- Biomedical Research (2014) Volume 25, Issue 4
Lipid profile and its effect on kidney in pregnancy-induced preeclampsia: A prospective case-controlled study on patients of Riyadh, Saudi Arabia.
Noura Al-Jameil1*, Hajera Tabassum1, Mir Naiman Ali2 and Mohammed Abdul Qadeer3
1Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Saud University, Riyadh, K.S.A
2Department of Microbiology, Mumtaz Degree & P.G. College, Hyderabad, India
3Cytocare Technologies Private Limited, Hyderabad, India
- *Corresponding Author:
- Noura Al Jameil
Department of Clinical Laboratory Sciences
College of Applied Medical Sciences
King Saud University, Riyadh, Saudi Arabia
Accepted date: June 21 2014
Abstract
Preeclampsia is characterized by development of high blood pressure and proteinuria. It affects 5–8% of all pregnancies and is a major contributor to maternal and fetal morbidity and mortality. In Saudi Arabia, the incidence of preeclampsia is extrapolated to 13,876 out of a population of 25,795,938.In view of increasing number of preeclamptic cases in women of Riyadh, Saudi Arabia, the objectives of this work was to quantitate and find out the significance of lipid profile levels in preeclamptic pregnancy in comparison with normal pregnancy and also to study the relation between the lipoprotein levels and their effects on kidney. A total of 120 pregnant women were selected in this case-controlled study and divided into three groups; control, high-risk of preeclampsia (HR) and preeclampsia (PET) of 40 each. Blood samples were obtained from all the patients, and the serum levels of triglycerides (Tg), total cholesterol (TC), low-density lipoproteins (LDL), high-density lipoproteins (HDL) and very low- density lipoproteins (VLDL), platelet count, 24-hour urinary protein and creatinine were determined. Comparison of different biochemical parameters among the three groups was made by one -way ANOVA. There was significant increase (p<0.001) in levels of VLDL-C, LDL-C and triglyceride levels in the women with preeclampsia compared to healthy pregnant women and the high-risk group. Levels of HDL-C was found to decrease significantly (p<0.001) in preeclampsia group compared to control and high-risk group. There was positive correlation between urinary creatinine and different types of lipoproteins, triglycerides and cholesterol. Negative correlation was observed between VLDL and triglyceride with proteinuria and a positive correlation between cholesterol, LDL and HDL with proteinuria was evident in preeclampsia cases. In conclusion, our data suggest that pregnant women with preeclampsia have abnormal lipid profile compared to normal pregnant women. In patients with preeclampsia, increased levels of LDL and cholesterol were positively correlated with urinary creatinine and protein. These observations indicate that the pregnant woman who has elevated lipid levels and abnormal increase in excretion of protein and creatinine are more susceptible to cardiovascular disorders and consequently preeclampsia. Hence, the abnormal lipid profile in pregnant women with increased blood pressure acts as predictor in diagnosis of preeclampsia in early stages of pregnancy.
Keywords
Abnormal lipid profile, Proteinuria, Preeclampsia, Lipoproteins
Introduction
Preeclampsia is a common medical complication of pregnancy. In Saudi Arabia, the incidence of preeclampsia is extrapolated to 13, 876 out of a population of 25,795,938 [1]. Preeclampsia is a non-convulsive form of pregnancy-induced hypertension and accounts for maternal and fetal morbidity and mortality [2]. Preeclampsia when complicated with convulsion is called eclampsia. In healthy pregnancies, adaptive changes take place in women’s physiology to meet the demands of the rapidly developing fetus. In pregnancies complicated by preeclampsia, these normal adaptive metabolic responses are further exaggerated [3]. Preeclampsia occurs during second and third trimester of pregnancy and is more common in nulliparous women. It is characterized by development of high blood pressure (hypertension) and proteinuria after 20 weeks of gestation and affects about 5-8% of all pregnancies. In preeclampsia, the systolic and diastolic BP are 140 mmHg and 90 mmHg, respectively with proteinuria 0.3 gm in a 24-hour urine sample or equal to 1+ or 100 mg/dl by dipstick response. Severe preeclampsia is associated with either or both of the elevated BP (160 mmHg systolic/110 mmHg diastolic) with proteinuria >5 g in a 24-hour urine collection [4]. During pregnancy, estrogen induces hepatic biosynthesis of endogenous triglycerides, which is carried by VLDL and leads to triglyceridemia. This may also be due to hyper-insulinism found in pregnancy [5,6]. Triglyceriderich lipoproteins may also trigger endothelial dysfunction and atherothrombosis [7]. Endothelial cell dysfunction is a key feature in the pathogenesis of preeclampsia. Pathophysiological abnormalities seen in preeclampsia including placental ischemia, generalized vasospasm, abnormal hemostasis with activation of the coagulation system, abnormal lipid metabolism with proteinuria may be explained by endothelial dysfunction. An abnormal lipid profile is known to be strongly associated with atherosclerotic cardiovascular disease and has a direct effect on endothelial function [8].
