Research Article - Journal Clinical Psychiatry and Cognitive Psychology (2022) Volume 6, Issue 3
Knowledge and awareness of juvenile myoclonic epilepsy-A survey.
Lakshmi Narayanan* and Sreekala Priyadharsini
Department of Pharmacology, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Poonamallee High Road, Chennai, Tamil Nadu, India
- Corresponding Author:
- Lakshmi Narayanan
Department of Pharmacology, Saveetha Dental College and Hospitals
Saveetha Institute of Medical and Technical Sciences
Saveetha University, Poonamallee High Road
Chennai, Tamil Nadu, India
E-mail: lakshmi167.sdc@gmail.com
Received: 01-May-2022, Manuscript No. AACPCP-22-63331; Editor assigned: 03-Apr-2022, PreQC No. AACPCP-22-63331 (PQ); Reviewed: 17-May-2022, QC No.AACPCP-22-63331; Revised: 18-May-2022, Manuscript No. AACPCP-22-63331 (R); Published: 25-May-2022, DOI:10.35841/aacpcp- 6.3.113
Citation:Narayanan L, Priyadharsini S. Knowledge and awareness of juvenile myoclonic Epilepsy- A survey. J Clin Psychiatry Cog Psychol.2022;6(3):113
Abstract
Juvenile Myoclonic Epilepsy (JME), otherwise known as Janz syndrome and impulsive petit mal, is an idiopathic, hereditary, and generalized form of epilepsy. Characterized by myoclonic jerks of the arms or legs, generalised tonic clonic seizures and sometimes, absence seizures. The seizures of juvenile myoclonic epilepsy often occur when people first awaken in the morning. Seizures can be triggered by lack of sleep, extreme fatigue, stress, or alcohol consumption. Onset typically occurs around adolescence in otherwise healthy children. The causes of juvenile myoclonic epilepsy are very complex and not completely understood. Aim: To assess the knowledge and awareness of juvenile myoclonic epilepsy among the general public. Materials and methods: A survey based questionnaire was prepared through google forms and circulated via Whatsapp. A total response of 100 was collected and the results were analysed using SPSS statistical software. Results: Based on the survey, it is inferred that around 59% of the general public were aware of juvenile myoclonic epilepsy. Conclusion: Juvenile myoclonic epilepsy (JME) is a common idiopathic generalized epileptic syndrome that occurs in about 5-11% of all the epileptic subjects. Hence it is important to have a knowledge about juvenile myoclonic epilepsy among the general public
Keywords
Knowledge, Awareness, Juvenile myoclonic Epilepsy, General public, Seizures.
Introduction
Juvenile myoclonic epilepsy (JME) is a common idiopathic generalized epileptic syndrome [1]. That occurs in about 5-11% of all the epileptic subjects. JME has a prevalence of 0.5 to 1.0 per 1000 with age of onset between 12 and 18 years. Seizures are characterized by sudden myoclonic jerks of shoulders and arms that usually appear shortly after awakening. A majority of patients also experience generalized tonic–clonic seizures (GTCS). Myoclonic jerks generally precede GTCS. Up to 1/3rd of patients also experience absence attacks. The majority of patients with JME start having seizures between the ages of 12 to 18 with a mean age of onset around 15 years. But, the onset can be early or later with an age range between 5-34 years. As mentioned in the introduction, JME is characterized by the presence of absence, myoclonic, and generalized tonicclonic (GTC) seizures. All patients with JME have myoclonic seizures, 85%-90% of patients have GTC seizures, and about 20-40% of patients have absence seizures [2-5].
Myoclonic seizures are the hallmark of this condition and are necessary for the diagnosis of JME. Only occasionally they will manifest as the only seizure type. They are described as brief, usually bilateral, jerking movements of the arms and sometimes legs with preservation of consciousness. The misdiagnosis of myoclonic seizures is possible as twitches or anxiety/nervousness. Many a time, increasing intensity and frequency of myoclonic jerks in clusters evolve into GTC seizures. GTC seizures usually occur a few months after the onset of myoclonic seizures. Absence seizures are generally the first seizures to manifest in JME. They tend to occur 3 to 5 years before the onset of myoclonic or GTC seizures, sometimes as early as 5 to 6 years of age. The early and isolated occurrence of absence seizures frequently leads to a diagnosis of childhood or juvenile absence epilepsy. Seizures with JME occur 30 minutes to an hour after morning awakening, especially the myoclonic seizures. Usual triggers for JME are sleep deprivation, alcohol intake, emotional stress, anxiety, fatigue. A large proportion (30%-40%) of JME patients exhibit photosensitivity and flashing lights, sunlight, TV, and computer can trigger seizures. Photosensitive patients tend to have an earlier onset of seizures.
