Research Article - Biomedical Research (2017) Volume 28, Issue 5
Medium and long-term follow-up in Chinese patients receiving low intensity warfarin anticoagulation treatment with low International Normalized Ratio (INR) after mechanical mitral valve replacement
Peizhe Tang1*, Qing Chang2, Mingfeng Dong1, Shengjun Ma11Department of Cardiovascular Surgery, Liaocheng People‘s Hospital, Liaocheng, PR China
2Department of Cardiovascular Surgery, Affiliated Hospital of Qiingdao University, PR China
- *Corresponding Author:
- Peizhe Tang
Department of Cardiovascular Surgery
Liaocheng People‘s Hospital, PR China
Accepted date: October 28, 2016
Abstract
Anticoagulation therapy was generally used in patients receiving mechanical mitral valve replacement. However, due to the increased risk of bleeding of anticoagulation therapy, it is important to control the International Normalized Ratio (INR) in a lower range to decrease the risk of bleeding. Therefore, the aim of this study was to investigate to which level the INR should be controlled and explore a suitable intensity of anticoagulation in Chinese Han population after mechanical mitral valve replacement. The experience of low INR anticoagulation using warfarin will also be summarized. We retrospectively recruited 747 patients who underwent mechanical cardiac mitral valve replacement in our institute. All of the patients received oral warfarin anticoagulation therapy with a target INR between 2.0 and 2.5 as the intensity of anticoagulation. Throughout the duration of follow-up, anticoagulation intensity reflected by INR and incidence of complications related to anticoagulation were analysed. The recruited patients were followed up for 6 to 135 months with an overall follow-up rate of 94.1% and a total of 1669.4 patient-years. Ten cases (0.60% patient-years) of cerebral emboli were diagnosed during the follow-up. Haemorrhage secondary to anticoagulation was observed in 39 cases (2.34% patient-years). A low INR oral warfarin anticoagulation therapy achieved satisfactory clinical effects with a low incidence of thromboembolism in Chinese Han population undergone mechanical mitral valve replacement. Thus, there may be room for reducing the intensity of anticoagulation to decrease the incidence of haemorrhagic complications in these patients.
Keywords
Prosthetic mechanical cardiac valve, Follow-up study, Anticoagulation treatment, Low international normalized ratio, Warfarin.
Introduction
Lifetime use of anticoagulants is unavoidable for patients who have undergone prosthetic mechanical mitral valve replacement [1,2]. In clinical practice, warfarin is the most commonly used oral anticoagulant. However, overanticoagulation still exists in the aspect of warfarin usage although International Normalized Ratio (INR) is being monitored [3,4]. Studies have shown that Asian patients with mechanical cardiac valves are more vulnerable to haemorrhage than thrombosis, which was opposite in patients from Europe and North America [5,6]. Therefore, we believe that a unified agreement or guideline particularly for anticoagulation treatment in Chinese population is desired and could be valuable in clinical practice although a number of guidelines for warfarin anticoagulation treatment for western countries patients have been published [7,8]. Thus, the purpose of the current study was to summarize our experience in a less intense oral warfarin anticoagulation therapy targeting low INR in Chinese population who had undergone mechanical mitral valve replacement, as well as to explore the suitable intensity of anticoagulation in this population.
Subjects and Methods
Patients
From January 2004 to December 2014, a total of 794 patients (395 male and 399 female) who received prosthetic mechanical mitral valve replacements and of Han origin were recruited in this study. The average pre-operative disease course was 11.3 ± 8.8 years. All patients were diagnosed with valvular heart disease by echocardiography before surgery. Male patients>45 years and female patients>50 years underwent either preoperative coronary artery CTA or coronary angiography to assess coronary artery disease. Patients with combined coronary artery disease and evidence of revascularization underwent concomitant coronary artery bypass grafting. Patients who had coronary artery disease combined with atrial fibrillation underwent a maze procedure or an intra-operative radiofrequency maze procedure. The study was approved by the Ethical Committee of the Institute.
Inclusion and exclusion criteria
The inclusion criteria included 1) Han patients born and mainly residing within the boundaries of Shandong, Hebei, and Henan Provinces 2) receiving medical mechanical valve 3) a past medical history without active rheumatism, bleeding events or haemorrhagic diseases before surgery, 4) normal liver and kidney function and 5) normal clotting time. The exclusion criteria included 1) those who received a Beijing GK or Medtronic unicuspid valve, 2) those who underwent AVR or TVR, 3) those with poor compliance, 4) those with cognitive disorder, mental, hearing or language disorders, and 5) those who declined to participate at any time during the study.
