bones. One treatment option for this condition is that the daily injection of human parathormone fragment (1-34) (hPTH(1-34)) as an anabolic therapy, stimulating osteoblastic bone formation [9]. This therapy can last up to 2 years, which makes patient compliance problematic. The first-in-human testing of a wirelessly controlled drug delivery microchip was utilized in the treatment of osteoporosis; the device consisted of silicon chips with multiple individual reservoirs crammed with concentrated hPTH(1-34) solution. Pharmacokinetic evaluation showed that the microchip produced similar results to multiple daily injections, with bone marker evaluation indicating that bone formation increased albeit a fibrous capsule developed round the implanted device. This study is a superb example of where implantable devices are often utilized to beat difficult treatment regimens and poor patient compliance. Implantable technology and cancer monitoring/treatment Tumor physiology differs markedly from normal tissue physiology. Tumors are characterized by areas of O2 depletion (hypoxia and anoxia), glucose and energy deprivation, extracellular acidosis, high lactate levels and interstitial hypertension. This unique tumour microenvironment (TME) is essentially determined by an abnormal tumor vasculature and its heterogeneous microcirculation [10].The TME strongly influences a tumors response to radiotherapy (RT) and chemotherapy; therefore, monitoring the TME surely biomarkers, pH, O2, cancer metabolites or chemotherapeutic drug concentrations could allow observation of the treatment response, and improve the detection of recurrence or metastasis. An implantable diagnostic device placed within the tumour, or the encompassing tissue, following surgery or at the time of biopsy would be one potential method to realize this type of precision monitoring (Figure 7).
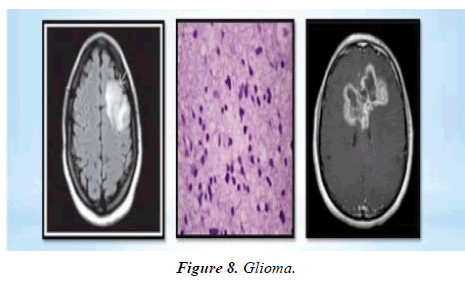
Could Monitor Cancer Activity this is able to be especially useful in areas where imaging (e.g. MRI/CT) makes it difficult to differentiate between the tumour recurrence, fibrosis, necrosis or benign lesions from previous surgery and/or chemoradiotherapy treatment (e.g. glioma) (Figure 8).
The first in vivo description of such a tool was wont to detect soluble cancer biomarkers (the β subunit of human chorionic gonadotrophin) in mice, using nanoparticle magnetic relaxation switches (MRSw) enclosed within an implantable device by a membrane.
Conclusion
Biosensors are analytical devices containing a biological detector that transforms a biological respond into electrical signals. Biosensors have a variety of applications, from environmental monitoring and food safety, to security and defence; however, the utilization of biosensors for medical diagnostics represents the foremost imperative driver for biosensor development and application today. Different molecular recognition elements are often employed, including enzymes, nucleic acids, antibodies, proteins and peptides. Enduring implantable CGM systems are beneficial to patients because the quantity of times the sensor must get replaced is decreased, thus reducing warm-up procedures and thus the danger of sensor damage from each implantation. Biosensors and drug delivery contrary types of biosensors feature drug delivery systems are urbanized with the power to deliver drugs in response to biosensor readings. Micro-electromechanical systems (MEMS)-based drug delivery devices are example, with BioMEMS defined as a category of MEMS which incorporate biological entities or have a biological application.
References
- Cohen-Karni T., Langer R, Kohane DS, The Smartest Materials: The Future of Nanoelectronics in Medicine, ACS Nano. 2012; 6(8):6541-6545. doi: 10.1021/nn302915s
- Rushdi M, On the Aspects of Memory, Shared Memory, and Artificial Consciousness in the Virternity Project. 2017. doi: 10.13140/RG.2.2.23528.78083
- Mehrotra P, Biosensors and their applications – A review, J Oral Biol Craniofac Res. 2016;6(2): 153–159. doi: 10.1016/j.jobcr.2015.12.002
- Morales MA, Halpern JM, Guide to Selecting a Biorecognition Element for Biosensors, Bioconjug Chem. 2018;29(10):3231–3239. doi: 10.1021/acs.bioconjchem.8b00592
- Florea DA, Melinte G , Simon I, et al.,Electrochemical Biosensors as Potential Diagnostic Devices for Autoimmune, Biosensors(mdpi). 2019.
- Vaddiraju S, Burgess DJ, Tomazos I, et al., Technologies for Continuous Glucose Monitoring: Current Problems and Future Promises, J Diabetes Sci Technol. 2010;4(6):1540–1562. doi:10.1177/193229681000400632
- Lundt A, Wormuth C, Siwek ME, et al., EEG Radiotelemetry in Small Laboratory Rodents: A Powerful State-of-the Art Approach in Neuropsychiatric, Neurodegenerative, and Epilepsy Research,Neural Plasticity. 2016.
- Papazoglou A, Lundt A, Wormuth C, et al., Non-restraining EEG Radiotelemetry: Epidural and Deep Intracerebral Stereotaxic EEG Electrode Placement, J Vis Exp. 2016;(112). doi: 10.3791/5421654216.
- Asafo-Adjei TA, Chen AJ, Najarzadeh A, et al., Advances in Controlled Drug Delivery for Treatment of Osteoporosis, Curr Osteoporos Rep. 2016;14(5):226–238. doi: 10.1007/s11914-016-0321-4
- Siemann DW, Horsman MR, Modulation of the Tumor Vasculature and Oxygenation to Improve Therapy, Pharmacol Ther. Author manuscript; available in PMC 2016 Sep 1. Pharmacol Ther. 2015;153:107–124. doi: 10.1016/j.pharmthera.2015.06.006