Physical examination remains normal, and cognition well preserved. Studies suggest an increased prevalence of psychiatric disorders, including anxiety, mood disorder, and personality disorders in patients with JME. JME is relatively easy to control with anti-epilepsy drugs (AEDs), and most patients respond to monotherapy. Valproic acid is the most effective medication and drug of choice for treatment of JME. It is a broad-spectrum medication and treats all seizure types of JME. Its use requires a great deal of caution in women of childbearing age, given its wellknown teratogenicity potential. Seizures are usually well controlled with medications in a great majority of patients with JME and generally improve after the fourth decade of life. But, JME requires lifelong treatment (even after a long seizure-free interval), as the chances of relapse are high if medications are discontinued. Our team has extensive knowledge and research experience that has translate into high quality publications [6-15]. The aim of our study is to assess the knowledge and awareness of juvenile myoclonic epilepsy among the general public.
Materials and Methods
This cross-sectional study was conducted among the undergraduate and postgraduate students of Saveetha Dental College and Hospital, Chennai. To evaluate the knowledge of juvenile myclonic epilepsy, a self-administered questionnaire containing 14 questions was prepared. The questions revolved around the awareness of the artifacts in cytology. The questionnaire was validated and later distributed to the participants. A web-based questionnaire was also developed using Google forms and was circulated. The participation of the subjects was kept voluntary and nobody was not obligated to fill the form. Questions were answered with “yes” or “no” or by marking the correct responses. Frequency analysis and percentage analysis were done with the obtained results. Data was recorded in Microsoft Excel and exported to the statistical package of social science for windows (SPSS) and subjected to statistical analysis. Descriptive statistics were done and Chi square tests are used for comparison
Results
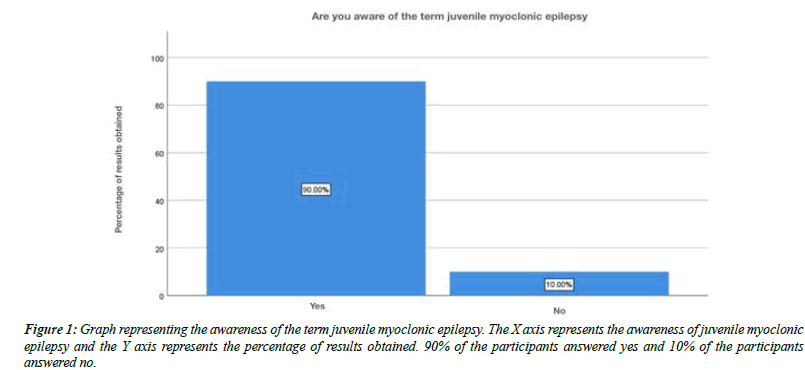
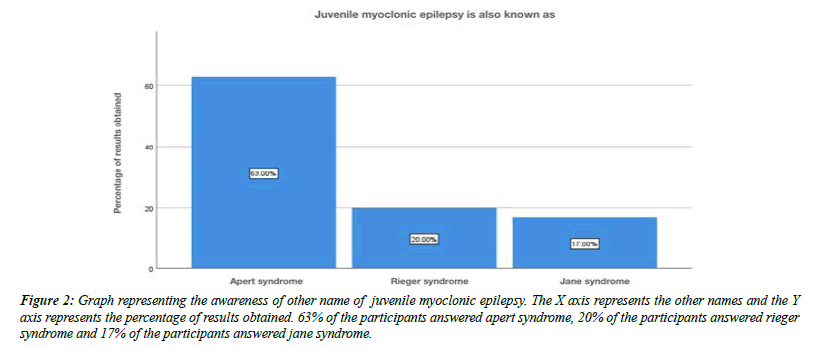
The overall response and the percent analysis were calculated for each question. The first question tests the awareness of the term juvenile myoclonic epilepsy. About 90% of the participants were aware, 10% were not aware of the term juvenile myoclonic epilepsy (Figure 1). The next question tests the other name for juvenile myoclonic epilepsy. About 63% of the participants answered apert syndrome, 20% of the participants answered rieger syndrome and about 17 % of the participants answered jane syndrome (Figure 2).