Anticoagulation treatment
An initial dose of 2.5 mg/d warfarin was given 1 d after surgery in patients who did not have contraindication of anticoagulation treatment. The coagulation status of patients was monitored every day while hospitalized. The INR was used to monitor anticoagulation intensity, and served as the basis for the oral warfarin dose adjustment until the standard was met. The target INR of Mitral Valve Replacement (MVR) patients was 2.3 (ranging from 2.0-2.5). Patients who met anticoagulation standards had their INR followed-up twice per week for 2 weeks after being discharged from the hospital and followed by the recommended schedule as follows: once every other day × 3 (week 1) → twice per week × 4 (weeks 2 and 3) → once per week × 4 (weeks 4, 5, 6, and 7) → once every 2 weeks × 4 (week 8) → once per month for life. INR test was performed once every other day after each dose adjustment.
Observation indices
The complications related to anticoagulation after cardiac valve replacement were determined using the specific guideline by the American Association for Thoracic Surgery (AATS). In the absence of infection, thromboembolism after valve replacement included central and peripheral emboli. Anticoagulation-related haemorrhage included massive internal and external bleeding that resulted in death, hospitalization, permanent organ damage (such as anopia), or requiring blood transfusion. Cerebral haemorrhage included all intracranial haemorrhage confirmed by imaging examinations.
Follow-ups
Patients were followed up on a regular basis according to the schedule mentioned in “anticoagulation treatment” section and the data were collected. Follow-up was carried out either over the telephone or by outpatient visit. Briefly, follow-ups of outpatient f of both local and out-of-town patients were carried out by their re-visit to our hospital or to other affiliated secondline hospital. For those were not able to conduct the follow-ups by revisiting hospital mentioned above, telephone call would be performed in this case.
Statistical analysis
SPSS16.0 was used in all statistical analysis in this study. Continuous parameters were presented as mean ± Standard Deviation (SD), and categorical data were shown in number and percentage (%).
Results
Patient clinical characteristics
325 out of the 794 patients were male patients and the average age of the overall population recruited in this study was 42.26 ± 11.33 years old. 392 out of 794 of these patients (49.4%) had a history of smoking and 392 out of 794 of these patients (53.8%) had a history of alcohol consumption. The coexistence of other cardiovascular diseases including atrial fibrillation, coronary artery disease, heart failure and diabetes were 208 (26.2%), 31 (3.9%), 103 (13.0%) and 11(1.4%) (Table 1). The etiology of their mitral valve disease included rheumatic disease, degenerative diseases, congenital diseases, aortic dissection, Marfan syndrome and aortic root aneurysm (Table 1).
| General information | |
|---|---|
| Gender (Male) | 325 (43.5) |
| Age (years) | 42.26 ± 11.33 |
| Height (m) | 1.72 ± 0.37 |
| Weight (kg) | 60.36 ± 20.53 |
| Smoking (yes) | 355 (47.5) |
| Alcohol consumption (yes) | 320 (42.8) |
| Cardiovascular disease n (%) | 208 (27.8) |
| Atrial fibrillation | 31 (4.1) |
| CHD | 103 (13.8) |
| Heart Failure | 11 (1.5) |
| Diabetes | |
| Etiology n (%) | |
| Rheumatic | 437 (58.50%) |
| Degenerative | 117 (15.66%) |
| Infective endocarditis | 94(12.58%) |
| Marfan syndrome | 18 (2.41%) |
| Aortic dissection | 29 (1.44%) |
| CHD: Coronary Heart Disease. | |
Table 1: General patient information and etiology.
Follow-ups of INR level, haemorrhagic and thrombosis complications
Among these 794 patients, 747 patients completed follow-up with an overall follow-up rate of 94.08%. No death was observed in these patients who finished the follow-up process. The duration of follow-up ranged from 6 months to 120 months with an average of 28.3 ± 16.2 months. This equalled to a total of 1669.4 patient-years. There were no massive haemorrhagic complications in all patients during their followups. INR values ranged mostly (90%) between 2.0 and 2.5, with an average of 2.3 ± 0.5. Ten cases of cerebral embolism were diagnosed during follow-up with incidence of 0.60% patient-years (Table 2). Incidence of other anticoagulation related complications were listed in Table 2. Briefly, haemorrhage events secondary to anticoagulation treatment occurred in 39 cases, and the incidence of severe haemorrhagic complications of long-term postoperative anticoagulation treatment was 2.34% patient-years (Table 2). In addition, the average INR of patients with haemorrhage complications was 3.07, while the average INR of patients with thrombus complications was 1.26.
| Anticoagulation-related complications | n (n/1669.4 • 100% patient-years) |
|---|---|
| Cerebral infarction | 10 (0.60%) |
| Hemarthrosis | 12 (0.72%) |
| Epistaxis | 10 (0.60%) |
| Bleeding gums | 7 (0.42%) |
| Gastrointestinal bleeding | 5 (0.30%) |
| Uterine bleeding | 4(0.24%) |
| Ophthalmorrhagia | 1 (0.06%) |
Table 2: Anticoagulation complications.
