Research Article - Biomedical Research (2017) Volume 28, Issue 1
Microbiological examination to investigate the differences in microorganisms and antibiotic sensitivity of head and neck space infections
Lin Ye1, Yan-bo Liu1*, Ao-lei Geng1, Hai-Yan Fu21Department of Pharmacy, Xiangyang Hospital Affiliated to Hubei University of Medicine, Xiangyang, Hubei 441000, PR China
2Department of Clinical Laboratory, Yantaishan Hospital, Yantai, Shandong 264000, PR China
- *Corresponding Author:
- Yan-bo Liu
Department of Pharmacy
Xiangyang Hospital Affiliated to Hubei University of
Medicine, PR China
Accepted on June 02, 2016
Abstract
The present study aimed to evaluate the prevalence and antimicrobial susceptibility, of aerobic and anaerobic strains in 100 patients with head and neck (single and multiple) abscesses. In total, 114 microbes were isolated. The sensitivity test was performed for both aerobic and anaerobic isolates. Out of 100 patients (65 males, 35 females), 74 presented with single space abscess and 26 patients with multiple space involvement. The submandibular space was the most frequent location for single space and multiple abscesses (76.92%; 84.92%). Predominantly 48 aerobic and 65 anaerobic bacteria were isolated; the most common bacteria being Klebsiella, Clostridium tertium, Staphylococcus aureus and Actinomyces odontolyticus. The anaerobic bacteria were found to be 100% resistant to amoxicillin and penicillin, whereas they were found to be susceptible to erythromycin (100%), cefotaxime (98.48%) and clindamycin (96.96%) respectively. On the other hand, the aerobic strains were resistant to penicillin (47.91%) and sensitive to amoxicillin (79.16%) respectively. Hence, the antibiotics alone cannot determine the odontogenic infection satisfactorily. For immediate recovery of patients, proper basic management of the disease is also equally important.
Keywords
Odontogenic infection, Antibiotics, Sensitivity, Resistance.
Introduction
The incidence, severity, morbidity, and mortality of odontogenic infections have declined dramatically over the years. This reduction in mortality is not attributed to the use of penicillin but is due to the application of the principles of the initial establishment of airway security, followed by early and aggressive surgical drainage of all anatomical spaces affected by cellulitis or abscesses. With the use of antibiotics and advanced medical supportive care, mortality associated with Ludwig’s angina has reduced to 4% [1].
Head and neck abscesses are less common today than they were in the past. The impact of antibiotic treatment and improved dental care are the most likely reasons for this change. However, in spite of the widespread use of antibiotics, deep neck infections remain one of the difficult emergencies encountered in daily clinical practice. Once an abscess occupies one of the deep neck spaces, the infection can spread across the spaces or damage the adjacent vital neural or vascular structures. The extent and severity of the illness could become life threatening. In addition to the systemic toxicity and localized respiratory and digestive tract disturbance, more serious complications, including air- way obstruction, pneumonia, lung abscess, mediastinitis, pericarditis, internal jugular vein thrombosis, and carotid artery erosion may also occur [2-5].
Besides adequate drainage of the abscess, antibiotic therapy is essential for the successful treatment. To administer antimicrobial agents effectively to a patient, microbiological data on the abscess must be obtained. However, it usually takes several days to get the necessary data, and consequently empiric antimicrobial therapy is frequently launched before the definite culture result is available. Most head and neck infections are endogenous and mixed [6]. So, the antibacterial treatment of mixed infections should cover both aerobes and anaerobes. Resistance rates for anaerobes vary within species as well as within sources of the isolates. According to several authors [7-9], resistance rates to some antibacterial agents (such as ampicillin/sulbactam and clindamycin) have shown a tendency to increase.
Recent reports have confirmed that oral/dental infections are polymicrobial, including facultative anaerobes, such as Viridans group streptococci and the Streptococcus anginosus group, with predominantly strict anaerobes, such as anaerobic cocci, Prevotella and Fusobacterium species. The use of sophisticated non-culture methods has identified a wider range of organisms, such as Treponema species and anaerobic Grampositive rods such as Bulleidia extructa, Cryptobacterium curtum, and Mogibacterium timidum [10]. So, in the present study an attempt was made to find out the efficacy of standard antibiotics against the odontogenic infection causing microbes.