Preeclampsia may affect functioning of various organs involved in lipid and lipoprotein metabolism. Several studies have shown that endothelial dysfunction is related to hyperlipidemia [9]. Abnormal lipid profiles and species may have a role in the promotion of oxidative stress and vascular dysfunctions seen in preeclampsia. Disorders of lipoprotein metabolism are considered to be a major cause of hypertension and proteinuria in preeclampsia. The cause of the endothelial cell injury is probably multifactorial, but poor placenta perfusion plays a major role [10]. In preeclampsia, characteristic pathological lesions in the placenta are fibrin deposits, acute atherosis and thrombosis. Increased triglycerides (Tg), found in pregnancy induced -hypertension is likely to be deposited in predisposed vessels, such as the uterine spiral arteries and contributes to the endothelial dysfunction, both directly and indirectly through generation of small dense LDL. Increased triglycerides may also be associated with hypercoagulability [10].
Lipid deposits are known to get accumulated in the glomeruli of preeclamptic patients, a finding known as glomerular endotheliosis. Glomerular lesions are accompanied with proteinuria, a predictive indicator and marker of disease severity. The severity of both hypertension and proteinuria seems to reflect the degree of endothelial damage and the possible correlation between the altered lipid profile and the severity of renal lesions, as reflected by proteinuria, may contribute to clarify the complex pathophysiology of preeclampsia. Thus, estimation of lipid profile may have a predictive role in the assessment of the extent of endothelial damage in preeclampsia and may help patient by preventing or foreseeing the effects of complications in preeclampsia.
In view of above and growing number of cases of preeclampsia in Riyadh, which is a central province of Kingdom of Saudi Arabia, the present study was undertaken to a) analyze the lipid profile (total cholesterol (TC), triglycerides (Tg), HDL-cholesterol (HDL-C), LDL-cholesterol (LDL-C) and VLDL-cholesterol (VLDL-C) in normal pregnant and preeclamptic women and women at high risk of the disease and b) to correlate the effect of different lipoproteins and cholesterol on excretion of creatinine and protein in preeclamptic women compared to normal pregnancy.
Materials and Methods
Study population
This cross sectional study was carried out in the Department of Clinical Laboratory Sciences, King Saud University and Section of Obstetrics and Gynecology, King Saud Medical City Hospital, Riyadh from September 2012 to December 2013. Hospital’s ethics committee approved the study. Informed consent was obtained from patients before blood sampling.
A total of 120 pregnant women were enrolled in this casecontrolled study and divided into three groups of 40 each; healthy normotensive pregnant women (control group), pregnant women at high risk of preeclampsia (HR group) and women with preeclampia (PET group). All study subjects were attending antenatal OPD or labor room in their third trimester of pregnancy.
Inclusion criteria
Control; pregnant women with normal BP, absence of proteinuria and without any other systemic or endocrine disorder and age-matched with the cases. All subjects included were in their third trimester (gestational age of =24 weeks).
High-Risk group; Women in high risk group were included based on the following criteria: pregnant women with body mass index (BMI) of 35 or more, with mild hypertension or those with Preeclampsia, gestational diabetes, IUGR (intrauterine growth restriction) or preterm delivery in previous pregnancies and those with family history of preeclampsia.
PET group; Selection and the diagnosis of preeclamptic group were based on the definition of American College of Obstetrics and Gynecologists [11].
Exclusion criteria
Patients with obesity, severe anemia or with dyslipidemia were excluded from the study.
Blood samples were obtained from patients attending OPD or admitted into hospital. On admission, venous serum samples were collected when the patients were in the supine position prior to commencement of intravenous therapy. At the time of blood collection, urine protein was measured by dipstick and was graded on a scale of 0-4+ (0,none; 1+,30 mg/dl; 2+,100 mg/dl; 3+,300-1,999 mg/dl; 4+,at least 2,000 mg/dl). Blood samples were analyzed for complete blood count, determination of lipid profile, serum and urinary protein.