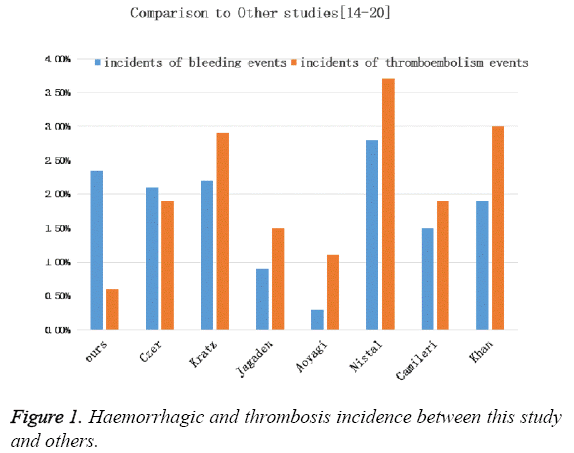
Since patients with atrial fibrillation need restrict INR control, we further compare warfarin dose, INR level and haemorrhage or thrombosis incidence between patient with and without atrial fibrillation (Table 3). We found that 27.84% of the population recruited had atrial fibrillation and there was no difference in warfarin dose, INR level and complication incidence when compared with those without atrial fibrillation (Table 3). We further compare the incidence of haemorrhagic and thrombosis events in our population and several reports from others and found that a relative low INR level did not resulted in an increased risk of haemorrhage (Figure 1).
| Atrial fibrillation | n (%) | Warfarin dose (mg/d) | INR | Haemorrhage (%) | Thrombus (%) |
|---|---|---|---|---|---|
| Yes | 208 (27.84%) | 2.42±0.59 | 2.39±0.57 | 4 (1.92%) | 3 (1.44%) |
| No | 539 (72.15%) | 2.11±0.83 | 2.21±0.52 | 6 (1.44%) | 5 (1.20%) |
Table 3: Warfarin doses, INR values, and complication rate of patients with and without atrial fibrillation.
Discussion
Prosthetic cardiac valve replacement is the one of the most preferred approaches to treat severe valvular heart disease. However, histological difference does exist between prosthetic cardiac valves and normal human valves. A prosthetic mechanical cardiac valve is not covered with endothelial cells, the most important cellular type in activating coagulation cascade and inducing thrombi formation on the prosthetic valve. Such process could largely affect the opening and closing of valve leaflets, and thus resulting in valve dysfunction. Once the severe thrombosis took place, the thrombi would completely block the opening of the valve orifice such that it ultimately led to heart failure or even sudden death. Thus, patients with prosthetic cardiac valve replacement should receive anticoagulation treatment. Although new anticoagulation medicines including Factor X (rivaroxaban and apixaban) and thrombin inhibitors have become used in clinics recently, the indications for these new anticoagulants were limited to hip and knee joint replacement. Thus, the traditionally used anticoagulant warfarin is currently the only effective medication for patients with prosthetic cardiac valve replacement supported by large scale clinical studies. However, despite of the fact that warfarin is inexpensive and commonly used oral anticoagulant in clinical practice, it has a relatively narrow safety range because its therapeutic dose is close to the toxic dose and large individualto- individual dose effect difference exists. Therefore, carelessness in monitoring the dose and INR level may result in complications [2,9] such as haemorrhage due to overanticoagulation or thromboembolism due to underanticoagulation [1,10,11]. The dose of warfarin had been adjusted by monitoring its influence on the extrinsic coagulation system (Prothrombin Time (PT)) after oral administration. However, the PT values determined in different laboratories using different reagents are not comparable with each other. Hence, the INR is currently used as the monitoring index as a more accurate parameter in reflecting the anticoagulation treatment intensity. The safety and effectiveness of warfarin treatment mainly relies on whether or not INR is maintained within the safe and effective therapeutic range. Therefore, the anticoagulation intensity of the optimum efficacy and the lowest risk needs to be identified. In terms of the optimum anticoagulation treatment intensity standard, the guidelines from the European Society of Cardiology (ESC) recommend that the anticoagulation intensity should be proportional to the degree of thromboembolic risk. The recommended INR of first-generation valves is 3.0-4.5, 3.0-3.5 for second-generation valves and mitral valves, and 2.5-3.0 for aortic valve. According to the guidelines from the American College of Chest Physicians (ACCP) published in 2008, the INR value should be maintained between 2.5 and 3.5 for most mechanical valves and 2.0-3.0 for low-risk patients with a bioprosthetic or aortic mechanical valve [12].