Figure 2: Graph representing the awareness of other name of juvenile myoclonic epilepsy. The X axis represents the other names and the Y axis represents the percentage of results obtained. 63% of the participants answered apert syndrome, 20% of the participants answered rieger syndrome and 17% of the participants answered jane syndrome.
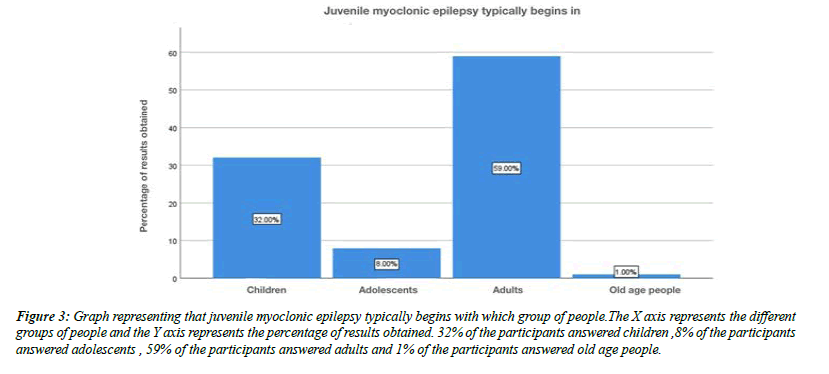
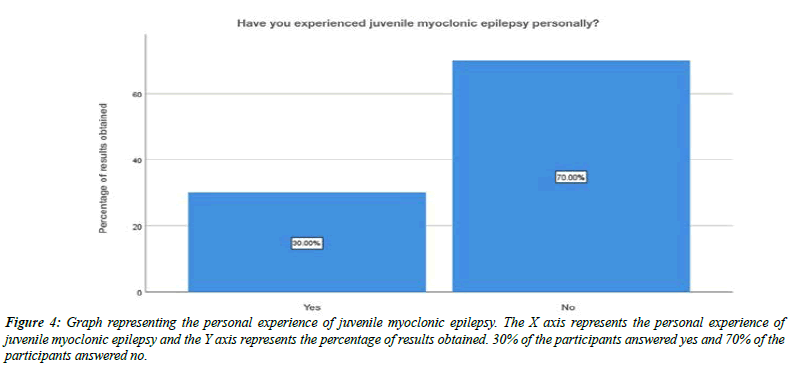
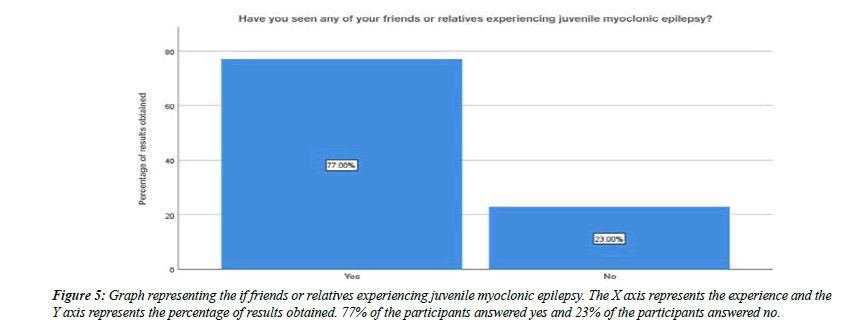
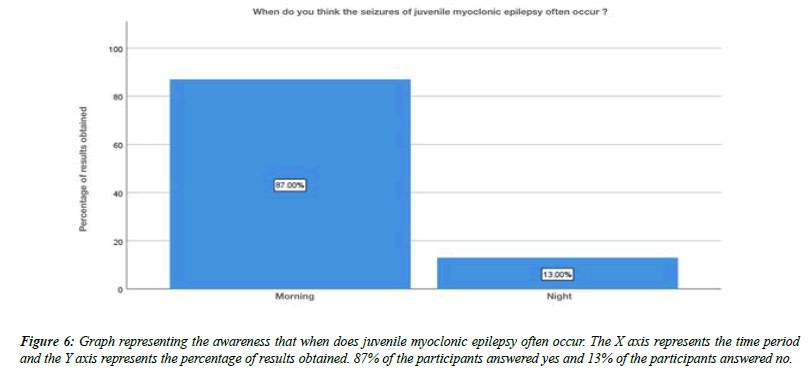
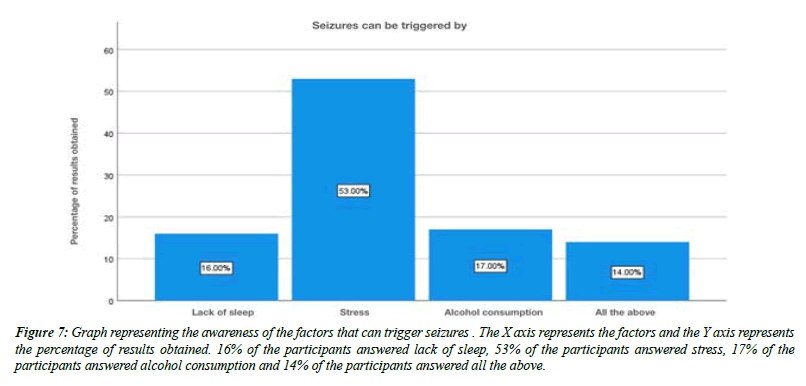
About 32% of the participants were aware that juvenile myoclonic epilepsy typically begins in children, 8% of the participants said that it beings in adolescents, 59% of the participants said that it begins in adults and about 1% of the participants answered that it begins with old age people (Figure 3). Moving onto the question of experiencing juvenile myoclonic epilepsy personally, 30% of the participants answered yes, 70% of the participants answered no (Figure 4). For the question, have you seen any of your friends or relatives experiencing juvenile myoclonic epilepsy, 77% of the participants answered yes, 23% of the participants answered no (Figure 5).For the question, when do you think the seizures of juvenile myoclonic epilepsy often occur, 87% of the participants answered occurs in the morning and 13% of the participants answered it occurs during night (Figure 6). For the question, which of the following do you think that triggered seizures, 16% of the participants answered lack of sleep, 53% of the participants answered stress, 17% of the participant answered alcohol consumption and 14% of the participants answered all the above (Figure 7).