Analysis of lipoproteins and Urinary protein
All biochemical parameters were determined in the biochemical analyzer, COBAS INTEGRA Autoanalyzer 800 (Roche, Germany). Total cholesterol was estimated by CHOD-PAP Generation 2., ID/MS in COBAS INTEGRA Autoanalyzer 800.Triglycerides were estimated by GPO-PAP method. LDL-C was estimated using 2nd generation and HDL-C using 3rd generation COBAS INTEGRA Autoanalyzer 800. Serum and urinary total protein was determined by biuret Gen 2 method and creatinine was estimated by jaffes Gen 2 method. Hematocrit (Hct) concentration was measured in automated Cell Dyne 3700 analyzer and platelet count was obtained using automatic reader, STA compact, Mediserv, UK.
Calculation of VLDL-C: VLDL-C level in serum is derived by dividing serum triglycerides by 5 using Friedwald’s formula [12].
Statistical analysis
The results were expressed as Mean ± S.D. Statistical analyses were performed using SPSS software. Comparison of clinical characteristics and biochemical parameters of cases with control among the groups was performed by one way-ANOVA followed by Holm Sidak test. The correlation between mean values of lipoproteins, urinary protein and creatinine in preeclamptic group were evaluated with Pearson’s correlation coefficient. P < 0.05 was considered to be statistically significant.
Results
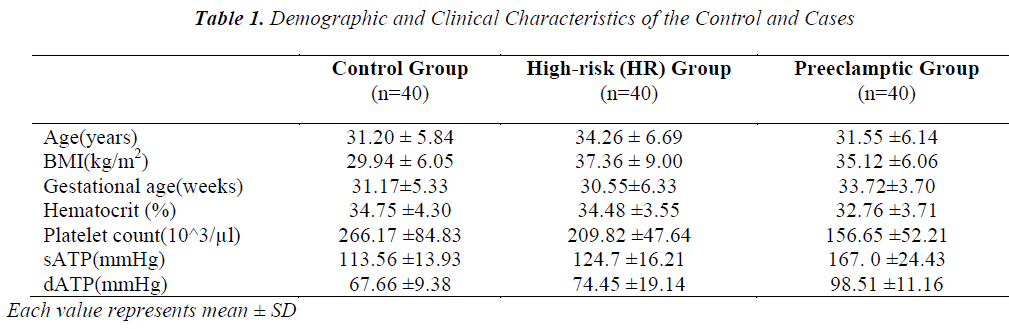
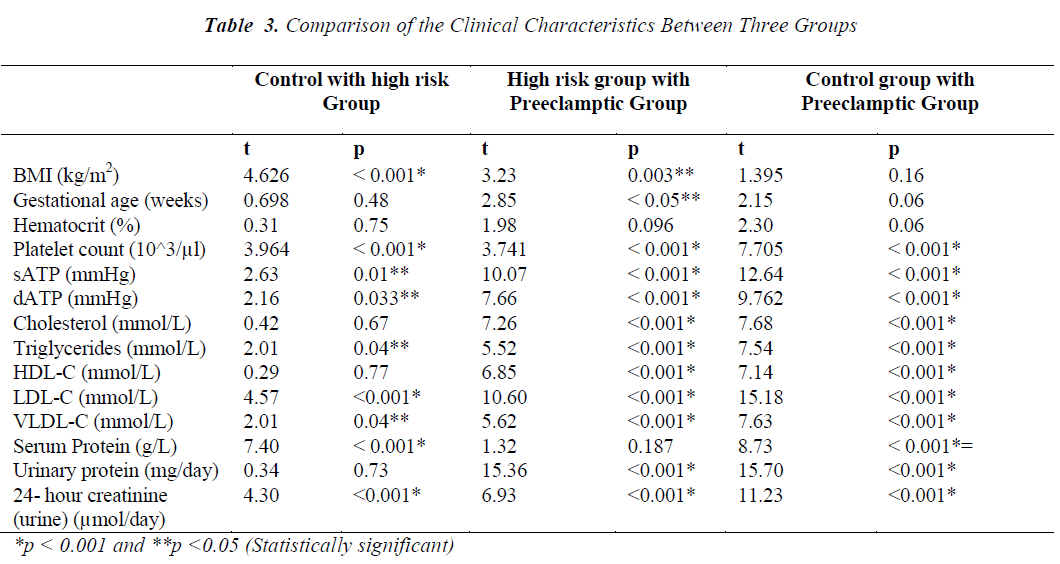
The present study enrolled 120 pregnant women divided into three groups; control, HR and PET. Table 1 depicts values (mean ± SD) of the clinical characteristics of control and cases. Age and hematocrit values among control, HR and PET were not found to be significantly different. Preeclamptic group has high gestational age compared to control and HR group. BMI of HR group (37.36 ± 9.005 kg/m2) was found to be high compared to control and PET groups (29.94 ± 6.05 and 35.12 ± 6.06 kg/m2, respectively). BMI of HR group was