Material and Methods
Samples and isolation of microorganisms
Patients those reported Department of Clinical Laboratory, Yantai Yuhuangding Hospital, Qingdao University, Yantai, Shandong, China during 2013-14 were enrolled in the present study. Pus samples were collected from 100 odontogenic infected patients by aspirating abscess using sterile 18/22- gauge needle and 5 ml syringe either intraorally or extraorally. After aspiration, a drop of specimen was immediately inoculated in sterile Robertson cooked meat broth (RCM) for isolation of anaerobic organisms. The inoculated RCM was incubated at 37ºC for 48 h and subcultured into solid media. The plate was incubated anaerobically in McIntosh field jar at 37ºC for 48 h. For aerobic culture the samples were inoculated on blood agar (BA), McConveys agar (MA), and nutrient broth. Incubation was done aerobically at 37ºC for 24 h. The colonies were then sub cultured into nutrient agar.
Antibiotic sensitivity test
In order to prepare the bacterial inoculum, a single colony was inoculated in nutrient broth and incubated overnight. After that, the turbidity was adjusted using Mc Farland standards. The Muller Hinton agar medium (MHA) was prepared as per manufacturer’s instructions (Hi-Media). The susceptibility testing was done using disc diffusion method of Kirby-Bauer. The discs (30 mcg) used for both aerobic and anaerobic bacteria were of amoxicillin, ampicillin, clindamycin, ciprofloxacin, ceprotaxime, penicillin, erythromycin, gentamicin and methicillin (Hi-Media) and sensitivity was accessed according to manufacturer instructions. The experimental plates were incubated at 37ºC for 24 h. All experiments were conducted in triplicates and the results were concurrent [11].
Results
Baseline characteristics of patients
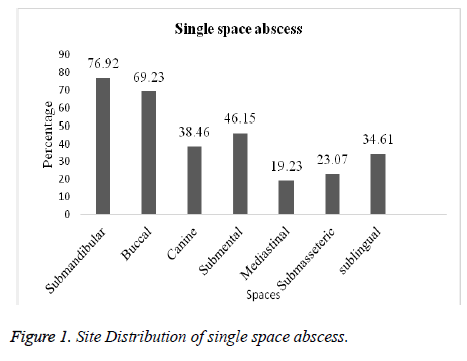
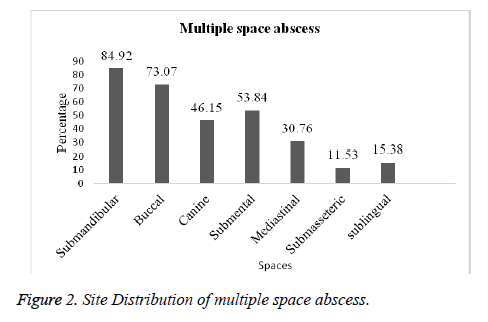
Amongst the 100 patients that were investigated, 74 were found to have single space abscess and 26 multiple abscess. The submandibular space was the most frequent location for single and multiple space abscesses (76.92%; 84.92%) followed by buccal (69.23%; 73.07%), submental (46.15%; 53.84%) and canine (38.46%; 46.15%) respectively. In case of other spaces like sublingual, mediastinal and submasseteric infection was slightly less in both single and multiple spaces (Figures 1 and 2).
Distribution of microorganisms
From 100 patients, 114 microorganisms were isolated. Among them 52 was gram positive, 14 were gram negative anaerobes. A total of 48 aerobes were obtained including 44 gram positive and 4 gram negative. The predominantly isolated microorganism was Klebsiella (17.54%) followed by Clostridium tertium (14.03%), Staphylococcus aureus (14.03%), Actinomyces odontolyticus (12.28%), Lactobacillus spp. (8.77%), Peptostreptococcus sp. (7.01%), E. coli (5.26%), Streptococcus sp. (4.38%), Streptococcus milleri (3.50%), Bacteriodes melaninogenicus (3.50%), Pseudomonas aeuroginosa (3.50%), Streptococcus mutans (1.75%) and Fusobacterium nucleatum (0.877 %), respectively (Table 1).
| Microorganisms | Instances (%) |
|---|---|
| Gram positive anaerobes | |
| Clostridium tertium | 16 (14.03) |
| Lactobacillus spp. | 10 (08.77) |
| Streptococcusmilleri | 04 (03.50) |
| Actinomycesodontolyticus | 14 (12.28) |
| Peptostreptococcus sp. | 08 (07.01) |
| Gram negative anaerobes | |
| Fusobacteriumnucleatum | 01 (0.877) |
| Escherichia coli | 06 (05.26) |
| Prevotellamelaninogenica | 01 (0.877) |
| Bacteriodesmelaninogenicus | 04 (03.50) |
| Bacteriodescorrodens | 02 (01.75) |
| Gram positive aerobes | |
| Streptococcus sp. | 05 (04.38) |
| Klebsiella sp. | 20 (17.54) |
| Streptococcusmutans | 03 (02.63) |
| Staphylococcus aureus | 16 (14.03) |
| Gram negative aerobes | |
| Pseudomonas aeuroginosa | 04 (03.50) |
| Total | 114 |
Table 1. Predominantly isolated aerobic and anaerobic microorganisms.