Due to the diversity of ethnicities, regions, and dietary habits, coagulation function varies between different populations. Therefore, optimized anticoagulation treatment practices in western countries should simply apply to Chinese population with caution. It was reported that coagulation time of the Asian ethnics is slower than patients from western countries, and thus, Asians are more vulnerable to haemorrhage [13]. Some clinical studies have proposed that low intensity anticoagulation treatment should be used for Chinese patients who have undergone cardiac valve replacement (i.e., the INR should be maintained between 1.5 and 2.0). The optimum value for the upper limit of the INR should be 2.5 for Chinese patients treated with warfarin, and the highest threshold should be 3.0 according to the research from Chinese populations. According to the study conducted by Liu et al. from Beijing Anzhen Hospital, warfarin anticoagulation treatment after prosthetic mechanical cardiac valve replacement with an INR maintained between 1.3 and 2.3 could achieve satisfactory prevention of thromboembolism and reduce the incidence of haemorrhagic complications related to anticoagulation [14-16]. The study conducted by Liu et al. [17] from Guangdong Provincial Cardiovascular Institute in 2010 also showed that the incidence of thromboembolic complications and severe haemorrhage events were similar between low intensity anticoagulation therapy which controls the INR between 1.5-2.0 and standard intensity anticoagulation therapy which controls INR between 2.5-3.5.
In the current study where the follow-up data of 747 patients who underwent prosthetic mechanical mitral valve replacement was analysed, the INR was control between 2.0-2.5, representing the low intensity anticoagulation treatment. We found that the anticoagulation intensities of 626 patients (83.80%) were within the expected anticoagulation range, 82 (10.98%) were below the expected anticoagulation criteria, and 39 (5.22%) exceeded the expected anticoagulation criteria, thus basically achieving the expected anticoagulation goal.
Among the patients included in this study, 10 cases of cerebral embolism were diagnosed during the follow-up and the incidence was as low as 0.60% patient-years which could possibly due to the insufficient post-operative anticoagulation intensity, the left atrial thrombosis due to atrial fibrillation or a relatively low trans-mitral blood flow velocity after MVR. Among the included patients, haemorrhage secondary to anticoagulation was observed in 39 cases, and the incidence of severe haemorrhagic complications in patients with long-term post-operative anticoagulation treatment was 2.34% patientyears possibly due to the relatively low intensity of anticoagulation treatment in this study, the lack of imaging methodology to confirm haemorrhage other than intracranial haemorrhage based on AATS guideline or low compliance of patients. Thus, the follow-up rate in the current study was 94.08%, which may have affected the overall incidence of haemorrhage.
To further compare our data from those of with western countries [9,18-20], the incidence of total thromboembolism and haemorrhage complications was 2.94%/person-years. However, a much lower incidence of thromboembolism was achieved with a similar incidence of haemorrhage in our study. Thus, our current anticoagulation standard to control INR in the range of 2.0-2.5 met the anticoagulation requirements for patients after MVR.
There are several limitations in this study. First, this study is a single center study with a relatively small size of the samples and the short follow-up period. Therefore the findings from this study need to be further confirmed by large-scale multicenter clinical studies. Secondly, for the same reason, whether or not the target INR needs to be adjusted to an even lower level to reduce the incidence of haemorrhage also needs to be determined through long-term follow-up with larger samples. Third, we were not able to analyse the survival outcome or report Kaplan-Meier curves in this study due to a relatively shorter period of follow-up. We are planning a longer period of follow-up study to overcome the limitation of this study.
Acknowledgments
None
Conflict of Interest
No conflict of interest associated with this work.
Contribution of Authors
We declare that this work was done by the author(s) named in this article and all liabilities pertaining to claims relating to the content of this article will be borne by the authors.
References
- Yang JC, Makaryus AN. Warfarin Anticoagulation and Spontaneous Pectoral Haematomas. Heart Lung Circ 2016; 25: e81-84.