significantly different from control and PET groups. BMI was not significantly different between control and PET groups. The mean value of systolic arterial blood pressure (sATP) of control group and HR group was 113.56 ± 13.93 mmHg and 124.70 ± 16.21mmHg, respectively while in PET group the sATP was 167.00 ± 24.43 mmHg. There was significant difference of sATP between the control and HR groups and similar differences were observed between the control and PET groups also between HR and PET groups. The diastolic arterial blood pressure (dATP) was found to be high in PET group (98.51 ± 11.16) compared to control and HR groups (67.66 ±9.38 and 74.45 ± 19.14, respectively). We observed a significant difference in dATP between control and PET, HR and PET group (p < 0.001) also a significant difference between control and HR group (p < 0.05). Similarly, when platelet count was compared between the three groups, we found that platelet count decreased in PET group compared to control and HR groups and this decrease was observed to be statistically significant (p< 0.001).
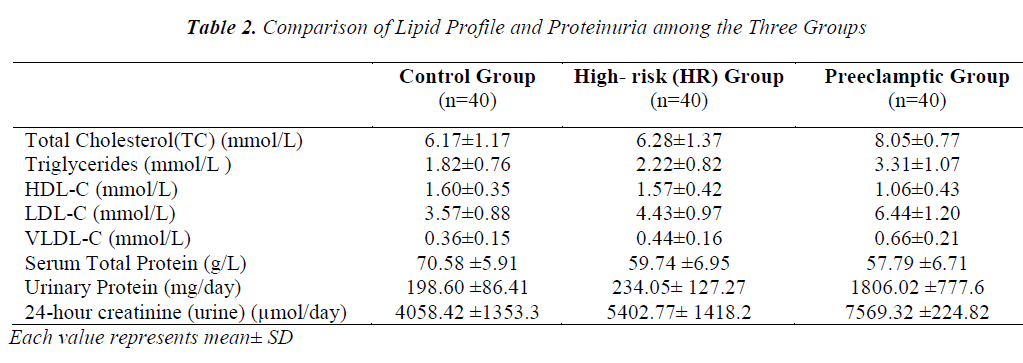
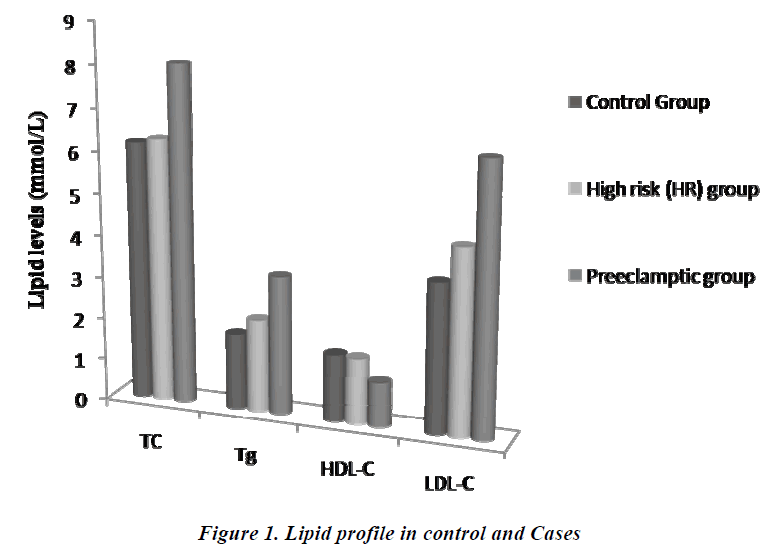
The comparison of lipid profile within three groups is represented in Table-2. The mean value of total cholesterol (TC) in PET group (8.05±0.78mmol/L) was significantly higher (p<0.001) than control and HR groups (6.17±1.17 and 6.28±1.37mmol/L, respectively). The results of ANOVA for individual parameters in the control, HR and the PET groups are shown in Table-3. Total cholesterol levels were not significantly different between control and HR groups, but found to be significantly different (P<0.001) between HR and PET groups. Similarly, the mean value of serum Tg in PET group (3.31±1.07) was significantly higher (p<0.001) than control and HR groups (1.80± 0.76 and 2.22 ± 0.82, respectively). Tg levels were found to be statistically significant (p<0.05) between the control and HR groups.