Antibiotic resistance among isolates
The susceptibility and resistance patterns were checked using standard antibiotics against the gram positive, gram negative aerobic and anaerobic bacteria. The anaerobes were found to be 100 % resistant to ampicillin and penicillin, whereas only 47.91 % of aerobic bacteria exhibited resistance to penicillin followed by 27.08% being resistant to ampicillin, 4.54% to gentamicin and 1.51% to amoxicillin. The aerobic bacteria were found to be resistant against cefotaxime and ciprofloxacin. There was no resistance observed against clindamycin, erythromycin and methicillin in both aerobic and anaerobic microbes. The anaerobes were found to be susceptible to erythomycin (100 %) followed by cefotaxime (98.48 %), clindamycin (96.96 %) and amoxicillin (60.60 %) respectively. The susceptibility to methicillin (22.72%), gentamicin (22.72%) and ciprofloxacin (9.06%) was less as compared to the above mentioned drugs. Further, anaerobes were not susceptible to ampicillin and penicillin. However, the aerobes were susceptible to amoxicillin (79.16%), erythromycin (45.83%), methicillin (37.50%), clindamycin (31.25%), penicillin (20.83%), gentamicin (18.75%), cefotaxime (16.66%) and ampicillin (12.50%) respectively. They were not susceptible to ampicillin (Table 2).
| Antibiotics | Microbes | |||
|---|---|---|---|---|
| No. of Sensitivity cases (%) | No. of Resistance cases (%) | |||
| Aerobes | Anaerobes | Aerobes | Anaerobes | |
| Amoxicillin | 38 (79.16) | 40 (60.60) | 0 | 01 (1.51) |
| Ampicillin | 06 (12.50) | 0 | 13 (27.08) | 66 (100) |
| Clindamycin | 15 (31.25) | 64 (96.96) | 0 | 0 |
| Ciprofloxacin | 0 | 06 (09.06) | 01 (2.08) | 05 (7.57) |
| Cefotaxime | 08 (16.66) | 65 (98.48) | 01 (2.08) | 0 |
| Penicillin | 10 (20.83) | 0 | 23 (47.91) | 66 (100) |
| Erythromycin | 22 (45.83) | 66 (100) | 0 | 0 |
| Gentamicin | 09 (18.75) | 10 (15.15) | 0 | 03 (4.54) |
| Methicillin | 18 (37.50) | 15 (22.72) | 0 | 0 |
| (Aerobe n=48; Anaerobe n=66) | ||||
Table 2. Antibiotic sensitivity, resistance pattern of aerobic and anaerobic strains.
Discussion
In the early 1960s, teeth were listed as the fifth or sixth leading cause of death recorded on the London Bills of Mortality [12]. At the beginning of the twentieth century, dental infections were found to be associated with a mortality rate of 10-40%. Odontogenic bacterial infection is still reported as being the most common cause of orofacial sepsis. This was illustrated by Amaidas [13] who showed the prevalence of 56.7% for odontogenic only causes and 21.7%, where the tooth was left in the jaw line of fracture. Flynn et al. [14] found that 65% of odontogenic abscesses were caused by caries, 22% by pericoronitis and 22% by periodontal infections. Other studies also supported the finding that odontogenic infections are the most common cause of orofacial sepsis [15-17]. Head and neck infections of odontogenic origins are routinely treated. Untreated or rapidly spreading odontogenic infections can be potentially life threatening secondary to airway compromise or septicemia [18].