Figure 3: Graph representing that juvenile myoclonic epilepsy typically begins with which group of people.The X axis represents the different groups of people and the Y axis represents the percentage of results obtained. 32% of the participants answered children ,8% of the participants answered adolescents , 59% of the participants answered adults and 1% of the participants answered old age people.
Figure 4: Graph representing the personal experience of juvenile myoclonic epilepsy. The X axis represents the personal experience of juvenile myoclonic epilepsy and the Y axis represents the percentage of results obtained. 30% of the participants answered yes and 70% of the participants answered no.
Figure 7: Graph representing the awareness of the factors that can trigger seizures . The X axis represents the factors and the Y axis represents the percentage of results obtained. 16% of the participants answered lack of sleep, 53% of the participants answered stress, 17% of the participants answered alcohol consumption and 14% of the participants answered all the above.
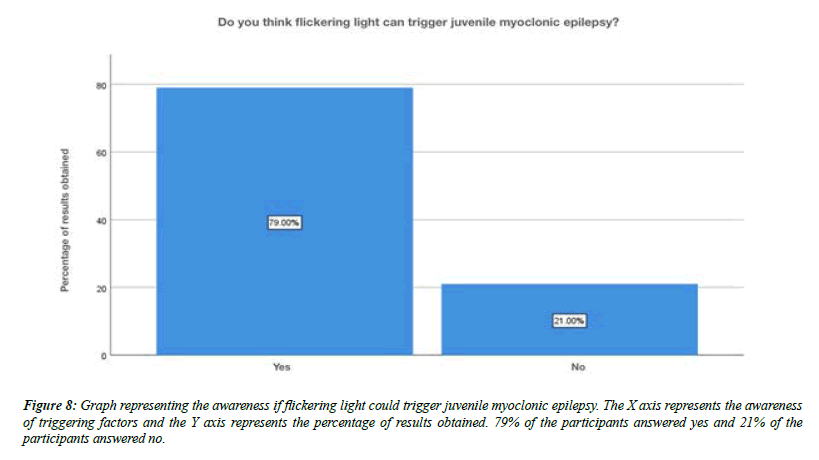
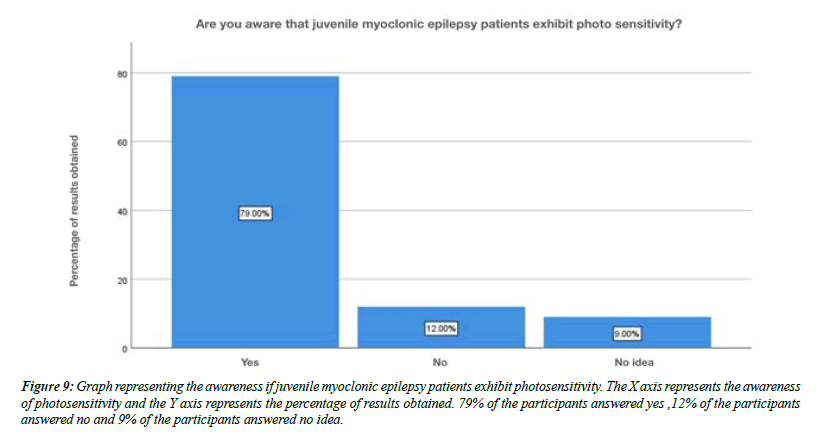
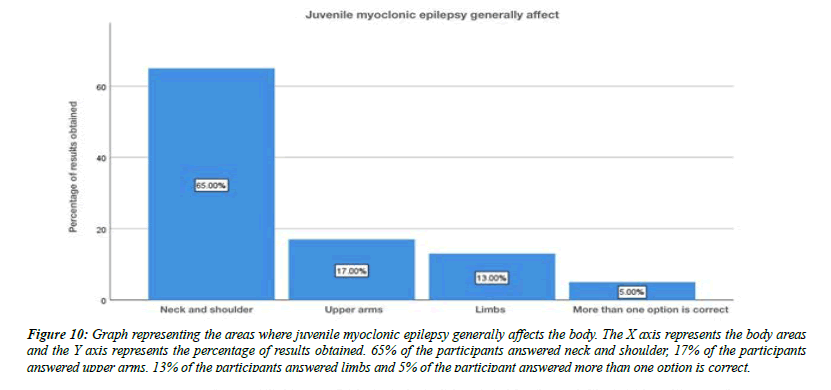
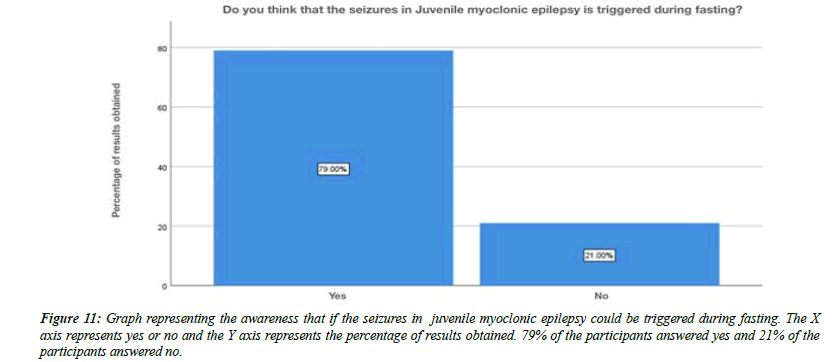
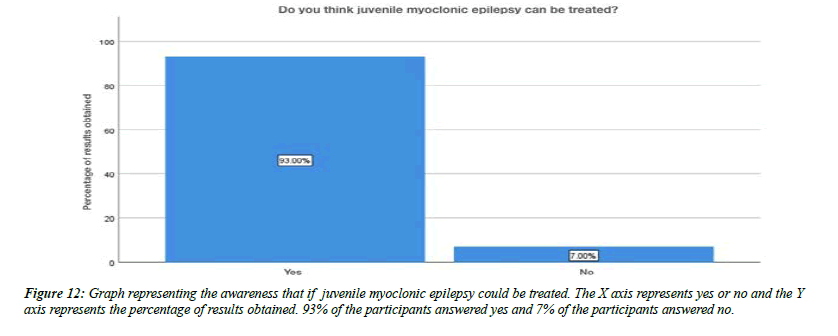
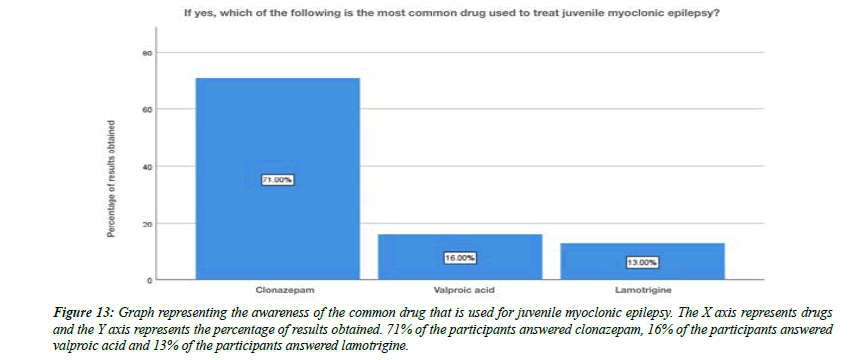
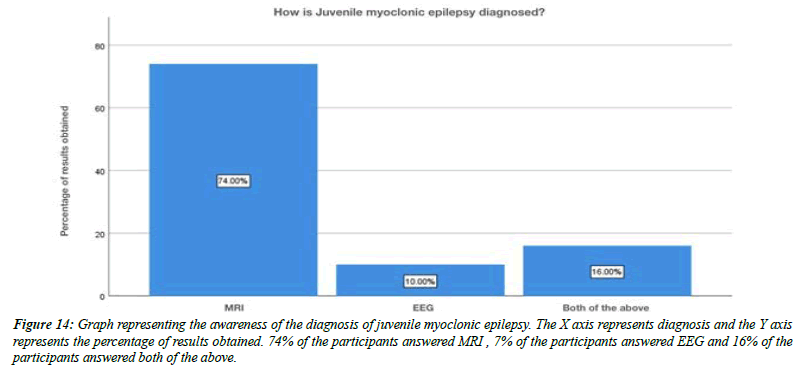
For the question, do you think flickering light can trigger juvenile myoclonic epilepsy, 79% of the participants answered yes , 21% of the participants answered no (Figure 8). For the question, are you aware that Juvenile myoclonic epilepsy patients