The mean value of HDL-C in PET group (1.06 ±0.43mmol/L) was significantly decreased (p<0.001) compared to control and HR groups (1.60±0.35 and 1.57±0.42 mmol/L, respectively).The decrease in HDL-C was also found to be statistically significant (p<0.001) between HR and PET groups. In contrast to HDL-C, the mean value of LDL-C (6.44±1.20mmol/L) was increased significantly (p<0.001) in PET group compared to control and HR groups (3.57±0.88 and 4.43±0.97, respectively). This increase in LDL-C was also found to be statistically significant (P<0.001) between the control and HR groups. Like LDL-C, the mean value of VLDL-C in PET groups (0.66±0.21) was increased significantly (p<0.001) compared to control and HR groups (0.36±0.15 and 0.44 ±0.16, respectively). Levels of VLDL-C between the control and HR groups (p<0.05) were found to be significantly different too.
In contrast to serum LDL-C and VLDL-C, the mean value of serum total protein (57.79 ±6.71 mmol/L) was found to be significantly lower (p<0.001) in PET group compared to control and HR groups (70.58±5.91 and 59.74±6.95 mmol/L, respectively). The decrease in serum total proteins was also found to be significantly different (p<0.001) between control and HR groups. Unlike serum proteins, the mean value of protein in 24 hour- urine of PET group (1806.02±777.6 mg/day) was increased significantly (p<0.001) compared to control and HR groups (198.60±86.41 and 234.05±127.27 mg/day, respectively). However, no such change was evident between the control and HR groups. Similarly, the mean value of urinary creatinine increased significantly in PET group (7569.32 ± 224.82 µmol/l) compared to control and HR groups (4058.42 ± 1353.39 and 5402.77 ± 1418.24 µmol/l, respectively). The increase in levels of urinary creatinine was found to be statistically significant (p < 0.001) among all the groups.
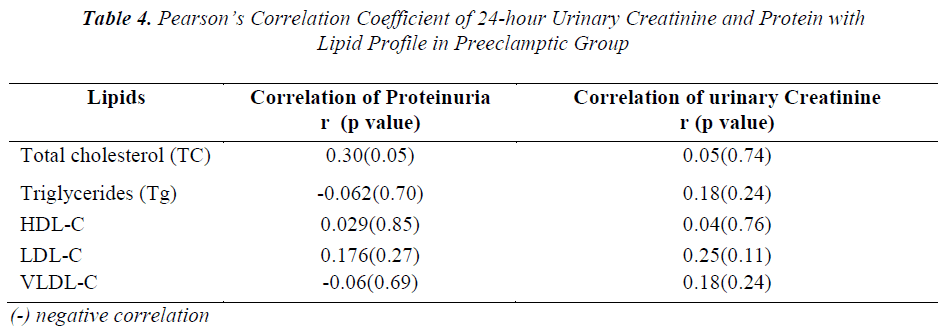
To know the correlation between the serum levels of total cholesterol, triglycerides and lipoproteins with urinary protein and creatinine in preeclampsia cases, Pearson’s correlation coefficient was performed and the results are depicted in Table-4. A positive correlation was evident between levels of urinary creatinine and serum cholesterol, Tg, LDL,VLDL,HDL and between urinary protein and serum cholesterol, LDL and HDL levels; whereas negative correlation was observed between VLDL-C and Tg with proteinuria in preeclamptic group.