In the present investigation 100 patients with odontogenic space infections were considered. Out of these 65 were males and 35 females. Both were infected by single and multiple space abscesses. Walia et al. [18] reported that out of the 42 patients, 28 (66.66 %) were presented with single space involvement and 14 patients with multiple space involvement, with a total of 64 spaces being involved. Male patients were more common than female patients [19-20]. An odontogenic infection spreads to fascial spaces because the anatomy of the fascial planes of the head and neck is such that it has an ineffective barrier to the spread of infection [21], and plays a vital role in the clinical localization of an abscess. The involvement of the fascial planes by cellulitis, aids in the surgical drainage. Of the 28 patients with single space involvement, submandibular space was involved in 8 patients (28.57 %), buccal space in 6 patients (21.42 %) and, canine space in 5 patients (17.85 %) [18].
Likewise in the present study in single space, submadibular space location was 76.92% followed by buccal 69.23%, submental 46.15%, canine 38.46%, sublingual 34.61%, submasseteric 23.07% and Mediastinal 19.23%. In multiple space, submandibular was 84.92%, buccal 73.07%, submental 53.84%, canine 53.84%, mediastinal 30.76 %, sublingual 15.38 % and submasseteric 11.53% respectively. This is contradictory to the incidence documented by Storoe et al. [22] at the same time correlated with Walia et al. [18]. Predominantly 114 microbes were isolated, among them 57.85% were anaerobes and 42.08 % were aerobes. From the anaerobes Clostridium tertium was mostly isolated (14.03%) followed by Actinomyces odontolyticus (12.28%), Lactobacillus spp. (8.77%), Peptostreptococcus sp. (7.01%), E. coli (5.26%), Bacteriodes melaninogenicus& Streptococcus milleri (3.50%) and Prevotella melaninogenica & Fusobacterium nucleatum (0.877%). Many investigations have demonstrated that Streptococcus viridans, Peptostreptococcus, Prevotella, Porphyromonas, and Fusobacterium are frequently isolated from odontogenic infections [20, 23-25].
The most commonly isolated organisms from aerobic bacteria were Klebsiella sp. (17.54%), Staphylococcus aureus (14.03%), Streptococcus sp. (4.38%), Pseudomonas aeuroginosa (3.50%) and Streptococcus mutans (2.63%). This finding was concurrent with that of Santosh et al. In the present study, all anaerobic bacteria were 100% resistant to ampicillin and penicillin. They were highly sensitive to Erythromycin 66 (100%) followed by Clindamycin 65 (98.48%) Cefotaxime 64 (96.96%) and Amoxicillin 40 (60.60%). At the same time it was less susceptible to Methicillin 15 (22.72%) and Gentamicin 10 (15.15%). This study was compared with Kuriyama et al. [26], Flynn et al. [14] and Santosh et al. [20]. Aerobic strains were highly susceptible to Amoxicillin 38 (79.16%) and moderate to other antibiotics. They were only 47.91% resistant create for Penicillin and for other antibiotics the resistance level was very less. In the present investigation, selected antibiotics are found to actively work against anaerobic bacteria as compared to aerobic bacterial strains.
The microbial infection accounts for most single and multiple head and neck abscesses. Gram-positive, gram-negative aerobes and anaerobes are most common pathogens for head and neck space infections. Erythromycin, cefotaxime, clindamycin and amoxicillin could be good candidates for treating both aerobic and anaerobic head and neck abscess involvement. One thing to remember, the antibiotic alone cannot determine the odontogenic infection satisfactorily, without forgetting the importance of early surgical intervention to reduce morbidity and complications. For immediate recovery of patients, proper basic management of the disease is also equally important.
Acknowledgement
The authors are thankful to Department of Clinical Laboratory, Yantai Yuhuangding Hospital, Affiliated Hospital of Medical College, Qingdao University, Yantai, Shandong 264000, China for their support.
References
- Hought RT, Fitzgerald BE, Latta JE, Zallen RD. Ludwig’s angina: report of two cases and review of the literature from 1945 to January 1979. J Oral Surg 1980; 38: 849-855.
- Sethi DS, Stanley RE. Deep neck abscesses-changing trends. J LaryngolOtol 1994; 108: 138-143.
- el-Sayed Y, al Dousary S. Deep-neck space abscesses. J Otolaryngol 1996; 25: 227-233.
- Sakaguchi M, Sato S, Ishiyama T, Katsuno S, Taguchi K. Characterization and management of deep neck infections. Int J Oral MaxillofacSurg 1997; 26: 131-134.
- Bottin R, Marioni G, Rinaldi R, Boninsegna M, Salvadori L, Staffieri A. Deep neck infection: a present- day complication. A retrospective review of 83 cases (1998-2001). Eur Arch Otorhinolaryngol 2003; 260: 576-579.