exhibit photosensitivity, 79% of the participants answered yes, and 12% of the participants answered no and 9% of the participants answered no idea (Figure 9). For the question, which of the following do you think that juvenile myoclonic epilepsy generally affect , 65% of the participants answered neck and shoulder, 17% of the participants answered upper arms, 13% of the participants answered limbs and 5% of the participants answered more than one option is correct (Figure 10). For the question, do you think that the seizures in Juvenile myoclonic epilepsy are triggered during fasting, 79% of the participants answered yes and 21% of the participants answered no (Figure 11). For the question, do you think juvenile myoclonic epilepsy can be treated, 93% of the participants answered yes and 7% of the participants answered no (Figure 12). For the question , which of the following is the most common drug used to treat juvenile myoclonic epilepsy , 71% of the participants answered clonazepam, 16% of the participants answered valproic acid and 13% of the participants answered lamotrigine (Figure 13). For the question, how is Juvenile myoclonic epilepsy diagnosed, 74% of the participants answered MRI, 10% of the participants answered EEG and 16% of the participants answered both of the above (Figure 14).
Figure 8: Graph representing the awareness if flickering light could trigger juvenile myoclonic epilepsy. The X axis represents the awareness of triggering factors and the Y axis represents the percentage of results obtained. 79% of the participants answered yes and 21% of the participants answered no.