Discussion
Preeclampsia is a hypertensive disorder of pregnancy characterized by vasospasm, proteinuria and edema. Endothelial dysfunction plays a vital role in the development of preeclampsia. Multiple circulating factors may provoke endothelial changes, including altered profile of lipoproteins. Serum lipids have a direct effect on endothelial dysfunction. Lipid peroxides are normally present in lipoproteins and seem to contribute to vascular tone regulation. An abnormal lipid profile is known to be strongly related with proatherogenic lipid profiles indicating high risk for coronary artery diseases. Altered lipid synthesis may be important in pathogenesis of preeclampsia. Although no agreement exists in the literature about changes in LDL and HDL-C in preeclampsia, different lines of evidence indicate that abnormal lipid metabolism is not merely a manifestation of preeclampsia, however, it is directly involved in its pathogenesis [13].
Lipid metabolism is dramatically altered during pregnancy. There is an increase in activity of hepatic lipase and decrease in lipoprotein lipase activity [14]. Hepatic lipase is responsible for increased synthesis of triglycerides at the hepatic level, whereas the decreased activity of lipoprotein lipase is responsible for the decreased catabolism at the adipose tissue level, the net effect of which will be an increase in circulating triglycerides. Hypertriglyceridemia is probably the consequence of competition between chylomicrons and very low-density lipoprotein cholesterol for the lipoprotein lipase. Chylomicron clearance occurs in two sequential steps: triglycerides hydrolysis by lipoprotein lipase and uptake of the remnant by the liver. Delay in the second step leads to accumulation of remnants in plasma and is generally thought to represent the atherogenic risk of hypertriglyceridemia. Because of decrease in the activity of lipoprotein lipase, VLDL remains in the plasma for longer and leads to the accumulation of LDL. An increase in LDL is associated with the development of atherosclerosis [15]. Women with preeclampsia display additional alterations in blood lipids reflecting disordered lipid and lipoprotein metabolism. In the present study, serum Tg levels were found to be high in preeclampsia group compared to control and the HR groups. This is in agreement with earlier studies [16,17]. High triglyceride levels seem to increase the risk of placental vascular disorders, which trigger endothelial dysfunction, atherosclerosis and thrombosis [18]. The development of atherosclerosis in the placental spiral arteries of preeclamptic women indicates that elevated levels of triglycerides are involved in this disorder.
In our study, there was significant increase (P<0.001) in TC levels in the PET group when compared to control and HR groups, which was identical to other report [19]. LDL-C and VLDL-C levels were observed to increase significantly in PET group as compared to controls. The results obtained are in concordance with previous report too [20]. In contrast to TC and LDL-C, there was significant fall in HDL-C in PET group compared to control and HR group. Decreased level of HDL-C in PET group could be due to insulin resistance and the decrease in activity of lipoprotein lipase may result in further lowering the HDL-C in preeclamptic patients [17].Therefore, altered lipid metabolism or the abnormal lipid profile was found to be associated with patients at high-risk and in PET groups. Abnormal lipid profile in HR group observed in this study compared to control is an indication or an alarm for progression of the disease to severe preeclampsia.
Women with preeclampsia are known to develop arterial lesions at the utero-placental implantation site. These morphological lesions are usually observed in cases of acute atherosclerosis, and are characterized by areas with fibrinoid necrosis surrounded by lipid-laden macrophages. Lipid deposits are also seen in the glomeruli of preeclamptic patients, a finding known as glomerular endotheliosis. Glomerular lesions are associated with proteinuria, a predictive indicator and marker of disease severity [21]. It has also been suggested that LDL and Tg may be involved in this renal damage [22]. In our study when different lipids and lipoproteins were correlated with proteinuria and urinary creatinine, we observed a positive correlation between urinary creatinine and protein with respect to cholesterol, LDL-C and HDL-C (decrease levels of HDL-C and increase levels of LDL-C). Tg and urinary creatinine were also positively correlated. The increased excretion of protein and creatinine by preeclamptic patients could be due to increased levels of Tg and LDL-C in serum. These findings suggest that these lipids may be involved in the endothelial damage observed in preeclamptic patients.
Based on our findings, we suggest that the pregnant women with slight variation in the blood pressure should go for complete analysis of lipid profile. Estimation of lipid profile may have a predictive role in the assessment of the extent of endothelial damage in preeclampsia and may help the patient by preventing or foreseeing the effects of complications in preeclampsia.