- Kuriyama T, Karasawa T, Nakagawa K, Yamamoto E, Nakamura S. Incidence of b-lactamase production and antimicrobial susceptibility of anaerobic Gram-negative rods isolated from pus specimens of orofacialodontogenic infections. Oral MicrobiolImmunol 2001; 16: 10-15.
- Aldridge KE, Ashcraft D, Cambre K, Pierson CL, Jenkins SG, Rosenblatt JE. Multicenter survey of the changing in vitro antimicrobial susceptibilities of clinical isolates of Bacteroidesfragilis group, Prevotella, Fusobacterium, Porphyromonas, and Peptostreptococcus species. Antimicrob Agents Chemother 2001; 45: 1238-1243.
- Jousimies-Somer H, Summanen P, Citron D, Baron EJ, Wexler HM, Finegold SM, Wadsworth KTL. Anaerobic Bacteriology Manual. Belmont, CA: Star Publishing 2002.
- Papaparaskevas J, Pantazatou A, Katsandri A, Legakis NJ, Avlamis A. Hellenic Study Group for Gram-Negative Anaerobic Bacteria. Multicentre survey of the in-vitro activity of seven antimicrobial agents, including ertapenem, against recently isolated Gram-negative anaerobic bacteria in Greece. ClinMicrobiol Infect 2005; 11: 820-824.
- Andersson L. Oral and Maxillofacial Surgery. In Andersson L, Kahnberg KE, Pog‐rel MA eds, Wiley Blackwell 2010, 280-314.
- Kora AJ, Manjusha R, Arunachalam J. Superior bactericidal activity of SDS capped silver nanoparticles: Synthesis and characterization. Mater SciEng C 2009; 29: 2104-2109.
- Clarke JH. Toothaches and death. J Hist Dent 1999; 47: 11-13.
- Amaidas VD. Cervicofacial infections. A three year retrospective study. Mmed MFOS thesis. Department of Library and Information Science, University of the Western Cape, 1990.
- Flynn TR, Shanti MR, Levi HM, Adamo AK, Kraut AR, Trieger N. Severe Odontogenic Infections, Part 1: Prospective Report. J Oral MaxillofacSurg 2006; 64: 1093-1103.
- Woods R. Pyogenic dental infections: A ten year review. Aust Dental J 1978; 23: 107-111.
- Morey GF, Moule EJ, Higgins TJ. Pyogenic dental infections, a restrospective analysis. Aust Dent J 1984; 29: 150-153.
- Guralnick W. Odontogenic Infections. Br Dent J 1984; 156: 440-447.
- Walia IS, Borle RM, Mehendiratta D, Yadav AO. Microbiology and Antibiotic Sensitivity of Head and Neck Space Infections of Odontogenic Origin. J Maxillofac Oral Surg 2014; 13:16-21.
- Rega AJ, Aziz SR, Ziccardi VB. Microbiology and antibiotic sensitivity of head and neck space infections of odontogenic origin. J Oral MaxillofacSurg 2006; 64: 1377-1380.
- Santosh AN, Viresh AN, Sharmada BK. Microbiology and antibiotic sensitivity of odontogenic space infection. IJMDS 2014; 3: 303-313.
- Grodinsky M, Holyoke EA. The fasciae and fascial spaces of the head, neck and adjacent regions. Am J Anat 1938; 63: 367-408.
- Storoe W, Haug RH, Lillich TT. The changing face of odontogenic infections. J Oral Maxillofacial Surg 2001; 59: 739-748.
- Sandor GKB, Low DE, Judd PL, Davidson RJ. Antimicrobial Treatment Options in the Management of Odontogenic Infections. J Can Dent Assoc 2009; 7: 508-514.
- Kuriyama T, Nakagawa K, Karasawa T, Saiki Y, Yamamoto E, Nakamura S. Past administration of Beta lactam antibiotics and increase in the emergence of beta lactamase producing bacteria in patients with orofacialodontogenic infections. Oral Surg Oral Med Oral Pathol Oral RadiolEndod 2000; 89: 186-192.
- Baker KA,Fotos PG. The management of odontogenic infections. A rationale for appropriate chemotheraphy. Dent Clin North Am 1994; 38: 689-706.
- Kuriyama T, Karasawa T, Nakagawa K, Nakamura S, Yamamoto E. Antimicrobial susceptibility of major pathogens of orofacialodontogenic infections to 11 beta- lactam antibiotics. Oral MicrobiolImmunol 2002; 5: 285-289.