Figure 9: Graph representing the awareness if juvenile myoclonic epilepsy patients exhibit photosensitivity. The X axis represents the awareness of photosensitivity and the Y axis represents the percentage of results obtained. 79% of the participants answered yes ,12% of the participants answered no and 9% of the participants answered no idea.
Figure 10: Graph representing the areas where juvenile myoclonic epilepsy generally affects the body. The X axis represents the body areas and the Y axis represents the percentage of results obtained. 65% of the participants answered neck and shoulder, 17% of the participants answered upper arms, 13% of the participants answered limbs and 5% of the participant answered more than one option is correct.
Figure 11: Graph representing the awareness that if the seizures in juvenile myoclonic epilepsy could be triggered during fasting. The X axis represents yes or no and the Y axis represents the percentage of results obtained. 79% of the participants answered yes and 21% of the participants answered no.
Figure 13: Graph representing the awareness of the common drug that is used for juvenile myoclonic epilepsy. The X axis represents drugs and the Y axis represents the percentage of results obtained. 71% of the participants answered clonazepam, 16% of the participants answered valproic acid and 13% of the participants answered lamotrigine.
Figure 14: Graph representing the awareness of the diagnosis of juvenile myoclonic epilepsy. The X axis represents diagnosis and the Y axis represents the percentage of results obtained. 74% of the participants answered MRI , 7% of the participants answered EEG and 16% of the participants answered both of the above.