- Bazan NS, Sabry NA, Rizk A, Mokhtar S, Badary OA. Factors affecting warfarin dose requirements and quality of anticoagulation in adult Egyptian patients: role of gene polymorphism. Ir J Med Sci 2014; 183: 161-172.
- McGuinn TL, Scherr S. Anticoagulation clinic versus a traditional warfarin management model. Nurse Pract 2014; 39: 40-46.
- Eikelboom JW, Hart RG. Intensity and quality of warfarin anticoagulation in Chinese patients: setting the record straight. Stroke 2015; 46: 5-6.
- Tatsuno SY, Tatsuno EM. Does ethnicity play a role in the dosing of warfarin in Hawaii? Hawaii J Med Public Health 2014; 73: 76-79.
- Shen AY, Chen W, Yao JF, Brar SS, Wang X. Effect of race/ethnicity on the efficacy of warfarin: potential implications for prevention of stroke in patients with atrial fibrillation. CNS Drugs 2008; 22: 815-825.
- Salem DN, Stein PD, Al-Ahmad A, Bussey HI, Horstkotte D, Miller N, Pauker SG. Antithrombotic therapy in valvular heart disease-native and prosthetic: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126: 457-482.
- Bavishi C, Koulova A, Bangalore S, Sawant A, Chatterjee S. Evaluation of the efficacy and safety of dual antiplatelet therapy with or without warfarin in patients with a clinical indication for DAPT and chronic anticoagulation: A meta-analysis of observational studies. Catheter CardiovascInterv 2016; 88: E12-22.
- Arbring K, Uppugunduri S, Lindahl TL. Comparison of prothrombin time (INR) results and main characteristics of patients on warfarin treatment in primary health care centers and anticoagulation clinics. BMC Health Serv Res 2013; 13: 85.
- Bishop MA, Streiff MB. Effects of anticoagulation provider continuity on time in therapeutic range for warfarin patients. J Thromb Thrombolysis 2016; 42: 283-287.
- Yamashita T, Inoue H, Okumura K, Atarashi H, Origasa H. Warfarin anticoagulation intensity in Japanese non-valvular atrial fibrillation patients: a J-RHYTHM Registry analysis. J Cardiol 2015; 65: 175-177.
- Salem DN, OGara PT, Madias C, Pauker SG. Valvular and structural heart disease: American College of Chest Physicians evidence-based clinical practice guidelines Chest (8th Edn.) 2008; 133: 593S-629S.
- Mori T, Asano M, Ohtake H, Bitoh A, Sekiguchi S, Matsuo Y, Aiba M, Yamada M, Kawada T, Takaba T. Anticoagulant therapy after prosthetic valve replacement -optimal PT-INR in Japanese patients. Ann ThoracCardiovascSurg 2002; 8: 83-87.
- Meng XLJ, Liu Y, Zhang HB. The uniform standard intensity of oral anticoagulant therapy for the patients with mechanical heart valve prostheses. Chinese J Cardiol 2004; 32: 618-621.
- Haibo Z, Jinzhong L, Yan L, Xu M. Low-intensity international normalized ratio (INR) oral anticoagulant therapy in Chinese patients with mechanical heart valve prostheses. Cell BiochemBiophys 2012; 62: 147-151.
- Liu YMX, Chen B. Clinical results of the low intensity of oral anticoagulant therapy for patients with mechanical heart valve prostheses. Chinese J ThoracCardiovasclSurg 2001; 5: 263.
- Liu YYX, Zhong SL, Yang M, Tan HH, Fei HW, Chen JY. Clinical application of anticoagulation treatment with warfarin after prosthetic heart valve replacement: a single center-based survey. J South Med Univ 2010; 30: 2242-2245.
- Haines DE, Mead-Salley M, Salazar M, Marchlinski FE, Zado E, Calkins H, Yarmohammadi H, Nademanee K, Amnueypol M, Skanes AC, Saklani P. Dabigatran versus warfarin anticoagulation before and after catheter ablation for the treatment of atrial fibrillation. J Interv Card Electrophysiol 2013; 37: 233-239.
- Camilleri LF, Bailly P, Legault BJ, Miguel B, DAgrosa-Boiteux MC, de Riberolles CM. Mitral and mitro-aortic valve replacement with SorinBicarbon valves compared with St. Jude Medical valves. CardiovascSurg 2001; 9: 272-280.
- Khan SS, Trento A, DeRobertis M, Kass RM, Sandhu M. Twenty-year comparison of tissue and mechanical valve replacement. J ThoracCardiovascSurg 2001; 122: 257-269.