Conclusion
Based on the data obtained in our study, we conclude that the women who develop preeclampsia has abnormal lipid profile. Increased triglycerides and LDL-C levels and decreased levels of good cholesterol (HDL) along with high blood pressure play a crucial role in the development of preeclampsia. This may add to better understanding of the pathological process of preeclampsia which could open doors for development of new therapeutic strategies for prevention and early diagnosis of preeclampsia. Therefore, pregnant women should receive adequate counseling to urge them to adopt healthier habits and lifestyles and to seek periodic checkups, in order to detect cardiovascular disease in its early stages, before irreparable damage.
Acknowledgements
The authors are grateful to the “Research Center of the ‘Center for Female Scientific and Medical Colleges’, Deanship of Scientific Research, King Saud University” for the grant. Authors are also thankful to Dr. Huda Al- Mayouf, Section of Obstetrics and Gynecology and the Management, King Saud Medical City Hospital, Riyadh, Kingdom of Saudi Arabia, for providing samples and facilities for carrying out the study.
References
- Statistics by country http://www.rightdiagnosis.com/p/preeclampsia/ stats-country.htm (03-12-2013).
- Vanderjagt DJ, Patel RJ, El-Nafaty AU, et al. High density lipoprotein and homocysteine levels correlate inversely in preeclamptic women in northern Nigeria. Acta Obstet Gyneacol Scand 2004; 83: 536-542.
- Jimenez DM, Pocovi M, Ramon-Cajal J,et al. Longitudinal study of plasma lipids and lipoprotein cholesterol in normal pregnancy and puerperium. Gynecol Obstet Invest 1988; 25: 158-164.
- Ray JG, Diamond P, Singh G, et al.Brief overview of maternal triglycerides as a risk factor for preeclampsia. Br J Obstet Gynaecol 2006; 113: 379-386.
- Glueck CJ, Fallet RW, Scheel D. Effects of oestrogenic compounds on triglyceride kinetics. Metabolism 1975; 24: 537-545.
- Adegoke OA, Iyare EE, Gbenebitse SO. Fasting plasma glucose and cholesterol levels in pregnant Nigerian women. Niger Postgrad Med J 2003; 10: 32-36
- Kugiyama K, Doi H, MotoyamaT,etal. Association of remnant lipoprotein levels withimpairment of endothelium dependent vasomotor function in human coronary arteries. Circulation 1998 ;97: 2519-2526
- Stewart DJ, Monge JC. Hyperlipidemia and endothelial dysfunction. Curr Opin Lipidol 1993; 4: 319-324.
- Roberts JM, Taylor RN, Musci TJ, et al. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol 1989; 161:1200-1204.
- Kokia E, Barkai G, Reichman B, et al. Maternal serum lipid profile in pregnancies complicated by hypertensive disorders. J Perinat Med 1990; 18:473-478.
- ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol 2002; 99: 159-167
- William TF, Robert IL, Donald SF.Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. ClinChem1972; 18: 499-502.
- Gratacos E. Lipid-mediated endothelial dysfunction: a common factor to preeclampsia and chronic vascular disease. Eur J Obstet Gynecol Reprod Biol 2000; 92: 63-66.
- Kaaja R, Tikkanen MJ. Serum lipoproteins, insulin and urinary prostanoid metabolites in normal and hypertensive pregnant women. Obstet Gynecol 1995; 85: 253-256.
- Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med 1999; 340: 115-126
- Enquobahrie DA, Williams MA, Butler CL, et al. Maternal plasma lipid concentrations in early pregnancy and risk of preeclampsia. Am J Hypertens 2004;17: 574–581
- Cekmen MB, Erbagci AB. plasma lipid and lipoprotein concentration in pregnancy induced hypertention. Clin Biochem 2003; 36: 575-578.
- Patrizia B, Giancarlo T, Franca E, et al. Lipoprotein metabolism during normal pregnancy. Am J Obstet Gynecol 1999; 181: 430-434.
- Rubina A, Tabassum M. Pre-eclampsia and lipid profile. Pak J Med Sci 2007; 23: 751-754.
- Gratacos E, Casals E, Gomez O, et al. Increased susceptibility to low density lipoprotein oxidationin women with a history of preeclampsia. Br J Obstet Gynaecol 2003; 110: 400-404
- Airoldi J, Weinstein L. Clinical significance of proteinuria in pregnancy. Obstet Gynecol Surv 2007;62:117-124
- Hubel CA, Lyall F, Weissfeld L, et al. Small lowdensity lipoproteins and vascular cell adhesion molecule-1 are increased in association with hyperlipidemia in preeclampsia. Metabolism 1998; 47:1281-1288.