Discussion
The official definition of JME in the ILAE classification of epilepsies is Impulsive Petit Mal appears around puberty and is characterized by seizures with bilateral, single or repetitive, arrhythmic, irregular myoclonic jerks, predominantly in the arms. Jerks may cause some patients to fall suddenly. No disturbance of consciousness is noticeable. The disorder may be inherited, and sex distribution is equal. Often, there are GTC seizures and, less often, infrequent absences. The seizures usually occur shortly after awakening and are often precipitated by sleep deprivation. In our study about 16% of the participants were aware that lack of sleep may be one of the causes for juvenile myoclonic epilepsy (Figure 7). Photosensitivity, clinically quite unexpressed, is described from 38% of some series up to 50–90% of the cases depending on age, treatment, and modality of photic stimulation. In our study, for the question, are you aware that Juvenile myoclonic epilepsy patients exhibit photosensitivity, 79% of the participants answered yes, and 12% of the participants answered no and 9% of the participants answered no idea (Figure 9). Classic JME, defined as adolescent onset of isolated awakening myoclonic seizures appearing as the first seizure type or following GTC seizures in 72% of the patients. In our study about 32% of the participants were aware that juvenile myoclonic epilepsy typically begins in children, 8% of the participants said that it beings in adolescents, 59% of the participants said that it begins in adults and about 1% of the participants answered that it begins with old age people (Figure 3). Patients with JME often respond to low VPA doses of 1000 mg daily or less and a satisfactory response to a low dose (400–600mg) may occur. Presently, VPA is the first choice AED in men with JME, since evidence suggests that VPA should be avoided in women of childbearing age because of significantly dose-dependent increased risks of fetal malformations and impaired postnatal cognitive development, including autism spectrum disorder. In our study For the question , which of the following is the most common drug used to treat juvenile myoclonic epilepsy , 71% of the participants answered clonazepam, 16% of the participants answered valproic acid and 13% of the participants answered lamotrigine (Figure 13).
Among the factors of worse prognosis in JME should be emphasized: long duration of the disease, combination of the three seizure types, non-classical clinical presentations, as absences in general evolving to JME, focal EEG abnormalities, psychiatric comorbidities, expression of reflex traits and variants. Despite these evidences, anticipation of who will have refractory seizures in JME early phase is still a challenge in this complex epileptic syndrome [16-29].
Conclusion
JME appears no longer as a homogeneous condition but as a disease with a phenotypic spectrum that comprises different combinations of the main seizure type , presence or absence of localized features in semiology or EEG, remarkable psychopathology and neuropsychological traits and varying treatment response. This diversity seems to be due to a combination and interaction of genetically determined endophenotypes along with quantitative differences in the expression of the predominant pathogenic mechanisms. In our study it is conclusive that there is some awareness about juvenile myoclonic epilepsy among the general public.
References
- Eslava‐Cobos J, Naririo D. Experience with the International League Against Epilepsy proposals for classification of epileptic seizures and the epilepsies and epileptic syndromes in a pediatric outpatient epilepsy clinic. Epilepsia. 1989;30(1):112-5.
- Janz D. Epilepsy with impulsive petit mal (juvenile myoclonic epilepsy). Acta Neurol. Scand. 1985;72(5):449-59...
- Serratosa JM, Delgado‐Escueta AV, Medina MT, et al. Clinical and genetic analysis of a large pedigree with juvenile myoclonic epilepsy. Ann. Neurol. 1996;39(2):187-95.
- Rajeshkumar S, Kumar SV, Ramaiah A, et al. Biosynthesis of zinc oxide nanoparticles usingMangifera indica leaves and evaluation of their antioxidant and cytotoxic properties in lung cancer (A549) cells.Enzyme Microb. Technol. 2018;117:91-5.
- Nandhini NT, Rajeshkumar S, Mythili S. The possible mechanism of eco-friendly synthesized nanoparticles on hazardous dyes degradation. Biocatal. Agric. Biotechnol.2019;19:101138.
- Durner M, Janz D, Zingsem J, et al. Possible association of juvenile myoclonic epilepsy with HLA‐DRw6. Epilepsia. 1992;33(5):814-6.
- Vairavel M, Devaraj E, Shanmugam R. An eco-friendly synthesis of Enterococcus sp.–mediated gold nanoparticle induces cytotoxicity in human colorectal cancer cells. Environ. Sci. Pollut. Res.2020;27(8):8166-75.
- Canevini MP, Mai R, Di Marco C, et al. Juvenile myoclonic epilepsy of Janz: clinical observations in 60 patients. Seizure.1992;1(4):291-8.
- Rajkumar PV, Prakasam A, Rajeshkumar S, et al. Green synthesis of silver nanoparticles using Gymnema sylvestre leaf extract and evaluation of its antibacterial activity.S. Afr. J. Chem. Eng.2020;32(1):1-4.
- Vijai J, Cherian PJ, Sylaja PN, et al. Clinical characteristics of a South Indian cohort of juvenile myoclonic epilepsy probands. Seizure.2003;12(7):490-6.
- Rajasekaran S, Damodharan D, Gopal K, et al. Collective influence of 1-decanol addition, injection pressure and EGR on diesel engine characteristics fueled with diesel/LDPE oil blends. Fuel.2020;277:118166.
- Raj R K. β‐Sitosterol‐assisted silver nanoparticles activates Nrf2 and triggers mitochondrial apoptosis via oxidative stress in human hepatocellular cancer cell line. J. Biomed. Mater. Res. 2020;108(9):1899-908.
- Saravanan M, Arokiyaraj S, Lakshmi T, et al. Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microbial pathogenesis. 2018;117:68-72.
- Liu M, Concha L, Beaulieu C, et al. Distinct white matter abnormalities in different idiopathic generalized epilepsy syndromes. Epilepsia. 2011;52(12):2267-75.
- Gheena S, Ezhilarasan D. Syringic acid triggers reactive oxygen species–mediated cytotoxicity in HepG2 cells. Hum Exp Toxicol. 2019;38(6):694-702.
- Ezhilarasan D, Sokal E, Najimi M. Hepatic fibrosis: It is time to go with hepatic stellate cell-specific therapeutic targets. HBPD. 2018;17(3):192-7.
- Dua K, Hansbro PM, Wadhwa R, et al. Targeting Chronic Inflammatory Lung Diseases Using Advanced Drug Delivery Systems. AP; 2020.
- Moschetta S, Fiore LA, Fuentes D, et al. Personality traits in patients with juvenile myoclonic epilepsy. Epilepsy & Behavior. 2011;21(4):473-7.
- Murthy JM, Rao CM, Meena AK. Clinical observations of juvenile myoclonic epilepsy in 131 patients: a study in South India. Seizure. 1998;7(1):43–7.
- Tomson T, Battino D, Perucca E. Valproic acid after five decades of use in epilepsy: time to reconsider the indications of a time-honoured drug. The Lancet Neurology. 2016;15(2):210-8.
- Baykan B, Altindag EA, Bebek N, et al. Myoclonic seizures subside in the fourth decade in juvenile myoclonic epilepsy. Neurology. 2008;70:2123-9.
- Martínez-Juárez IE, Alonso ME, Medina MT, et al. Juvenile myoclonic epilepsy subsyndromes: family studies and long-term follow-up. Brain. 2006;129(5):1269-80.
- Rajeshkumar S, Kumar SV, Ramaiah A, et al. Biosynthesis of zinc oxide nanoparticles usingMangifera indica leaves and evaluation of their antioxidant and cytotoxic properties in lung cancer (A549) cells. Enzyme Microb. Technol. 2018;117:91-5.
- Nandhini NT, Rajeshkumar S, Mythili S. The possible mechanism of eco-friendly synthesized nanoparticles on hazardous dyes degradation. Biocatal. Agric. Biotechnol. 2019;19:101138.
- Vairavel M, Devaraj E, Shanmugam R. An eco-friendly synthesis of Enterococcus sp.–mediated gold nanoparticle induces cytotoxicity in human colorectal cancer cells. Environ. Sci. Pollut. Res. 2020;27(8):8166-75.
- Rajasekaran S, Damodharan D, Gopal K, et al. Collective influence of 1-decanol addition, injection pressure and EGR on diesel engine characteristics fueled with diesel/LDPE oil blends. Fuel. 2020;277:118166.
- Raj R K. β‐Sitosterol‐assisted silver nanoparticles activates Nrf2 and triggers mitochondrial apoptosis via oxidative stress in human hepatocellular cancer cell line.J. Biomed. Mater.Res.2020;108(9):1899-908.
- Saravanan M, Arokiyaraj S, Lakshmi T, et al. Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microb. Pathog. 2018;117:68-72.
- Ezhilarasan D, Sokal E, Najimi M. Hepatic fibrosis: It is time to go with hepatic stellate cell-specific therapeutic targets. HBPD. 2018;17(3):192-7.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.
Indexed at, Google Scholar, Cross Ref.